Abstract
AIM: To elucidate the interaction between non-parenchymal cells, extracellular matrix and oval cells during the restituting process of liver injury induced by partial hepatectomy (PH).
METHODS: We examined the localization of oval cells, non-parenchymal cells, and the extracellular matrix components using immunohistochemical and double immunofluorescent analysis during the proliferation and differentiation of oval cells in N-2-acetylaminofluorene (2-AAF)/PH rat model.
RESULTS: By day 2 after PH, small oval cells began to proliferate around the portal area. Most of stellate cells and laminin were present along the hepatic sinusoids in the periportal area. Kupffer cells and fibronectin markedly increased in the whole hepatic lobule. From day 4 to 9, oval cells spread further into hepatic parenchyma, closely associated with stellate cells, fibronectin and laminin. Kupffer cells admixed with oval cells by day 6 and then decreased in the periportal zone. From day 12 to 15, most of hepatic stellate cells (HSCs), laminin and fibronectin located around the small hepatocyte nodus, and minority of them appeared in the nodus. Kupffer cells were mainly limited in the pericentral sinusoids. After day 18, the normal liver lobule structures began to recover.
CONCLUSION: Local hepatic microenvironment may participate in the oval cell-mediated liver regeneration through the cell-cell and cell-matrix interactions.
Keywords: Oval cells, Liver regeneration, Extracellular matrix, Hepatic stellate cells, Kupffer cells
INTRODUCTION
The capacity of hepatocytes and cholangiocytes to contribute to their own maintenance has long been recognized. They transiently enter the cell cycle and proliferate to restore the lost liver mass after parenchymal damage[1,2]. If the proliferation of hepatocytes is inhibited or blocked (such as by viral infection or chemicals), or if liver damage is extensive and chronic, putative liver progenitor cells are activated to proliferate into mature liver cells[3–7]. Liver progenitor cells are evident in both human pathologic ductular reactions and experimental animal models during hepatocarcinogenesis or as a part of the restituting response to liver injury[8–10]. After liver injury was induced by N-2-acetylaminofluorene (2-AAF)/partial hepatectomy (PH) in rodent models, the best candidate for liver progenitor cells would be the “oval cell” population, which is oval in shape, with a darkly staining nucleus and scant, basophilic cytoplasm. These cells radiate out from terminal biliary ductules, proliferate and migrate from portal regions into the parenchyma until liver regeneration is completed.
In recent years, attention has focused on the influence of the hepatic microenvironment on hepatic oval cell activation and proliferation[11–13]. The microenvironment comprises the extracellular matrix, epithelial and non-epithelial resident liver cells, and recruited inflammatory cells as well as the variety of growth-modulating molecules. It is conceived as a restricted locale in an organ that regulates liver progenitor cell division through microenvironmental signaling, supporting their self-renewal, inhibiting or maintaining normative baseline differentiation in normal physiological states, and promoting proliferation and differentiation in response to injury[14–16]. The modulation factors for oval cell proliferation and differentiation are now being identified. Although great progress has been made in the research of multiple growth modulators (priming factors, growth factors, inflammatory cytokines, chemokines and growth inhibitory factors) involved in oval cell regulation[13], the role of individual non-parenchymal cells and hepatic extracellular matrix in oval cell-mediated liver regeneration remains unclear.
In the present work, we present a detailed immuno-histochemical analysis of the involvement of non-parenchymal cells, hepatic extracellular matrix components and the interaction of these elements with oval cell and hepatocytes in oval cell-mediated liver regeneration induced by partial hepatectomy after 2-AAF treatment. To obtain detailed morphological assessment, the samples were analyzed under traditional light microscopy and confocal microscopy. The results indicated that different non-parenchymal cells and extracellular matrix component proliferation and migration were accompanied with the “ductular” periportal oval cell response. The local hepatic microenvironment may influence the oval cell response through the production of growth factors, expression of growth factor receptors and remodeling the hepatic extracellular matrix during the restitution process.
MATERIALS AND METHODS
Animal models
Male Sprague Dawley (SD) rats (8-9 wk of age; 120-150 g of body weight) were used. They were fed standard pelleted chow and had access to water ad libitum. Rats were maintained in a temperature-controlled room with a 12-h light/dark illumination cycle. To inhibit hepatocyte proliferation, all rats received daily oral gavage of 2-AAF (Sigma Chemical Co) at a dosage of 15 mg/kg body weight for 4 d before and up to 7 d after PH. 2-AAF was dissolved in dimethyl sulfoxide (DMSO, Sigma Chemical Co). After the first four daily gavages, all rats were anesthetized, and two-thirds partial hepatectomy was performed by surgical removal of the left and median liver lobes; no dosing was performed on the day of surgery. Three rats were killed at 2, 4, 6, 9, 12, 15, 18 and 21 d after PH. Formalin-fixed and paraffin-embedded serial liver tissue sections (4 μm) were used for immunohistochemical and double immunofluorescent analysis. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee.
Immunohistochemistry
Paraffin sections of formalin-fixed liver tissues were stained with mouse monoclonal antibody to ov-6 (MAB2020, R&D Systems, Inc), a marker of hepatic oval cells in ductular reaction; mouse monoclonal antibody to desmin (DE-R-11, DAKO Denmak), a marker of stellate (Ito) cells, smooth muscle cells, periportal fibroblasts; mouse monoclonal antibody to ED1 (MCA341GA, Serotec Ltd) recognizing the cytoplasmic antigen in circulating monocytes and small Kupffer cells in the liver; and polyclonal rabbit anti-laminin (Z0097, DAKO, Denmark) or polyclonal rabbit anti-fibronectin (A0245, DAKO Denmark). Tissue sections were rehydrated at descending concentrations of ethanol and endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol. Tissues used for ov-6 and desmin immunohistochemistry were microwaved to boiling for 15 min in 10 mmol/L Tris buffer, 1 mmol/L ethylenediamine tetra-acetic acid (EDTA), pH 9.0, for antigen retrieval. The sections labeled ED1, laminin or fibronectin were digested with Proteinase (10 μg/L, 5 min, 37°C). After antigen retrieval, the tissue sections were blocked with 10% normal serum from the donor species of the secondary antibodies for 15 min at room temperature, followed by incubation with primary antibodies overnight at 4°C. Primary antibody dilutions were as follows: anti-ov-6, 1:10; anti-desmin, 1:50; anti-ED1, 1:50; anti-laminin, 1:100 and anti-fibronectin, 1:50. After rinsing with phosphate buffered solution (PBS), primary antibodies were detected by incubation for 30 min with biotinylated rabbit anti-mouse or goat anti-rabbit immunoglobulins. After further rinsing with PBS, sections were incubated with horseradish peroxidase conjugated streptavidin/biotin complex (85-9843, Histostain Plus Kits, Zymed Laboratories Inc.). Peroxidase activity was developed with 0.05% diaminobenzidine and 0.03% H2O2. Finally, sections were counterstained for 5 min in hematoxylin, dehydrated through graded alcohols, and mounted under glass coverslips.
Double immunofluorescent analysis
After deparaffinization and hydration, liver sections were microwaved for 15 min in 10 mmol/L Tris buffer, 1 mmol/L EDTA, pH 9.0, for antigen retrieval. Proteinase digestion was added if the second primary antibodies were laminin, ED1 or fibronectin. Five pairs of double labeling (ov-6/desmin, ov-6/laminin, ov-6/ED1, ov-6/fibronectin, desmin/laminin) were performed. We used a vector Mouse-On-Mouse (MOM) immunodetection kit (FMK2201, Vector Laboratories Inc.) when both primary antibodies came from mice. After rinsing with PBS, tissue sections were incubated for 5 min in working solution of MOMTM diluent, followed by incubation with primary antibodies overnight at 4°C. Working solution of MOM biotinylated anti-mouse IgG was used as secondary antibodies. Then, sections were incubated with fluorescein avidin DCS for 5 min. Before incubating the second primary antibodies, both the avidin/biotin blocking step and the mouse Ig blocking step must be done to prevent the interaction of the second set of labeling reagents with the first set of labeling reagents if the two mouse monoclonal primary antibodies were used. If two primary antibodies came from different species, only the avidin/biotin blocking step was necessary. After the protein blocking step, sections were incubated with the second primary antibodies for 1 h at 37°C, followed by incubation with working solution of MOM biotinylated anti-mouse or anti-rabbit IgG (BA-1000, Vector Laboratories Inc.) for 10 min, then Texas Red Avidin DCS (A-2016, Vector Laboratories Inc.) for 10 min. All samples were analyzed by confocal laser-scanning microscopy using the Nikon Digital ECLIPSE C1 system (Nikon Corporation, Japan).
RESULTS
Ductular oval cell response
In the normal liver, ov-6 strongly labeled pre-existing intraportal bile ducts, ductules and terminal duct cells. There was no change from normal in the distribution and the expression pattern of the ov-6 (+) cells on day 1 after PH. On day 2 after PH, small cells with high nuclear/cytoplasmic ratio (oval cells) were evident in and around the portal area. Oval cells were close to hepatocytes at limiting plates (Figure 1A). On day 4 after PH, ov-6 positives cells could be seen protruding into the parenchyma. Pre-existing ducts stained more strongly than new ducts (Figure 1B). By day 6, there is further extension of ductular structures across the hepatic lobule. On day 9 after PH, multiple strings of ductular cells spread further into the midzone (Figure 1C). By day 12 and beyond, the periportal areas were colonized by small hepatocyte nodus, though small strings of ductular cells could still be discerned at the periphery of hepatocytes. Many ductular profiles exhibited what looked like clear intestinal differentiation. On day 15 after PH, there was a significant decrease in the ductular structure and increase in the new small hepatocyte nodus (Figure 1D). From day 18 to 21 after PH, small ov-6 (+) cells further decreased or disappeared. The normal liver lobule structures recovered.
Figure 1.
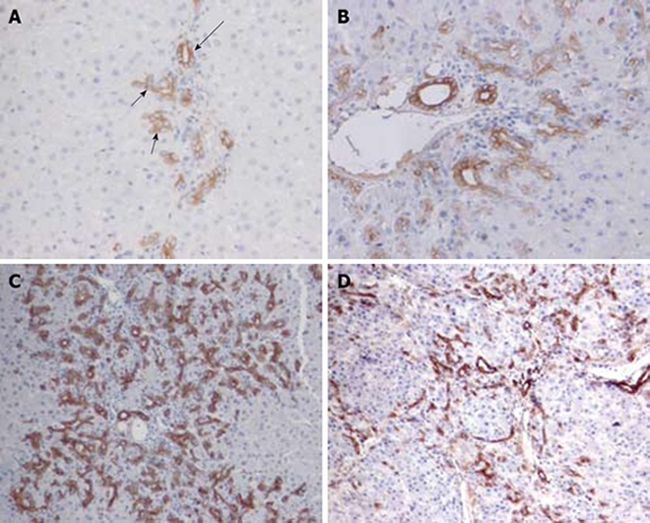
Ov-6 immunoreactivity of ductular cell reaction following PH of rats treated with AAF. A: Oval cells (short arrow) close to hepatocytes at limiting plates on day 2 after PH show much lighter labeling than pre-existing bile duct cells (long arrow) (× 200); B: On day 4 after PH, strongly stained cells are beginning to move into the parenchyma (× 200); C: On day 9 after PH, apparent long cords of ductular oval cells are fanning outward from each portal area (× 100); D: On day 15, the ov-6 (+) ductular structures are restricted to the periphery of small hepatocyte nodus (× 100).
Non-parenchymal cell response and interaction with oval cells
Desmin stained smooth muscle cells of the blood vessels and dendritic cells around the bile ducts and vessels, but not sinusoidal cells as in normal rat liver. However, by day 2 after PH, hepatic stellate cells (HSCs) were activated and expressed desmin. At this moment, a few oval cells began to proliferate from the portal areas. Most desmin (+) cells were located in the portal areas and minority of the HSCs were small interlobular sinusoidal cells (Figure 2A). There were no desmin (+) cells in the central zone. From day 4 to 6 after PH, desmin (+) cells could be seen protruding into the parenchyma and formed the organized meshwork arrangement (Figure 2B). As time elapsed, HSCs spread further into the parenchyma and reached the mid zonal parenchyma. The number of desmin (+) periportal stellate cells peaked on day 9 and 12 after PH and decreased afterward. During the course of proliferation, HSCs appeared to be closely associated with the oval cells. They appeared to admix with oval cells in the periportal zone and tightly surrounded the long strings of ductular oval cells (Figure 2C). Along with the formation of the small hepatocyte nodus, most of the desmin (+) cells were located around the nodus. There were few activated HSCs in the nodus (Figure 2D). In addition, increasing numbers of desmin (+) cells were seen in the interlobular sinusoidal zone. On day 15 after PH desmin (+) cells became far less prominent and accompanied with the ductular structure around small hepatocyte nodus (Figure 2E). The number of stellate cells decreased more significantly than the ductular oval cells. On day 18 after PH, the number of desmin (+) stellate cells further decreased or vanished completely (Figure 2F).
Figure 2.
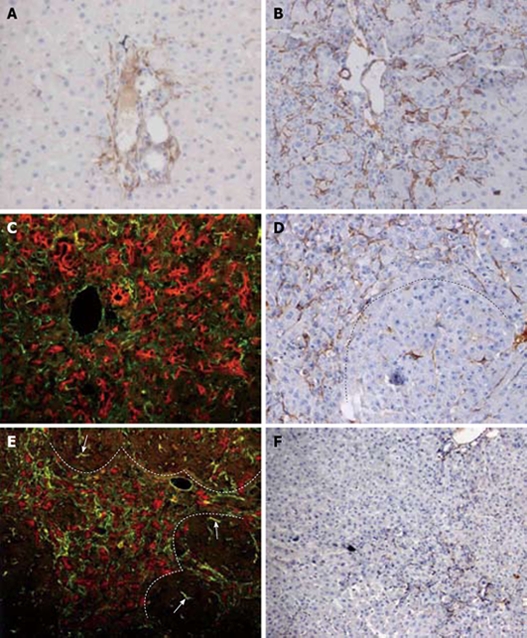
HSCs response and correlation with oval cell following PH of rats treated with AAF. A: Increase in number of desmin positive stellate cells in the portal areas. Very few of HSCs were small interlobular sinusoidal cells on day 2 after PH (× 200); B: Further increase in number of stellate cells at the periphery of the portal areas at day 6 after PH (× 200); C: Double immunofluorescent labelling for ov-6 (red) and desmin (green) on day 9 after PH. The strings of ductular oval cells are closely surrounded by the mesh-like desmin (+) stellate cells (× 400); D: On day 12 after PH, dash line marks the edge of a regenerative small hepatocyte focus. Desmin (+) cells are present around the focus, and occasionally in the focus (× 200); E: Double immunofluorescent labelling for ov-6 (red) and desmin (green) on day 12 after PH. Desmin (+) cells accompany the ductular structure around small hepatocytes focus. There are few HSCs in the focus negative for ov-6 (× 200); F: By day 18 after PH, the number of desmin (+) portal stellate cells is further decreased ( ×100).
ED1 stained the small rounded or spindle-like macrophages in the sinusoids with a cytoplasmic staining pattern in normal liver (Figure 3A). The number of ED1 (+) cells sharply increased not only in the pericentral zone but also in the periportal zone by day 2 after PH. At this time, no obvious ductular structures spread into the parenchyma (Figure 3B). On day 4, ED1 (+) cells began to decrease in both portal and central zones with an increase of the ductular oval cells. Infiltration of ED1 labelled macrophages into the ductular structures was seen in the periportal areas. A few scattered ED1 (+) cells around the portal zone appeared to admix with oval cells (Figure 3C and D). From day 6 to 9, there appeared to be different fate for central zone and portal zone macrophages. The number of ED1 (+) cells in the periportal zone decreased more markedly than in the pericentral zone as the ductular oval cells spread further into the mid zone (Figure 3E). On day 12, ED1 staining was limited to a few pericentral sinusoidal macrophages. There were very few ED1 (+) cells in the expanding ductular cells (Figure 3F). After day 15, ED1 (+) cells were seen mainly located between the central veins and small hepatocyte nodus as oval cells differentiated into small hepatocyte-like cells.
Figure 3.
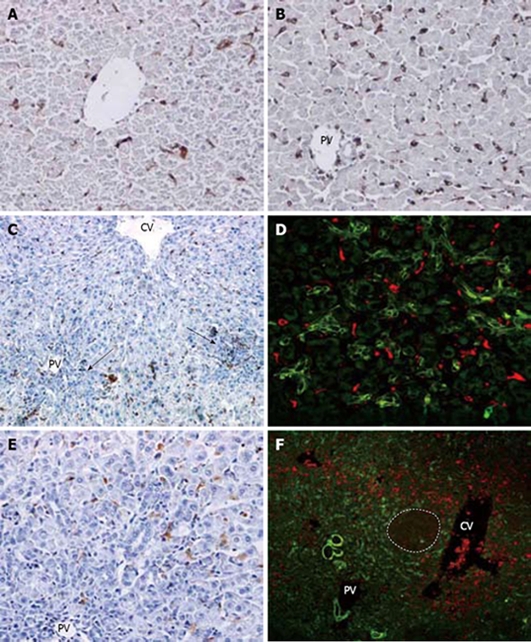
Kupffer cell response and correlation with oval cells following PH of rats treated with AAF. A: Normal rat liver. Small round or spindle-like cells in the sinusoids are labelled (× 200); B: On day 2 after PH, there is marked increase of ED1 (+) cells in periporal and interlobular sinusoids. No oval cells are seen in the poral areas (× 200); C: on day 4 after PH, number of pericentral and periportal ED1 (+) cells decreased along with the ductular oval cells(arrows) (× 100); D: double immunofluorescent labelling for ov-6 (green) and ED1 (red) at day 4 after PH. A few scattered ED1 (+) cells around the portal appeared to admix with ov-6 (+) oval cell (× 400); E: on day 9 after PH, very few ED1 (+) cells are visualized in the periportal ductular oval cells (× 200); F: double immunofluorescent labelling for ov-6 (green) and ED1 (red) on day 12 after PH. Dash line marks the edge of a regenerative small hepatocyte nodus. ED1 (+) cells are situated around the small hepatocte nodus and ductular ov-6 (+) cells (× 100).
Extracellular matrix changes and interaction with oval cells
Laminin normally stained around the bile ducts, blood vessels, and in the sinusoids. On day 2 after PH, laminin appeared along the hepatic sinusoids in the peirportal areas and in the cytoplasm of few nonparenchymal cells, as well (Figure 4A). During the course of oval cell proliferation, laminin-containing basement membrane surrounded the undifferentiated oval cells (Figure 4C). The cylinder formed by the basement membrane had an open end plugged by hepatocytes (Figure 4B), and desmin-positive stellate cells were accompanied with the ductular structures outside the basement membrane. In addition, laminin was found in the cytoplasm of many desmin (+) fusiform stellate cells (Figure 4D). No cells penetrating through the basement membrane could be observed. The maximum laminin staining was from day 9 to 12 after PH and decreased afterwards. The paucity or complete lack of laminin staining was characteristic of nodus as oval cells differentiated into small hepatocyte nodus on day 12 after PH. Numerous undifferentiated oval cells located outside nodus were still outlined brightly by laminin staining. After day 15, the ductular oval cells further decreased and the small hepatocyte-vascular relationship and hepatic architecture began to be restored. Straps of laminin were present in the periportal hepatocyte cluster in which there was no intervening sinusoids or extracellular matrix (ECM) in the early stage of regeneration (Figure 4E).
Figure 4.
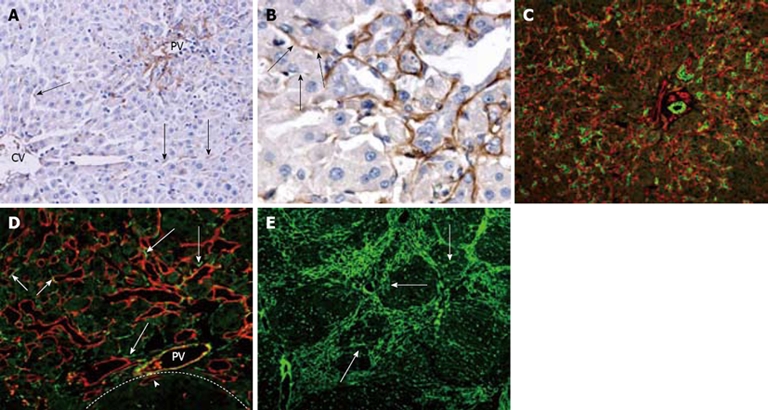
Laminin distribution and correlation with oval cell following PH of rats treated with AAF. A: On day 2 after PH, laminin is present along hepatic sinusoids in peirportal areas and in the cytoplasm of few nonparenchymal cells in the lobule (arrows) (× 200); B: On day 6 after PH, laminin positivity around the ductular oval cells. The proximal part of the ductule has continuous laminin staining, whereas distally the basement membrane is fragmented or absent (arrows) (× 400); C: Periportal area stained for OV-6 (green) and laminin (red) 12 d after PH. The bifurcating ductule strongly positive for OV-6, is surrounded by continuous basement membrane (× 200); D: Portal area 15 d after PH, stained for laminin (red) and desmin (green). Some of the desmin-positive cells are positioned closely to the laminin (+) basement membrane of ductules (long arrows). Some of the laminin-positive ductules also stained positively for desmin (short arrows). The focus (dash line marks the edge) is negative for laminin except some oval cell ductules enclosed in the focus (arrow head) (× 400); E: On day 15 after PH, most laminins are present around the focus, some laminins invading the hepatocyte clusters (arrows) (× 100).
In normal liver, fibronectin is present in the perisinusoidal space and codistributed with collagens in the ECM of the subcapsular region, portal triad, and central vein regions. In general, the cellular staining for fibronectin in the normal liver is very similar to that of ED1. On day 2 after PH, fibronectin staining became more prominent in sinusoidal cells and in the periportal and pericentral regions (Figure 5A). By day 4, fibronectin (+) cells increased further in the periportal areas accompanying with ductular oval cells as oval cells began to spread into the parenchyma, and there was a marked decrease of fibronectin staining in the sinusoids of the mid zone and the pericentral areas (Figure 5B). From day 6 to 9, fibronectin admixed with the proliferating ductual oval cells expanded further into the pericentral areas. The clumps of oval cells were closed associated with desmin, and fibronectin as well (Figure 5C). On day 12, fibronectin (+) cells were seen around the small hepatocyte nodus in the periportal areas and very little fibronectin was detected within the hepatocyte clusters (Figure 5D). The number of fibronectin (+) cells among the expanding ductules decreased as oval cells differentiated into the hepatocytes on day 15 (Figure 5E). Recovery of normal sinusoidal fibronectin expression occurred after day 18.
Figure 5.
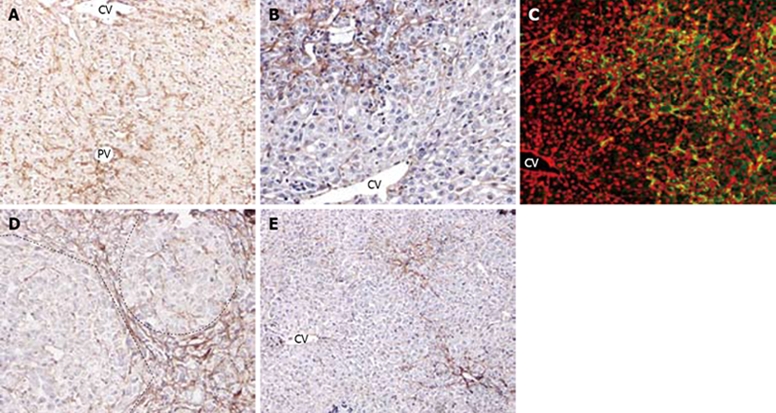
Fibronectin distribution and correlation with oval cell following PH of rats treated with AAF. A: On day 2 after PH, there is a marked increase of fibronectin in the periportal, pericentral areas and interlobular sinusoids (× 100); B: On day 4 after PH, there is a decrease in number of fibronectin in pericentral zone and increase of fibronectin in periportal areas (× 200); C: Double immunofluorescent labelling for ov-6 (green) and fibronectin (red) on day 9 after PH. The ductular oval cells fanning outward from portal area were closely surrounded by fibronectin (× 200); D: On day 12 after PH, dash line marks the edge of a regenerative small hepatocyte focus. Fibronectin was present around the focus, very few in the nodus (× 200); E: On day 15 after PH, there is notable decrease of fibronectin during recovery of normal hepatic architecture (× 100).
DISCUSSION
In this paper, we describe the proliferation of non-parenchymal cells and extracellular matrix components in the oval cell-mediated liver regeneration in a 2-AAF/PH rat model. In addition, the interaction of these elements with oval cell and hepatocytes was studied through confocal double immunofluorescent labeling. Our results show that the reconstitution of the liver parenchymal following 2-AAF/PH treatment involves not only the ductular oval cells but the Kupffer cells, hepatic stellate cells and ECM components (laminin and fibronectin). A close anatomical relationship among the hepatic oval cells, non-parenchymal cells and ECM was observed during the restituting process. These results suggest that ECM remodeling and production of growth factors and expression of growth factor receptors by non-parenchymal cells play an important role in the oval cell-mediated liver regeneration.
The effect of HSCs on oval cell proliferation and differentiation is complex, including elaboration and secretion of specific paracrine stimuli to proliferation in the early phase after partial hepatectomy, inhibition of proliferation in the later phase and matrix remodeling. During oval cell growth and ductule formation, HSCs are closely associated with oval cells, as suggested by Alison et al[17]. Both HSCs and oval cells produce and have receptors for a variety of overlapping growth factors[18]. Liver stem-like cells can differentiate into hepatocytes induced by coculture with hepatic stellate cells. These results suggest that, besides growth factors, cell-cell interaction through extracellular matrices produced by HSCs is also important for the induction of hepatocytic differentiation[19]. Another important role of HSCs is matrix remodeling by combination of proteolytic degradation of ECM and extracellular matrix synthesis. HSCs exhibit collagenase, stromelysins, metalloelastase and gelatinase A activity. Tissue inhibitors of metalloproteinases, also HSC derived, balance these activities. Matrix components, including laminins, fibronectins, collagens and proteoglycans, are largely but not exclusively HSC derived[20]. Our results show that after ductular oval cells differentiate into small hepatocyte nodus, the HSCs extend into the hepatocyte clusters without intervening sinusoids or ECM. HSCs gradually disappear as the normal liver lobule structures are restored. These results indicate that HSCs may play an important role in the restoration of normal hepatocyte-vascular relationships and overall hepatic architecture in the later stages of regeneration.
Concomitant with periportal oval cell proliferation, there is an increase in ED1 (+) Kupffer cells. Kupffer cells are mainly phagocytic cells also synthesize and secrete a number of cytokines, including tumor necrosis factor-α (TNF-α), IL-1 and IL-6[20–22]. Inhibition of TNF signaling results in a reduced progenitor cell response and impaired liver regeneration[23]. In our study, there was a quick increase in ED1 (+) Kupffer cells in the whole hepatic lobule by day 2 after PH, before obvious ductular structures were observed. From these results, we can speculate that Kupffer cells may play a crucial role in priming the oval cells and inducing DNA synthesis by secreting the priming factors (TNF-α and IL-6) in the early phase of oval cell-mediated liver regeneration. After 4 d, as the oval cells spread further into the liver parenchyma, Kupffer cells appeared to admix with the expanding oval cell population. The structural relationship of the interaction of the responding cells implies that there may also be a functional interaction. Takeishi et al[24] demonstrated that Kupffer cells play a stimulatory role in liver regeneration by enhancing hepatocyte growth factor (HGF) expression. In addition, studies indicate that HGF accelerates the proliferation of hepatic oval cells and promotes the differentiation to hepatocytes[25,26]. Apart from secreting growth factors, Kupffer cells may also produce metalloproteases, elastase, collagenase and fibronectin[27]. Thus, Kupffer cells and HSCs may act together to contribute to the dissolution of basement membrane around small ductules, perhaps allowing ductular oval cells to move into the adjacent hepatic lobe, providing mitogenic growth factor and remodeling ECM during the proliferation and differentiation of oval cells. Kupffer cells also play a role in terminating the surge of replication after partial hepatectomy[20]. One of powerful inhibitors of hepatocyte replication is TGF-β, produced most prominently by hepatic stellate cells and Kupffer cells. TGF-β has been proposed to play similar modulatory roles in oval cell-mediated liver regeneration[28].
The extracellualr matrix is a dynamic complex of macromolecules and plays a role not only in structural support, but also in cell proliferation, migration, and differentiation[29]. The morphological observations described above suggest a link between stroma in the hepatic microenvironment and the oval cell response. We speculate that ECM (laminin and fibronectin) may affect the oval cell response by: (1) Providing the growth factors for oval cells in the early stage of regeneration. The upregulation of urokinase-type plasminogen activator (uPA) mRNA accompanying oval cell proliferation has been reported, and infusion of uPA enhanced the mitogenic response of cells located near bile ducts[30]. uPA has been shown to initiate the degradation of the ECM through activation of metalloproteinase and the degradation leads to the release of bound HGF with a subsequent increase in serum HGF concentration[31]. Several growth factors involved in oval cell regeneration may be also regulated by this system[18]. Together, these findings support the overall concept of ECM remodeling as an important step in the growth phase of oval cell-mediated liver regeneration. (2) Influencing the differentiation state of the oval cell. Our results show that there is a distinct continuous laminin staining around the oval cells, and the disappearance of the basement membrane (BM) that surrounds the oval cell ductules is closely associated with initiation of the differentiation into hepatocytes. Yin
et al[32]reported that isolated hepatic stem cells expressed biliary or hepatocytic phenotypes in culture, depending on the presence or absence of basement membrane matrix (Matrigel). Matrigel was also found to play an important role in maintaining the biliary phenotype in tissue culture in other experimental systems[33,34]. Recently Leite et al[35] demonstrate that fibronectin and laminin can induce expression of islet cell markers in hepatic oval cells in culture. (3) Affecting the migration, proliferation and attachment of oval cells. Sánchez et al[36] demonstrate that fibronectin might regulate morphology, cell organization and gene expression of rat fetal hepatocytes in primary culture. Other studies show that the apparent affinity of hepatocytes to laminin increases during the prereplicative phase of rat liver regeneration and laminin is one of the most effective substrates in supporting the responstiveness of hepatocytes to the growth stimulus[37,38]. (4) Playing a role in the development of the hepatic sinusoidal vasculature. We observed that both laminin and fibronectin are present in the periportal hepatocyte cluster and when the maturation of the sinusoidal vasculature is complete, the expression is repressed. A resynthesis of the previously degraded perisinusoidal ECM is required for full regeneration. Epithelial cells require contact with ECM to inhibit detachment-induced apoptosis, and the reconstitution of a perisinusoidal ECM therefore will stabilize the newly formed hepatocyte population[39].
In summary, our results indicate that there is a close relationship between the non-parenchymal cells (HSCs and Kupffer cells), ECM components (laminin and fibronectin) and oval cells during the restitutive repair in 2-AAF/PH model, providing evidence that the local hepatic microenvironment may participate in the oval cell-mediated liver regeneration through the cell-cell and cell-matrix interactions. Furthermore, our results also suggest that the production of growth factors and extracellular matrix remodeling may be required to regulate the migration, proliferation and differentiation of oval cells and the process of liver regeneration. Further studies should focus on providing the direct evidence of hepatic microenvironment in regulating to oval cells via in vitro experiments and elucidating the molecular mechanisms.
COMMENTS
Background
Oval cells are thought to be the progeny of stem cells in adult liver, which are able to differentiate bipotentially into mature hepatocytes and billary epithelial cells when the proliferation of mature hepatocytes is inhibited or blocked. In recent years, attention has focused on the influence of the hepatic microenvironment on hepatic oval cell activation and proliferation.
Research frontiers
Although great progress has been made in the research of multiple growth modulators involved in oval cell regulation, the role of individual non-parenchymal cells and hepatic extracellular matrix in oval cell-mediated liver regeneration remains unclear.
Innovations and breakthroughs
The current study demonstrated that the local hepatic microenvironment may influence the oval cell response through the production of growth factors, expression of growth factor receptors and remodelling the hepatic extracellular matrix during the restitution process.
Applications
By observing the interaction of hepatic microenvironment with oval cells in oval cell-mediated liver regeneration, the existence and importance of liver stem cell niche have been further confirmed. This study has laid a foundation for researchers to elucidate the molecular mechanisms of hepatic microenvironment in regulating oval cells.
Terminology
Stem cell niche is conceived as a restricted locale in an organ that regulates stem cell division through microenvironmental signaling, supporting their self-renewal, inhibiting or maintaining normative baseline differentiation in normal physiological states, and promoting proliferation and differentiation in response to injury.
Peer review
The authors reported a close anatomical relationship between the hepatic oval cells, non-parenchymal cells and extracellular matrix (ECM), and they suggested that ECM remodeling and production of growth factors and expression of growth factor receptors by non-parenchymal cells may play an important role in the oval cell-mediated liver regeneration. Overall, the data are well presented, although some conclusions could not be supported as this is only a immunohistochemical and a double immunofluorescent analysis.
Supported by A grant from National Natural Science Foundation of China, 30430670
Peer reviewer: Maria Concepción Gutiérrez-Ruiz, PhD, Departamento de Ciencias de la Salud, Universidad Autónoma Metropolitana-Iztapalapa, DCBS, Av San Rafael Atlixco 186, Colonia Vicentina, México, DF 09340, México
S- Editor Li LF L- Editor Ma JY E- Editor Zheng XM
References
- 1.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 2.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 3.Theise ND. Liver stem cells. Cytotechnology. 2003;41:139–144. doi: 10.1023/A:1024826823194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117–130. doi: 10.1016/s0925-4773(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 5.Shafritz DA, Oertel M, Menthena A, Nierhoff D, Dabeva MD. Liver stem cells and prospects for liver reconstitution by transplanted cells. Hepatology. 2006;43:S89–S98. doi: 10.1002/hep.21047. [DOI] [PubMed] [Google Scholar]
- 6.Sell S. Heterogeneity and plasticity of hepatocyte lineage cells. Hepatology. 2001;33:738–750. doi: 10.1053/jhep.2001.21900. [DOI] [PubMed] [Google Scholar]
- 7.Alison M. Hepatic stem cells. Transplant Proc. 2002;34:2702–2705. doi: 10.1016/s0041-1345(02)03382-1. [DOI] [PubMed] [Google Scholar]
- 8.Roskams TA, Libbrecht L, Desmet VJ. Progenitor cells in diseased human liver. Semin Liver Dis. 2003;23:385–396. doi: 10.1055/s-2004-815564. [DOI] [PubMed] [Google Scholar]
- 9.Libbrecht L, Roskams T. Hepatic progenitor cells in human liver diseases. Semin Cell Dev Biol. 2002;13:389–396. doi: 10.1016/s1084952102001258. [DOI] [PubMed] [Google Scholar]
- 10.Shafritz DA, Dabeva MD. Liver stem cells and model systems for liver repopulation. J Hepatol. 2002;36:552–564. doi: 10.1016/s0168-8278(02)00013-2. [DOI] [PubMed] [Google Scholar]
- 11.Braun KM, Thompson AW, Sandgren EP. Hepatic microenvironment affects oval cell localization in albumin-urokinase-type plasminogen activator transgenic mice. Am J Pathol. 2003;162:195–202. doi: 10.1016/S0002-9440(10)63810-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin L, Lynch D, Ilic Z, Sell S. Proliferation and differentiation of ductular progenitor cells and littoral cells during the regeneration of the rat liver to CCl4/2-AAF injury. Histol Histopathol. 2002;17:65–81. doi: 10.14670/HH-17.65. [DOI] [PubMed] [Google Scholar]
- 13.Santoni-Rugiu E, Jelnes P, Thorgeirsson SS, Bisgaard HC. Progenitor cells in liver regeneration: molecular responses controlling their activation and expansion. APMIS. 2005;113:876–902. doi: 10.1111/j.1600-0463.2005.apm_386.x. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 15.Theise ND. Gastrointestinal stem cells. III. Emergent themes of liver stem cell biology: niche, quiescence, self-renewal, and plasticity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G189–G193. doi: 10.1152/ajpgi.00041.2005. [DOI] [PubMed] [Google Scholar]
- 16.Lemischka IR, Moore KA. Stem cells: interactive niches. Nature. 2003;425:778–779. doi: 10.1038/425778a. [DOI] [PubMed] [Google Scholar]
- 17.Alison MR, Golding MH, Sarraf CE. Pluripotential liver stem cells: facultative stem cells located in the biliary tree. Cell Prolif. 1996;29:373–402. doi: 10.1111/j.1365-2184.1996.tb00982.x. [DOI] [PubMed] [Google Scholar]
- 18.Lowes KN, Croager EJ, Olynyk JK, Abraham LJ, Yeoh GC. Oval cell-mediated liver regeneration: Role of cytokines and growth factors. J Gastroenterol Hepatol. 2003;18:4–12. doi: 10.1046/j.1440-1746.2003.02906.x. [DOI] [PubMed] [Google Scholar]
- 19.Nagai H, Terada K, Watanabe G, Ueno Y, Aiba N, Shibuya T, Kawagoe M, Kameda T, Sato M, Senoo H, et al. Differentiation of liver epithelial (stem-like) cells into hepatocytes induced by coculture with hepatic stellate cells. Biochem Biophys Res Commun. 2002;293:1420–1425. doi: 10.1016/S0006-291X(02)00406-0. [DOI] [PubMed] [Google Scholar]
- 20.Malik R, Selden C, Hodgson H. The role of non-parenchymal cells in liver growth. Semin Cell Dev Biol. 2002;13:425–431. doi: 10.1016/s1084952102001301. [DOI] [PubMed] [Google Scholar]
- 21.Meijer C, Wiezer MJ, Diehl AM, Schouten HJ, Schouten HJ, Meijer S, van Rooijen N, van Lambalgen AA, Dijkstra CD, van Leeuwen PA. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver. 2000;20:66–77. doi: 10.1034/j.1600-0676.2000.020001066.x. [DOI] [PubMed] [Google Scholar]
- 22.Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 23.Nagy P, Kiss A, Schnur J, Thorgeirsson SS. Dexamethasone inhibits the proliferation of hepatocytes and oval cells but not bile duct cells in rat liver. Hepatology. 1998;28:423–429. doi: 10.1002/hep.510280220. [DOI] [PubMed] [Google Scholar]
- 24.Takeishi T, Hirano K, Kobayashi T, Hasegawa G, Hatakeyama K, Naito M. The role of Kupffer cells in liver regeneration. Arch Histol Cytol. 1999;62:413–422. doi: 10.1679/aohc.62.413. [DOI] [PubMed] [Google Scholar]
- 25.Hasuike S, Ido A, Uto H, Moriuchi A, Tahara Y, Numata M, Nagata K, Hori T, Hayashi K, Tsubouchi H. Hepatocyte growth factor accelerates the proliferation of hepatic oval cells and possibly promotes the differentiation in a 2-acetylaminofluorene/partial hepatectomy model in rats. J Gastroenterol Hepatol. 2005;20:1753–1761. doi: 10.1111/j.1440-1746.2005.03922.x. [DOI] [PubMed] [Google Scholar]
- 26.Okano J, Shiota G, Matsumoto K, Yasui S, Kurimasa A, Hisatome I, Steinberg P, Murawaki Y. Hepatocyte growth factor exerts a proliferative effect on oval cells through the PI3K/AKT signaling pathway. Biochem Biophys Res Commun. 2003;309:298–304. doi: 10.1016/j.bbrc.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Decker K. Biologically active products of stimulated liver macrophages (Kupffer cells) Eur J Biochem. 1990;192:245–261. doi: 10.1111/j.1432-1033.1990.tb19222.x. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen LN, Furuya MH, Wolfraim LA, Nguyen AP, Holdren MS, Campbell JS, Knight B, Yeoh GC, Fausto N, Parks WT. Transforming growth factor-beta differentially regulates oval cell and hepatocyte proliferation. Hepatology. 2007;45:31–41. doi: 10.1002/hep.21466. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Hernandez A, Amenta PS. The extracellular matrix in hepatic regeneration. FASEB J. 1995;9:1401–1410. doi: 10.1096/fasebj.9.14.7589981. [DOI] [PubMed] [Google Scholar]
- 30.Bisgaard HC, Santoni-Rugiu E, Nagy P, Thorgeirsson SS. Modulation of the plasminogen activator/plasmin system in rat liver regenerating by recruitment of oval cells. Lab Invest. 1998;78:237–246. [PubMed] [Google Scholar]
- 31.Black D, Lyman S, Heider TR, Behrns KE. Molecular and cellular features of hepatic regeneration. J Surg Res. 2004;117:306–315. doi: 10.1016/j.jss.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Yin L, Sun M, Ilic Z, Leffert HL, Sell S. Derivation, characterization, and phenotypic variation of hepatic progenitor cell lines isolated from adult rats. Hepatology. 2002;35:315–324. doi: 10.1053/jhep.2002.31355. [DOI] [PubMed] [Google Scholar]
- 33.Couchie D, Holic N, Chobert MN, Corlu A, Laperche Y. In vitro differentiation of WB-F344 rat liver epithelial cells into the biliary lineage. Differentiation. 2002;69:209–215. doi: 10.1046/j.1432-0436.2002.690414.x. [DOI] [PubMed] [Google Scholar]
- 34.Strick-Marchand H, Weiss MC. Inducible differentiation and morphogenesis of bipotential liver cell lines from wild-type mouse embryos. Hepatology. 2002;36:794–804. doi: 10.1053/jhep.2002.36123. [DOI] [PubMed] [Google Scholar]
- 35.Leite AR, Corrêa-Giannella ML, Dagli ML, Fortes MA, Vegas VM, Giannella-Neto D. Fibronectin and laminin induce expression of islet cell markers in hepatic oval cells in culture. Cell Tissue Res. 2007;327:529–537. doi: 10.1007/s00441-006-0340-z. [DOI] [PubMed] [Google Scholar]
- 36.Sánchez A, Alvarez AM, Pagan R, Roncero C, Vilaró S, Benito M, Fabregat I. Fibronectin regulates morphology, cell organization and gene expression of rat fetal hepatocytes in primary culture. J Hepatol. 2000;32:242–250. doi: 10.1016/s0168-8278(00)80069-0. [DOI] [PubMed] [Google Scholar]
- 37.Kato S, Otsu K, Ohtake K, Kimura Y, Yashiro T, Suzuki T, Akamatsu N. Concurrent changes in sinusoidal expression of laminin and affinity of hepatocytes to laminin during rat liver regeneration. Exp Cell Res. 1992;198:59–68. doi: 10.1016/0014-4827(92)90149-3. [DOI] [PubMed] [Google Scholar]
- 38.Carlsson R, Engvall E, Freeman A, Ruoslahti E. Laminin and fibronectin in cell adhesion: enhanced adhesion of cells from regenerating liver to laminin. Proc Natl Acad Sci USA. 1981;78:2403–2406. doi: 10.1073/pnas.78.4.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimmermann A. Regulation of liver regeneration. Nephrol Dial Transplant. 2004;19 Suppl 4:iv6–iv10. doi: 10.1093/ndt/gfh1034. [DOI] [PubMed] [Google Scholar]


