Abstract
AIM: To investigate the prevalence of vacuolating cytotoxin (vacA), cytotoxin associated gene A (cagA) and blood adhesion binding antigen (babA2) genotypes of Helicobacter pylori (H pylori) isolates from Cuban dyspeptic patients.
METHODS: DNA was extracted from H pylori-positive cultures taken from 130 dyspeptic patients. Genotyping was performed by PCR, using specific primers for vacA (s1, s2, m1, m2), cagA and babA2 genes. Endoscopic observations and histological examinations were used to determine patient pathologies.
RESULTS: vacA alleles s1, s2, m1 and m2 were detected in 96 (73.8%), 34 (26.2%), 75 (57.7%) and 52 isolates (40%), respectively, while the cagA gene was detected in 95 isolates (73.2%). One hundred and seven isolates (82.3%) were babA2-positive. A significant correlation was observed between vacAs1m1 and cagA and between vacAs1m1 and babA2 genotypes (P < 0.001 and P < 0.05, respectively) and between babA2 genotype and cagA status (P < 0.05); but, no correlation was observed between vacAs1 and babA2 genotypes. Eighty five (65.4%) and 73 (56.2%) strains were type 1 (vacAs1-cagA-positive) and “triple-positive” (vacAs1-cagA-babA2-positive), respectively, and their presence was significantly associated with duodenal ulcer (P < 0.01 and P < 0.001, respectively).
CONCLUSION: The distribution of the main virulence factors in the Cuban strains in this study resembled that of the Western-type strains, and the more virulent H pylori isolates were significantly associated with duodenal ulcer, ulcer disease being the worst pathology observed in the group studied.
Keywords: Cuban dyspeptic patients, Helicobacter pylori, vacA, cagA and babA
INTRODUCTION
Helicobacter pylori (H pylori), a spiral-shaped microaerophilic bacterium infects more than 50% of the world’s population, the rate of infection being higher in developing countries[1]. H pylori is a major etiological agent in several gastroduodenal diseases, such as functional dyspepsia, peptic ulcer disease, gastric cancer and mucosa-associated lymphoid tissue lymphoma. The clinical outcome following infection with this pathogen has been related to environmental conditions, host immunological factors and microorganism virulence[2].
Vacuolating cytotoxin (VacA), cytotoxin associated gene A (cagA), and blood adhesion binding antigen (babA) are the most commonly studied virulence markers of H pylori. However, there are other bacterial proteins with pathogenic potential, such as sialic acid-binding adhesin (SabA), outer inflammatory protein (oipA), and duodenal ulcer promoting gene (dupA); but, the influence of these proteins on H pylori pathogenesis is still under study[3].
The VacA protein induces vacuolation and apoptotic processes in epithelial cells, as well as immunosuppressive actions in immunological cells[4]. The vacA gene comprises two main regions: the signal zone (s1 or s2) and the middle region (m1 or m2)[5]. The vacA s1m1 allelic combination exhibits the highest activity, while s2m2 and the rare s2m1 combinations are non-toxic[5]. Recently, a new polymorphic region in the vacA gene called the intermediate region (i) has been discovered and its i1 active allele seems to be a better predictor of gastric cancer than the s1 or m1 allele[6].
Hydrophilic protein CagA contains the so-called EPIYA motifs[7], which interact with several eukaryotic proteins, promoting changes in the signal transduction pathway, cytoskeletal plasticity and IL-8 secretion in epithelial cells[8]. CagA-positive H pylori isolates are associated with a higher rate of gastric inflammation and damage, when compared with CagA-negative strains[8,9]. The cagA gene is located at the end of the cag pathogenecity island, a system that introduces CagA and a peptidoglycan into epithelial cells[10]. Several epidemiological studies have shown the correlation between cagA-positive strains and a higher risk of developing peptic ulceration, gastric atrophy and gastric cancer[8,9].
The blood group binding antigen mediates adherence of H pylori to human gastric epithelium[11]. This antigen is encoded by the polymorphic gene called babA2, while allele babA1 is non-functional[11]. Some studies have suggested that BabA plays a crucial role in the development of severe functional dyspepsia, peptic ulcer and gastric adenocarcinoma[12,13]. Furthermore, the combined presence of vacAs1 and cagA genotypes (type 1 strains) or even the “triple-positive” strains (vacAs1, cagA and babA2), has shown a higher correlation with the appearance of peptic ulcer, intestinal metaplasia and gastric cancer[14].
The clinical outcome of this bacterial infection seems to be influenced by the distribution of the above-mentioned pathogenic factors in H pylori strains[15]; but, complete genotyping of Cuban H pylori strains has never been carried out. Therefore, the aim of this study was to determine the frequency of the main virulence factor genes in Cuban H pylori isolates and establish their associations with the clinical outcome.
MATERIALS AND METHODS
Patients
H pylori isolates were obtained from 130 consecutive H pylori-positive patients (77 male and 53 female) with a mean age of 49.1 years (range, 18 to 88) who underwent routine endoscopy due to dyspeptic complaints at CIMEQ Hospital, Havana, Cuba. Endoscopic observation and histological confirmations were used to determine patient pathologies. This study was approved by the ethics committee at CIMEQ Hospital. All patients provided informed consent to participate in the study.
Microorganism culture
Antrum gastric biopsy specimens obtained from all patients were homogenized, inoculated into Columbia agar base plates with 7% human blood and SR0147E selective supplement (Oxoid, England, UK), and grown under microaerophilic conditions at 37°C for 5 to 8 d. All H pylori isolates were positive for oxidase, catalase and urease. The reference strain J99[16] was kindly provided by Professor Francis Megraud from Pellegrin Hospital, Bordeaux, France.
DNA extraction and cagA, vacA and babA2 genotyping
Genomic DNA was extracted by CTAB methodology with phenol/chloroform and isopropanol precipitation as previously described[17]. Purified DNAs were stored at -20°C until use. In all cases, PCR amplification was carried out in a 25 μL reaction mixture containing 2.5 μL 10X PCR buffer (Roche, Germany), 0.2 mmol/L of each deoxynucleotide triphosphate, 0.6 mM sense and antisense primers, 4 mmol/L magnesium chloride, 1.25 U Taq DNA polymerase (CIGB, Cuba) and 100 ng genomic DNA. The PCR had an initial step at 94°C for 1 min, followed by 40 cycles at 94°C for 1 min, 60°C for 1 min and 72°C for 1 min, and a final extension at 72°C for 5 min, using a Master Cycler apparatus (Eppendorf, Germany).
The primers used and their details are shown in Table 1. Primers to the glmM gene of H pylori were used to control DNA integrity and specificity. PCR products were analyzed on 1.5% agarose gel electrophoresis with ethidium bromide. Images were taken through the Gene Genius system (Syngene, England, UK).
Table 1.
Primer used for PCR genotyping of Cuban H pylori strains
| Primer | Sequence (5’-3’) | AT °C | Size (bp) | Ref. |
| glmMF | CCCTCACGCCATCAGTCCCAAAAA | 60 | 417 | [18] |
| glmMR | AAGAAGTCAAAAACGCCCCAAAAC | |||
| cagF1 | GATAACAGGCAAGCTTTTGA | 60 | 349 | [7] |
| cagB1 | CTGCAAAAGATTGTTTGGCAGA | |||
| vacAsF | ATGGAAATACAACAAACACAC | 52 | s1-259/s2-286 | [20] |
| vacAsR | CTGCTTGAATGCGCCAAAC | |||
| vacAmF | CAATCTGTCCAATCAAGCGAG | 56 | m1-567/m2-642 | [20] |
| vacAmR | GCGTCAAAATAATTCCAAGG | |||
| bab7-F | CCAAACGAAACAAAAAGCGT | 60 | 271 | [21] |
| bab7-R | GCTTGTGTAAAAGCCGTCGT | |||
| babA2F1 | AATCCAAAAAGGAGAAAAAGTATGAAA | 60 | 832 | [13] |
| babA2R | TGTTAGTGATTTCGGTGTAGGACA | |||
| babA2R6072 | GTTTTCTTTGAGCGCGGGTAAGC | 60 | 607 | [14] |
Forward primer used with primer babA2R or babAR607 to amplify babA2 gene;
Five nucleotides (GTTTT) were added to the original primer designed by Zambon et al[14] to increase specificity.
Statistical analysis
Differences among groups were tested using the χ2 test. P values < 0.05 were considered to be significant. The statistic software, version 8 for Windows, was used for statistical analysis.
RESULTS
Detection of H pylori genotypes
H pylori was successfully cultured from 130 Cuban dyspeptic patients. DNA integrity and specificity was confirmed by glmM PCR, which rendered the expected 417 bp band from all isolates (data not shown). PCR product sizes of vacA s and m alleles were used to differentiate them in agarose gels (Figure 1, panel A). The most virulent vacAs1 allele was predominantly present in Cuban H pylori isolates (Table 2), and was visualized as a band of 259 bp on agarose gel electrophoresis (Figure 1, panel A), whereas 26.2% of isolates had the vacAs2 genotype (Table 2). The middle region of the vacA gene was detected in only 127 of the 130 isolates, m1 and m2 genotypes were more equally distributed than s genotypes (Table 2). On the other hand, s1m1 and s2m2 genotypes were the most common allelic combinations of the vacA gene among Cuban isolates, and only one strain harbored the s2m1 genotype (Table 2).
Figure 1.
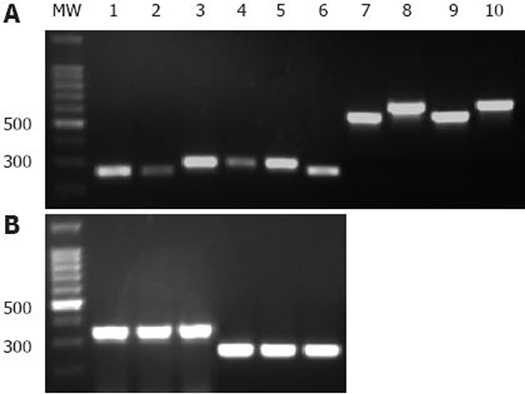
Genotyping of main virulence factor genes in Cuban H pylori isolates. The images shown are from a representative gel electrophoresis of two independent PCR amplification products of vacA (s1, s2, m1, m2), cagA and babA2 genes from Cuban isolates and J99 control strain. A: Lanes 1 and 7, reference strain J99 (vacAs1 and m1 alleles, respectively); Lanes 2 and 6, vacAs1 strains; Lanes 3-5, vacAs2 strains; Lanes 8 and 10 vacAm2 strains; Lane 9, vacAm1 strain. B: Lanes 1 and 4, J99 strain (cagA and babA2 gene, respectively); Lanes 2 and 3, cagA-positive strains; Lanes 5 and 6 babA2-positive strains. MW: 100 bp DNA Ladder (Promega, USA).
Table 2.
Correlation between vacA alleles and cagA and babA2 genotypes in 130 Cuban H pylori isolates
| vacA | s1m1 | s1m2 | s2m2 | s2m1 | s1m- | Total (%) |
| cagA+ | 70 | 14 | 8 | 1 | 2 | 95 (73.2) |
| cagA- | 4 | 5 | 25 | 0 | 1 | 35 (26.8) |
| babA2+ | 67 | 14 | 24 | 1 | 1 | 107 (82.3) |
| babA2- | 7 | 5 | 9 | 0 | 2 | 23 (17.7) |
| Total (%) | 74 (56.9) | 19 (14.6) | 33 (35.4) | 1 (0.8) | 3 (2.3) | 130 |
Amplification of the cagA gene was visualized as a band of 349 bp (Figure 1, panel B) and was present in 73.2% of the strains (Table 2). When primers babA7F/babA7R (Table 1) were used to amplify the babA2 gene, over 80% of the Cuban strains carried this gene (Table 2). In contrast, a low prevalence of babA2 genotype was observed among the Cuban isolates when using primers babA2F/babA2R and babA2F/babA2R607 (Table 1).
Combinations of vacA, cagA and babA2 genotypes
On examining the association of the main virulence genes in each strain, a statistically significant correlation was observed between s1m1 genotype and cagA status (P = 0.00001), between s1m1 and babA2 genotypes (P = 0.047), and between cagA and babA2 genotypes (P = 0.049). A significant association was also observed between vacAm1 allele and cagA status or babA2 genotype (P = 0.00001 and P = 0.035, respectively), while most s2m2 strains carried a cagA-negative genotype (Table 2). However, no correlation was observed between vacAs1 and babA2 genotypes (P = 0.12). Additionally, 85 isolates were classified as type 1 strains and 73 were triple-positive strains (Table 3).
Table 3.
Correlation between virulence factor genotypes and disease outcome
| Genotypes |
Pathologies |
||
| FD n = 51 (%) | GU n = 33 (%) | DU n = 46 (%) | |
| vacAs1m1 | 28 (54.9) | 16 (48.5) | 30 (65.2) |
| s1m2 | 5 (9.7) | 9 (27.3) | 5 (10.9) |
| s2m2 | 16 (31.4) | 6 (18.2) | 11 (23.9) |
| s1m- | 1 (2) | 2 (6) | - |
| s2m1 | 1 (2) | - | - |
| cagA+ | 36 (70.6) | 19 (57.6) | 40 (87) |
| babA2+ | 36 (70.6) | 28 (84.8) | 43 (93.5)b |
| Type 1 | 29 (56.9) | 16 (48.5) | 40 (87)d |
| Triple-positive | 24 (47.1) | 12 (36.4) | 37 (80.4)f |
FD: Functional dyspepsia; GU: Gastric Ulcer; DU: Duodenal Ulcer; P values were calculated with the χ2 test; b, d, fStatistically significant differences (P values < 0.01).
Relationship between genotypes and gastric diseases
Of the 130 H pylori infected patients studied, 39.2% were diagnosed with functional dyspepsia, 35.4% had a duodenal ulcer (DU) and 25.4% had a gastric ulcer (GU). Table 3 shows that the vacAs1m1 genotype was detected at a higher frequency in isolates from patients with DU, and in strains obtained from patients with functional dyspepsia; but, the presence of this genotype did not correlate with the presence of duodenal or gastric ulcer (P = 0.21 and P = 0.4, respectively). On the other hand, the vacA s1m1 genotype had a higher frequency in DU patients; but, no association was observed between s1m1 or any other vacA genotype, and the presence of severe pathologies in this study (Table 3). GU patients exhibited the highest frequency of s2m2 strains, followed by patients with functional dyspepsia (Table 3). No correlation was found between the cagA genotype and duodenal or gastric ulcer (P = 0.051 and P = 0.22, respectively); but, an association between cagA-positive strains and DU may be assumed as a clear tendency (Table 3). Meanwhile, the babA2 genotype was significantly associated with DU (P = 0.004), but not with GU (P = 0.13). Type 1 and triple-positive strains (Table 3) were also associated with DU (P = 0.001 and P = 0.0007, respectively) but not with GU (P = 0.45 and P = 0.33, respectively).
DISCUSSION
Several studies have shown that the incidence and/or severity of gastroduodenal pathologies related to H pylori may vary between geographic areas. This phenomenon is partly due to a different distribution of pathogenic markers in circulating strains[15]. Several pathogenic factors of H pylori have been described and their association with the clinical outcome studied[19–21]. Distribution of the main virulence factors around the world is summarized in Table 4, showing the high variation between geographic areas. This is the first report to examine the three main H pylori virulence associated genes, vacA, cagA and babA2 in Cuban isolates.
Table 4.
Worldwide distribution of main H pylori virulence factors
| World Area |
vacA alleles prevalence (%) |
References | cagAprevalence (%) cagA+ | babA2prevalence (%) babA2+ | |||
| s1 | s2 | m1 | m2 | ||||
| Europe | 48-89 | 11-51 | 37 | 63 | [14,23,26,27] | 66-73[14,23,26,27] | 34-72[13,14,34] |
| America | 57-68 | 16-48 | 37-44 | 29-63 | [19,20,24] | 57-75[7,19,24] | 46-69[24,32] |
| East Asia | 100 | 0 | 41-94 | 5-55 | [12,25,28] | 90-100[12,25,28,35] | 80-100[12,21,35] |
vacA alleles
The vacA s1 and s2 leader sequences are different in a small insert, totaling 27 bp, carried by the vacAs2 allele[20], which has a reduced capacity to secrete VacA toxin[22]. According to our results, the most virulent vacAs1 allele was predominant in Cuban H pylori isolates (Table 2), a finding which has also been observed in other studies of Western strains (Table 4)[23,24]. In the present study, the prevalence of vacAm1 and vacAm2 were similar compared to that of the s1 and s2 allele; meanwhile, the s1m1 and s2m2 genotypes were the most common allelic combinations of the vacA gene from Cuban isolates (Table 2), a finding reported in several studies from various countries[19,20]. Furthermore, the middle region of vacA was not detected in three isolates, while only one strain harbored the s2m1 genotype. Genotyping of the vacA middle region failed in three strains, probably due to heterogeneity in the vacA gene, a finding described previously[12,24]. Additionally, only one strain harbored the s2m1 genotype, the vacA allelic combination relating to lower incidence in several studies[23,24]. On the other hand, the vacA s1m1 genotype was noted at a higher frequency in DU patients; but, no significant correlation was observed between vacA genotypes and the appearance of peptic ulcer disease, which is in agreement with previous reports[19,25].
cagA genotype
H pylori cagA-positive strains have been associated with more severe gastroduodenal diseases[8,14,15]. Here, 73.2% of the H pylori strains were cagA-positive, a prevalence similar to that reported in many studies from Western countries (e.g. USA, 60%[7]; Spain, 66%[26]; and England, 68%[27]) but lower than that reported in some East Asian studies, which encountered over 90% of cagA-positive isolates (Table 4)[25,28]. In addition, a highly significant correlation was observed between cagA status and vacAs1 and vacAs1m1 genotypes (Table 2), which is commonly linked to an increase in H pylori virulence[13,14,29,30]. An association was also observed between the presence of the cagA gene and the babA2-positive genotype, due to the fact that most cagA-positive isolates carried the babA2 allele. Our data support the relationship between cagA and babA2 genes found in previous reports, which could be caused by selective pressure[13,14], although other authors, such as Mattar et al[24], did not find any correlation between these virulence factors in the isolates investigated. On the other hand, previous studies have shown a high association between the cagA-positive genotype and the appearance of DU[31,32]. In this study, however, no correlation was observed between cagA status and DU. Moreover, a high frequency of cagA-positive strains was observed in DU patients (Table 3), indicating that a statistical association could be reached by increasing the number of patients in future studies.
babA2 genotype
Adherence of H pylori to epithelial cells is a relevant step in the development of gastroduodenal pathologies. BabA2 attaches H pylori to these cells, allowing delivery of VacA and CagA toxins near the gastric epithelium and therefore enhancing gastric tissue damage[3,11]. Here, Cuban H pylori isolates exhibited a high frequency (82.3%) of the babA2 allele when the primers of Sheu et al[21] were used to amplify the gene. In contrast, a low prevalence of the babA2 genotype was observed when the primers reported by Gerhard et al[13] and a variant of Zambon et al[14] were used, respectively (Table 2). Interestingly, these last two primers are located in a high polymorphic zone of the babA gene[33], which should lead to an underestimation of babA2-positive strains. Our results add new data to previous observations[24,34] that support the ineffectiveness of the Gerhard et al[13] primers to detect the babA2 gene, and for the first time relate this to deficiencies in the primers used by Zambon et al[14]. Consequently, the low levels of babA2 alleles reported in several previous studies[13,14,32] may be underestimated, due to the use of Gerhard primers[13]. However, the prevalence of the babA2 gene was above 70% in Asian countries using the same primers[12,35], suggesting that underestimation due to allelic variation in the babA gene could have a variable impact in different geographic areas, as was previously suggested[34]. This study showed a high association between the presence of the babA2 allele and DU disease (Table 3), which is in agreement with several reports which associate the presence of this gene with the appearance of severe gastric damage[13,14]. However, other studies have claimed no association between this genotype and more severe pathologies[24].
Combination of virulence genotypes
Of the 130 Cuban H pylori isolates, 65.4% and 56.2% were type 1 and triple-positive strains, respectively. Infection with these strains has been associated with a higher degree of inflammation and gastroduodenal lesions[14]. Similar percentages of both types of strains were found in this study and in a previous report[13], while Brazilian dyspeptic patients seem to have a lower rate (32.6%) of triple-positive strains[24]. Our data indicate that type 1 and triple-positive strains increase the risk of developing DU in Cuban dyspeptic patients, a finding consistent with other studies, in which these types of strains were mainly found in subjects with peptic ulcer disease[13], and in patients with intestinal metaplasia and gastric atrophy[14].
We hypothesize that the absence of a correlation between the virulence genes analyzed and the development of GU might be influenced by the small number of patients with this pathology in our study, although several other studies have not found any correlation between the presence of H pylori main virulence factor genes (alone or in combinations) and peptic ulcer disease[24,25].
It is interesting to note that despite the high rate of H pylori infection in Cuban dyspeptic patients[36–38], and the relatively high pathogenic potential of Cuban isolates found previously[37,38] and in the present study, a low incidence of gastric adenocarcinoma has been found in Cuban patients with dyspepsia[36–38]. This reflects a general tendency in the Cuban population towards low levels of gastric cancer; in fact, a gastric cancer death rate of 7.1/100 000 was observed in Cuba in 2007 (http://www.sld.cu/servicios/estadisticas/). Future studies are required to elucidate the above-mentioned investigative problem, including a full characterization of Cuban H pylori isolates.
In conclusion, this study has shown a relatively high prevalence of the main virulence factor genes in Cuban H pylori isolates, which is similar to that found in the Western-type strains. In addition, a significant association was found between the virulence genes in Cuban strains. Consequently, the presence of more virulent type 1 and triple-positive strains was relatively high in Cuban dyspeptic patients, and increased their risk of developing duodenal ulcer. On the other hand, more severe gastroduodenal pathologies, such as intestinal metaplasia, gastric atrophy and gastric cancer were not found in this study, or in other similar studies and which might merit further research.
COMMENTS
Background
Cuba has a high incidence of H pylori infection. The presence and association of the main virulence factors VacA, CagA and BabA2 in H pylori strains influences the clinical outcome following infection with this pathogen. So far, no whole genotyping of Cuban H pylori strains has been carried out. This study addresses the frequency and association of the main virulence factor genes in H pylori isolates, and establishes their relationship to clinical outcome in a Cuban dyspeptic population.
Research frontiers
In a dyspeptic population in Cuba, the presence and association of the main virulence factor genes (vacA, cagA and babA2) in the infecting strains was significantly high, and their combined presence is a risk factor for duodenal ulcer (DU), but is not associated with gastric ulcer (GU). More severe pathologies, such as intestinal metaplasia, gastric atrophy and gastric cancer were not present in the group studied.
Innovations and breakthroughs
Studies of various populations have indicated an association between the presence of vacA, cagA and babA2 genes in H pylori isolates and the appearance of more severe gastroduodenal pathologies. The distribution of these virulence markers in H pylori strains varies among populations. The present study showed a relatively high prevalence of the main virulence factor genes in Cuban H pylori isolates, similar to that found in the Western-type strains. In addition, the study demonstrated a significant association between the virulence genes in the strains studied, which was related to the risk of developing DU, but not GU in dyspeptic patients. Furthermore, despite the relatively high virulence potential of Cuban H pylori isolates, pathologies such as intestinal metaplasia, gastric atrophy and gastric cancer were not present in the dyspeptic population studied.
Applications
In developing countries with a high incidence of H pylori infection and dyspepsia, it is important to screen the isolates for main virulence factors. The information generated here may be used to develop a procedure to detect H pylori pathogenic factors in a given population from biopsy samples. Intervention may then be concentrated on subjects with a higher risk of severe pathologies.
Peer review
This study determined the prevalence of main virulence factor genes vacA, cagA and babA2 in Cuban H pylori isolates and their association with gastroduodenal diseases.
Acknowledgments
We acknowledge Yampier Roblejo, Marcia Samada, Juan González, Rafael Fando, Margot Martínez and Orlando Reyes for their genuine contributions to endorse the data and conclusions of the manuscript. We are also grateful to Francis Megraud for the reference strain J99 and to Ana Laura Lopez for her technical assistance.
Footnotes
Supported by The National Centre for Scientific Research of Cuba, No. 220207
Peer reviewer:Harry HX Xia, PhD, MD, Novartis Pharmaceuticals Corporation, One Health Plaza, East Hanover, NJ 07936-1080, United States
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
References
- 1.1 Ahuja V, Sharma MP. High recurrence rate of Helicobacter pylori infection in developing countries. Gastroenterology. 2002;123:653–654. doi: 10.1053/gast.2002.35224. [DOI] [PubMed] [Google Scholar]
- 2.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 3.Figueiredo C, Machado JC, Yamaoka Y. Pathogenesis of Helicobacter pylori Infection. Helicobacter. 2005;10 Suppl 1:14–20. doi: 10.1111/j.1523-5378.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- 4.Gebert B, Fischer W, Haas R. The Helicobacter pylori vacuolating cytotoxin: from cellular vacuolation to immunosuppressive activities. Rev Physiol Biochem Pharmacol. 2004;152:205–220. doi: 10.1007/s10254-004-0027-3. [DOI] [PubMed] [Google Scholar]
- 5.Yang JC, Kuo CH, Wang HJ, Wang TC, Chang CS, Wang WC. Vacuolating toxin gene polymorphism among Helicobacter pylori clinical isolates and its association with m1, m2, or chimeric vacA middle types. Scand J Gastroenterol. 1998;33:1152–1157. doi: 10.1080/00365529850172494. [DOI] [PubMed] [Google Scholar]
- 6.Rhead JL, Letley DP, Mohammadi M, Hussein N, Mohagheghi MA, Eshagh Hosseini M, Atherton JC. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133:926–936. doi: 10.1053/j.gastro.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 7.Tummuru MK, Cover TL, Blaser MJ. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993;61:1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatakeyama M, Higashi H. Helicobacter pylori CagA: a new paradigm for bacterial carcinogenesis. Cancer Sci. 2005;96:835–843. doi: 10.1111/j.1349-7006.2005.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu AH, Crabtree JE, Bernstein L, Hawtin P, Cockburn M, Tseng CC, Forman D. Role of Helicobacter pylori CagA+ strains and risk of adenocarcinoma of the stomach and esophagus. Int J Cancer. 2003;103:815–821. doi: 10.1002/ijc.10887. [DOI] [PubMed] [Google Scholar]
- 10.Selbach M, Moese S, Meyer TF, Backert S. Functional analysis of the Helicobacter pylori cag pathogenicity island reveals both VirD4-CagA-dependent and VirD4-CagA-independent mechanisms. Infect Immun. 2002;70:665–671. doi: 10.1128/iai.70.2.665-671.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, Incecik ET, Berg DE, Covacci A, Engstrand L, Borén T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 12.Yu J, Leung WK, Go MY, Chan MC, To KF, Ng EK, Chan FK, Ling TK, Chung SC, Sung JJ. Relationship between Helicobacter pylori babA2 status with gastric epithelial cell turnover and premalignant gastric lesions. Gut. 2002;51:480–484. doi: 10.1136/gut.51.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerhard M, Lehn N, Neumayer N, Borén T, Rad R, Schepp W, Miehlke S, Classen M, Prinz C. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci USA. 1999;96:12778–12783. doi: 10.1073/pnas.96.22.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zambon CF, Navaglia F, Basso D, Rugge M, Plebani M. Helicobacter pylori babA2, cagA, and s1 vacA genes work synergistically in causing intestinal metaplasia. J Clin Pathol. 2003;56:287–291. doi: 10.1136/jcp.56.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaoka Y, Kato M, Asaka M. Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern Med. 2008;47:1077–1083. doi: 10.2169/internalmedicine.47.0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL, et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–80. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 17.Xu C, Li ZS, Tu ZX, Xu GM, Gong YF, Man XH. Distribution of cagG gene in Helicobacter pylori isolates from Chinese patients with different gastroduodenal diseases and its clinical and pathological significance. World J Gastroenterol. 2003;9:2258–2260. doi: 10.3748/wjg.v9.i10.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Musich PR, Ha T, Ferguson DA Jr, Patel NR, Chi DS, Thomas E. High prevalence of Helicobacter pylori in saliva demonstrated by a novel PCR assay. J Clin Pathol. 1995;48:662–666. doi: 10.1136/jcp.48.7.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faundez G, Troncoso M, Figueroa G. cagA and vacA in strains of Helicobacter pylori from ulcer and non-ulcerative dyspepsia patients. BMC Gastroenterol. 2002;2:20. doi: 10.1186/1471-230X-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atherton JC, Cao P, Peek RM Jr, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 21.Sheu BS, Sheu SM, Yang HB, Huang AH, Wu JJ. Host gastric Lewis expression determines the bacterial density of Helicobacter pylori in babA2 genopositive infection. Gut. 2003;52:927–932. doi: 10.1136/gut.52.7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClain MS, Cao P, Iwamoto H, Vinion-Dubiel AD, Szabo G, Shao Z, Cover TL. A 12-amino-acid segment, present in type s2 but not type s1 Helicobacter pylori VacA proteins, abolishes cytotoxin activity and alters membrane channel formation. J Bacteriol. 2001;183:6499–6508. doi: 10.1128/JB.183.22.6499-6508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudi J, Kolb C, Maiwald M, Kuck D, Sieg A, Galle PR, Stremmel W. Diversity of Helicobacter pylori vacA and cagA genes and relationship to VacA and CagA protein expression, cytotoxin production, and associated diseases. J Clin Microbiol. 1998;36:944–948. doi: 10.1128/jcm.36.4.944-948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattar R, dos Santos AF, Eisig JN, Rodrigues TN, Silva FM, Lupinacci RM, Iriya K, Carrilho FJ. No correlation of babA2 with vacA and cagA genotypes of Helicobacter pylori and grading of gastritis from peptic ulcer disease patients in Brazil. Helicobacter. 2005;10:601–608. doi: 10.1111/j.1523-5378.2005.00360.x. [DOI] [PubMed] [Google Scholar]
- 25.Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999;37:2274–2279. doi: 10.1128/jcm.37.7.2274-2279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alarcón T, Domingo D, Martinez MJ, López-Brea M. cagA gene and vacA alleles in Spanish Helicobacter pylori clinical isolates from patients of different ages. FEMS Immunol Med Microbiol. 1999;24:215–219. doi: 10.1111/j.1574-695X.1999.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 27.Warburton VJ, Everett S, Mapstone NP, Axon AT, Hawkey P, Dixon MF. Clinical and histological associations of cagA and vacA genotypes in Helicobacter pylori gastritis. J Clin Pathol. 1998;51:55–61. doi: 10.1136/jcp.51.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeda S, Ogura K, Yoshida H, Kanai F, Ikenoue T, Kato N, Shiratori Y, Omata M. Major virulence factors, VacA and CagA, are commonly positive in Helicobacter pylori isolates in Japan. Gut. 1998;42:338–343. doi: 10.1136/gut.42.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Doorn LJ, Figueiredo C, Sanna R, Blaser MJ, Quint WG. Distinct variants of Helicobacter pylori cagA are associated with vacA subtypes. J Clin Microbiol. 1999;37:2306–2311. doi: 10.1128/jcm.37.7.2306-2311.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kidd M, Lastovica AJ, Atherton JC, Louw JA. Conservation of the cag pathogenicity island is associated with vacA alleles and gastroduodenal disease in South African Helicobacter pylori isolates. Gut. 2001;49:11–17. doi: 10.1136/gut.49.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nomura AM, Pérez-Pérez GI, Lee J, Stemmermann G, Blaser MJ. Relation between Helicobacter pylori cagA status and risk of peptic ulcer disease. Am J Epidemiol. 2002;155:1054–1059. doi: 10.1093/aje/155.11.1054. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira AG, Santos A, Guerra JB, Rocha GA, Rocha AM, Oliveira CA, Cabral MM, Nogueira AM, Queiroz DM. babA2- and cagA-positive Helicobacter pylori strains are associated with duodenal ulcer and gastric carcinoma in Brazil. J Clin Microbiol. 2003;41:3964–3966. doi: 10.1128/JCM.41.8.3964-3966.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pride DT, Meinersmann RJ, Blaser MJ. Allelic Variation within Helicobacter pylori babA and babB. Infect Immun. 2001;69:1160–1171. doi: 10.1128/IAI.69.2.1160-1171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olfat FO, Zheng Q, Oleastro M, Voland P, Borén T, Karttunen R, Engstrand L, Rad R, Prinz C, Gerhard M. Correlation of the Helicobacter pylori adherence factor BabA with duodenal ulcer disease in four European countries. FEMS Immunol Med Microbiol. 2005;44:151–156. doi: 10.1016/j.femsim.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Mizushima T, Sugiyama T, Komatsu Y, Ishizuka J, Kato M, Asaka M. Clinical relevance of the babA2 genotype of Helicobacter pylori in Japanese clinical isolates. J Clin Microbiol. 2001;39:2463–2465. doi: 10.1128/JCM.39.7.2463-2465.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suárez RY, Samada M, Cansino JG, Sabatier CA, Arroyo MM, Marrero A, Fando R y Rodríguez BL. Comparación de métodos en el diagnóstico de la infección por Helicobacter pylori en pacientes con desórdenes gastroduodenales. Rev CNIC Cienc Biol. 2005;36:191–197. [Google Scholar]
- 37.Valmaseda T, Gisbert JP, Paniagua M, Pajares JM. [Helicobacter pylori CagA antibodies in various gastroduodenal diseases from 2 different populations] Med Clin (Barc) 2002;118:90–93. doi: 10.1016/s0025-7753(02)72295-1. [DOI] [PubMed] [Google Scholar]
- 38.Gutiérrez B, Vidal T, Valmaña CE, Camou-Juncas C, Santos A, Mégraud F, González N, Leonard I, Martínez R, Díaz-Canel O, et al. Helicobacter pylori infection in Havana, Cuba. Prevalence and cagA status of the strains. VacciMonitor. 2005;14:15–19. [Google Scholar]


