Abstract
We report a case of primary localized malignant biphasic mesothelioma of the liver in a 66-year-old man associated with asbestosis. The tumor was detected as a hepatic nodule, 4 cm in diameter, in the right lobe (S8 segment) on CT scan. Histopathological examination demonstrated an intrahepatic tumor with central necrosis consisting of a papillary epithelioid pattern on the surface of the liver, microcystic (microglandular or adenomatoid) pattern mainly in the subcapsular area and sarcomatoid pattern intermingled with microcystic pattern in the major part of the hepatic nodular tumor. Tumor cells, especially of epithelioid type, showed distinct immunoreactivity for mesothelial markers (WT-1, calretinin, D2-40, CK5/6, mesothelin, thrombomodulin) and no immunoreactivity for epithelial (adenocarcinoma) markers (CEA, CD15, BerEP4, BG8, MOC31). P53 immunoreactivity was detected focally in papillary epithelioid tumor cells and extensively in microcystic and sarcomatoid components, suggesting that the papillary epithelioid mesothelioma arose on the surface of the liver, and tumor cells showing microcystic and sarcomatoid patterns invaded and grew into the liver. To date, this is the first case of primary localized malignant biphasic mesothelioma of the liver, since all three primary hepatic mesotheliomas reported so far were epithelioid type.
Keywords: Malignant mesothelioma, Pulmonary asbestosis, Liver, Immunohistochemistry
INTRODUCTION
Malignant mesothelioma is a rare neoplasm of mesothelial cells arising most frequently in the parietal or visceral pleura and much less commonly in the peritoneum and pericardium[1–3]. In about 80%-90% of these patients, malignant mesothelioma is related to occupational exposure to asbestos in the air. The latent development period is long, about 25-40 years after initial exposure[3]. The disease is more common in men with a mean age of 58 years, with a male to female ratio of 4:1[1,4]. Mesothelioma occurring at various sites other than pleura, peritoneum and pericardium has been reported previously[4–7]. The mesothelial nature of the lesions is supported by immunohistochemical or electron microscopic evidence[3]. Most malignant mesotheliomas grow widely over the serosal membrane surfaces and tumors in the later stages eventually encase organs surrounding the involved site. Diffuse malignant mesothelioma typically has a poor clinical course with death occurring in most patients within 2 years of diagnosis[3]. In contrast, Allen et al[8] have recently reported a series of localized malignant mesotheliomas as uncommon sharply circumscribed tumors of the serosal membranes with the microscopic appearance of diffuse malignant mesothelioma. They proposed that localized malignant mesotheliomas should be separated from diffuse malignant mesotheliomas because of their localized presentation, quite different biological behavior, and far better prognosis[8].
Primary malignant mesothelioma arising in the liver is very rare and there have been only 3 adult cases in previous reports[9–12], to our knowledge. In addition, there is one reported case of malignant cystic mesothelioma in an infant[11]. Although several primary hepatic tumors termed “localized fibrous mesotheliomas” have been reported[2,13–17], this type of tumor is better classified as a solitary fibrous tumor rather than mesothelioma retrospectively, because of the characteristic CD34 immunoreactivity[16] and limited evidence of a mesothelial nature[18].
In this report, we describe a case of primary localized malignant biphasic mesothelioma of the liver showing a nodular hepatic tumor and histologically sarcomatoid and epithelioid patterns in a 66-year-old man associated with asbestosis, and review the literature on primary malignant mesothelioma of the liver.
CASE REPORT
Clinical Findings
The patient (66-year-old man) had been followed up for hypertension and pulmonary asbestosis caused by occupational exposure to asbestos. At the age of 66, CT revealed a hepatic nodule, 4 cm in diameter, in the S8 segment of the liver (Figure 1) on periodic examination for pulmonary asbestosis. The hepatic nodule was located just under the diaphragm, wedge-shaped and slightly low density compared to the surrounding hepatic parenchyma on plain CT. Peripheral staining of the hepatic nodule was seen on enhanced CT (Figure 1). Cholangiocarcinoma or inflammatory pseudotumor was suspected from CT findings. The findings signifying pulmonary asbestosis showed on chest X-ray and chest CT as previously detected, but there were no new lesions suggesting lung cancer or pleural mesothelioma (Figure 1). There was no finding suggesting peritoneal mesothelioma or a primary mesothelioma of the tunica vaginalis testis. Examination using [18F]-fluoro-deoxy-D-glucose positron emission tomography (FDG-PET) revealed no additional lesions other than the hepatic tumor. At that time, the serum levels of ALT, AST, Al-P, r-GTP and LDH were within the normal range. AFP, CEA and CA19-9 were negative. Viral markers related to hepatitis B virus (HBV) and hepatitis C virus (HCV) infection were negative. The patient was not a drinker. The needle biopsy revealed that the hepatic tumor was a sarcomatoid malignant tumor with immunoreactivity for cytokeratin and vimentin, and with faint and focal immunoreactivity for calretinin and D2-40. The papillary and microcystic components were not included in the biopsy. The patient underwent a hepatic segmentectomy (S8). A few sclerotic nodules attached to the diaphragm were also removed during the operation. Recurrence and metastasis of the tumor have not been detected by 6 mo after the operation.
Figure 1.
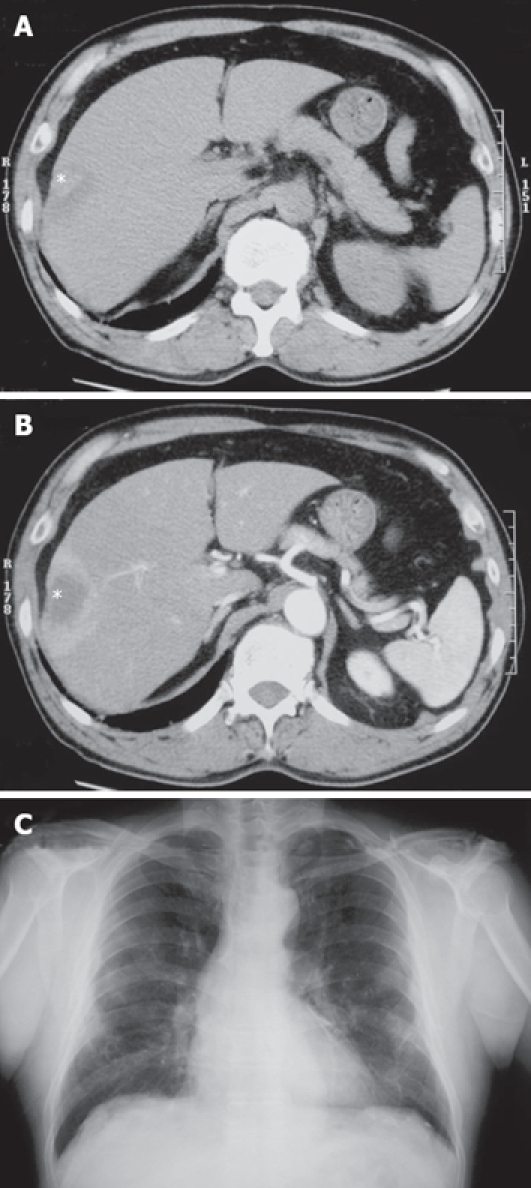
Radiological findings. A: Plain CT showed a low-density mass (asterisk) in S8 segment of the liver; B: The periphery of the mass (asterisk) was enhanced in the early phase of enhanced CT, suggesting abundant blood supply; C: Chest X-ray showed pulmonary asbestosis and pleural thickening, but there were no new lesions suggesting lung cancer or pleural mesothelioma.
Gross pathology examination
The resected specimen consisted of a 12.8 cm × 17 cm × 7 cm portion of the liver (445 g). On the surface faced to the diaphragm, a firm, white and slightly depressed lesion measuring 4.2 cm × 3 cm was identified (Figure 2). On sectioning of the liver, a yellowish-white tumor of 4.4 cm × 3.8 cm was identified just under the hepatic capsule. The tumor had well-defined borders and contained central necrosis (Figure 2). The tumor mainly grew within the hepatic parenchyma, not in the surface of liver, and therefore adhesion did not occur between the diaphragm and hepatic capsule. There were no daughter nodules in the liver and the surgical margin was free from tumor. The background liver appeared normal macroscopically.
Figure 2.
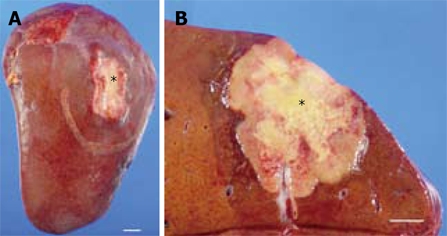
Gross appearance of the tumor. A: A firm, white and slightly depressed lesion (asterisk) measuring 4.2 cm × 3 cm was identified on the liver surface. B: Cross section of the tumor in the liver. A yellowish-white tumor of 4.4 cm × 3.8 cm was identified just under the hepatic capsule (asterisk). The tumor had well-defined borders and an area of central necrosis. Bar indicates 1 cm.
Histologic findings
Hematoxylin and eosin stained sections showed a tumor composed of three main components: (1) sarcomatoid, (2) papillary epithelioid and (3) microcystic (microglandular or adenomatoid) (Figure 3). The sarcomatoid component was predominant and was composed of tumor cells showing trabecular structures separated by bands of fibrous tissue (Figure 3A). The tumor cells had abundant eosinophilic or clear cytoplasm, indistinct cytoplasmic borders, round and atypical nuclei with vesicular fine chromatin and variably sized nucleoli (Figure 3A). There were many mitoses and atypical mitoses in tumor cells. The tumor contained broad coagulation necrosis. Although the tumor had well-defined borders, there was no fibrous capsule and hepatocytes and bile ducts were entrapped in infiltrating tumor cells. In the white lesion on the surface, papillary proliferation of epithelioid tumor cells was identified. Epithelioid cells had eosinophilic cytoplasm with bland nuclei and distinct nucleoli. Near the surface, tumor cells showed microcystic structures with a lace-like, adenoid cystic or signet ring appearance (microcystic, microglandular or adenomatoid component). The microcystic component was located adjacent to the papillary epithelioid component (Figure 3B). Furthermore, microcystic component was also intermingled with the sarcomatoid component even in the deeper area adjacent to the surrounding hepatic parenchyma (Figure 3C). The background liver showed mild steatofibrosis and there was no intrahepatic metastasis of the tumor. The biopsy site could not be identified by gross and histological examination in the surgical specimen.
Figure 3.
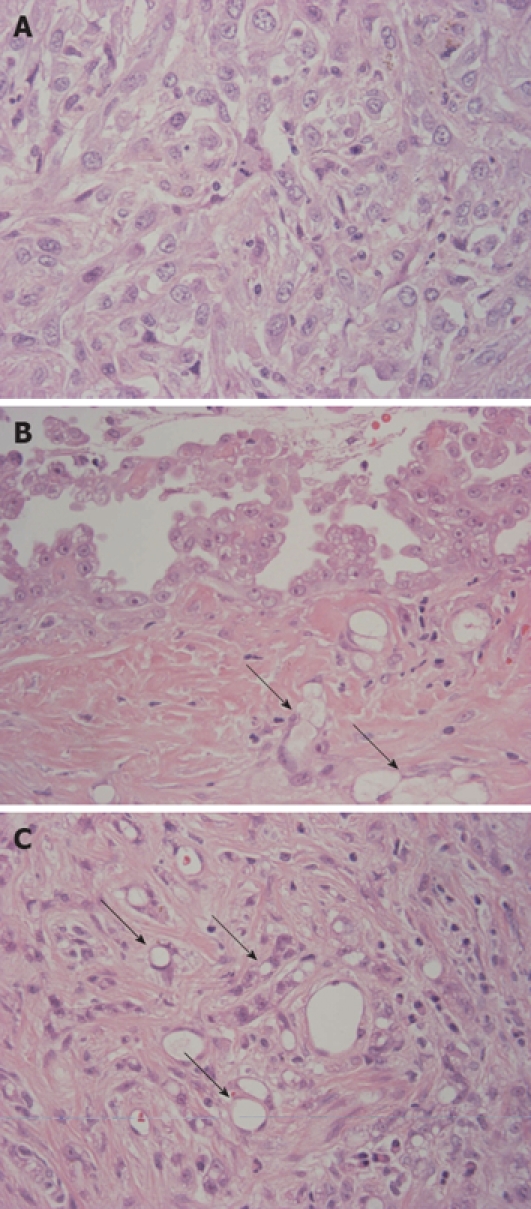
Histologic features of the tumor. A: Sarcomatoid component was predominant and was composed of tumor cells with abundant eosinophilic or clear cytoplasm, indistinct cytoplasmic borders, round and atypical nuclei with vesicular fine chromatin and variably sized nucleoli; B: Papillary proliferation of epithelioid tumor cells was identified on the surface of the tumor. Epithelioid cells had eosinophilic cytoplasm with bland nuclei and distinct nucleoli. Arrows indicate microcystic (microglandular or adenomatoid) component; C: In the area near the surface, a microcystic (microglandular or adenomatoid) component showing microcystic structures with lace-like, adenoid cystic or signet ring appearance was detected (arrows) (HE × 400).
A few sclerotic nodules attached to the diaphragm were composed of dense hyalinized fibrous tissue resembling pleural plaques. There were no tumor cells in the nodules.
Immunohistochemical and special stains
Formalin-fixed, paraffin-embedded sections were stained with periodic acid-Schiff (PAS) with and without diastase digestion, and alcian blue (pH 2.5) with and without hyaluronidase digestion. Immunohistochemical stains were performed using the Envision methods (Dako, Carpinteria, CA, USA) and antibodies as described previously[19]. The antibodies included WT-1 (6F-H2, Dako), calretinin (polyclonal; no dilution; Nichirei, Tokyo, Japan), D2-40 (D2-40; dilution 1/100; Dako), CK5/6 (D5/16B4, dilution 1/50; Dako), mesothelin (5B2, dilution 1/20; Lab vision, Fremont, CA, USA), thrombomodulin (1009, dilution 1/20; Dako), vimentin (3β4, dilution 1/600; Dako), CD34 (Immu133, dilution 1/200; Immunotech, Fullerton, CA, USA), p53 (DO-7; dilution 1/100; Dako), epithelial membrane antigen (EMA) (E29; dilution 1/200; Dako), MUC1 (DF3, Toray-Fuji Bionichs, Tokyo, Japan), Ber-EP4 (Ber-EP4; no dilution; Neomarkers, Fremont, CA, USA), cytokeratin (polyclonal; dilution 1/600; Dako), MOC-31 (MOC-31; dilution 1/50; Dako), CK7 (OV-TL 12/30; dilution 1/50; Dako), CK19 (RCK108; dilution 1/50; Dako), CEA (II-7, dilution 1/100, Dako), CA19-9 (C241:5:1:4, dilution 1/100; Novocastra, Newcastle, UK), CD15 (C3D-1; dilution 1/50; Dako), BG-8 (F-3; dilution 1/200, Signet, Dadham, MA, USA), HepPar1 (OCH1E5, dilution 1/100; Dako). Heat-induced epitope retrieval was used for all antibodies except CD34, MUC1, CA19-9 and BG-8. Appropriate positive and negative controls were used throughout.
Alcian blue showed positive staining on the surface of papillary epithelioid cells and the lumen of microcystic component, and this positive staining was sensitive to digestion with hyaluronidase. Neutral mucin stained by PAS stain was not detected on the surface of papillary epithelioid cells or the lumen of the microcystic component. Immunohistochemical stains confirmed the mesothelial features of the tumor showing membranous D2-40, mesothelin and thrombomodulin and nuclear WT-1, in addition to nuclear and cytoplasmic calretinin immunoreactivity, mainly in the papillary epithelioid and microcystic components (Figure 4). Sarcomatoid tumor cells showed focal immunoreactivity for WT-1, D2-40 and calretinin. When we examined the immunoreactivity for D2-40 and calretinin in 23 intrahepatic cholangiocarcinoma samples for comparison, none of the cholangiocarcinoma showed nuclear immunoreactivity for calretinin or immunoreactivity for D2-40. Four cholangiocarcinomas showed weak cytoplasmic immunoreactivity for calretinin. The tumor cells showed focal immunoreactivity for CK5/6. The tumor cells showed strong immunoreactivity for vimentin, CK7, CK19 and polyclonal cytokeratin (Figure 4). Strong EMA and MUC1 immunoreactivity was seen on the surface of papillary epithelioid cells, the lumen of the microcystic component and part of the sarcomatoid component (Figure 4). Sarcomatoid and microcystic components showed strong nuclear immunoreactivity for p53 (Figure 4). A few positive cells were also seen in the papillary epithelioid component. The tumors showed no immunoreactivity for CEA, Ber-EP4, MOC-31, CA19-9, CD15, BG-8, CD34 and HepPar1.
Figure 4.
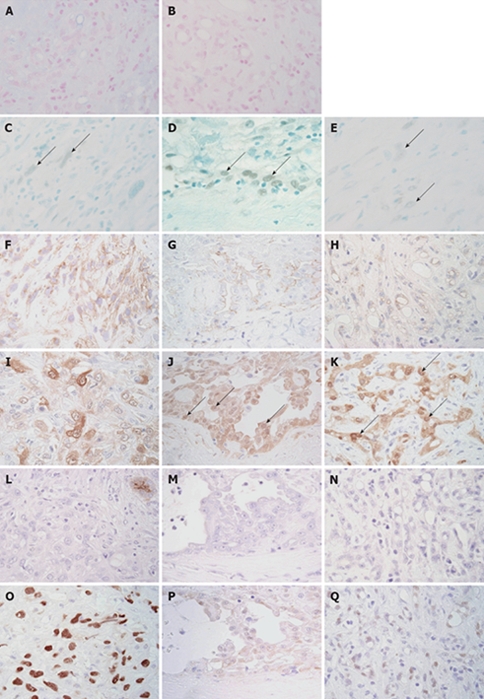
Immunohistochemical and special stains. Alcian blue (pH 2.5) showed positive staining on the lumen of adenomatoid tumors (A). This positive staining was sensitive to digestion with hyaluronidase (B). Nuclear WT-1 immunoreactivity (arrows) was detected focally in sarcomatoid (C), papillary epithelioid (D) and microcystic (E) components. Immunostaining for WT-1 and methyl green. Sarcomatoid tumor cells showed focal membranous immunoreactivity for D2-40 (F). Membranous D2-40 immunoreactivity was detected in the papillary epithelioid (G) and microcystic (H) components. Immunostaining for D2-40 and hematoxylin. Sarcomatoid tumor cells showed focal and rather weak immunoreactivity for calretinin (I). Nuclear calretinin immunoreactivity (arrows) was detected in the papillary epithelioid (J) and microcystic (K) components. Immunostaining for calretinin and hematoxylin. Immunoreactivity for CA19-9 was not detected in sarcomatoid (L), papillary epithelioid (M) and microcystic (N) components. The apical surface of the entrapped bile duct showed immunoreactivity for CA19-9 (L, upper right corner). Immunostaining for CA19-9 and hematoxylin. Sarcomatoid (O) and microcystic (P) components showed strong nuclear immunoreactivity for p53. A few papillary epithelioid cells showed nuclear immunoreactivity for p53 (Q). (Immunostaining for p53 and hematoxylin, × 400).
DISCUSSION
Primary malignant mesothelioma arising in the liver is rare and is currently not listed in the World Health Organization classification of hepatic tumors[20]. Review of the literature disclosed only three previously reported adult cases of primary malignant mesothelioma[9,10,12]. The previously reported cases were two men[9,10] and a woman[12], ranging in age from 54 to 64 years old (mean: 60 years old). Two patients had no history of asbestos exposure[9,10] and there was no indication in one patient[12]. One case was associated with cirrhosis due to hepatitis C viral infection[9]. All tumors arose in the right hepatic lobe, located in the subcapsular area of the liver and were 3.2 to 12 cm in diameter (mean: 7 cm).
Histologically malignant mesotheliomas conform to one of three patterns: epithelial (the most common type), sarcomatoid and biphasic (mixture of epithelioid and sarcomatoid) types[1,3,4]. All three reported mesotheliomas arising in the liver were of the epithelioid type[9,10,12]. One tumor was composed of sheets of cytologically bland polygonal cells containing multiple gland-like spaces and microcysts lined by cuboidal to columnar epithelium[12]. The other tumors displayed the tubular[9,10] and papillary proliferation[10] of epithelioid cells with a desmoplastic stroma[9] or surrounded by a densely mixed inflammatory infiltrate[10]. A panel of immunohistochemical markers was applied for differential diagnosis in all three tumors as discussed below and electron microscopic findings suggested a mesothelial cell origin in two tumors[10,12]. One tumor was speculated to originate from mesothelial cells of Glisson’s capsule[12]. However, no tumors were exposed to the peritoneal cavity on the hepatic capsule[9,10,12] and were thought to be localized mesothelioma of the liver, not to be localized mesothelioma of the peritoneum with hepatic invasion[9,10,12].
In this report, we have described for the first time a malignant mesothelioma of biphasic type arising in the liver in a 66-year-old man with a history of asbestos exposure. The tumor was detected as a hepatic nodular lesion in a follow-up examination for asbestosis and there was no other lesion suggesting primary tumor in the pleura and peritoneum. The tumor arose in the right lobe (S8 segment) of the liver in accordance with the reported cases[9,10,12]. In the major part of the hepatic nodular tumor, the tumor cells showed a sarcomatoid pattern and a microcystic pattern was intermingled with the sarcomatoid pattern. Tumor cells showed a papillary epithelioid pattern on the surface of the liver and a microcystic pattern was also seen in the subcapsular area near the surface. Since these tumor cells show the profile of mesothelioma even in the deeper area, this tumor is diagnosed as a malignant mesothelioma, not as another type of tumor with reactive mesothelial hyperplasia. The tumor in the present case may be categorized into the entity of localized malignant mesothelioma because of localized presentation[8], and better prognosis may be expected in the patient.
The origin of tumor cells showing mesothelial features in the liver is not clear, since mesothelial cells are not present in livers under normal physiological conditions. The mesothelioma cells might originate from other types by transition, although there has been no evidence of transition to mesothelial cells, so far. In contrast to the reported cases in which tumor cells did not expose to the peritoneal cavity, a papillary epithelioid component was also seen on the surface of the hepatic capsule adjacent to the hepatic nodular tumor. This finding suggests that the present tumor may originate from mesothelial cells of the Glisson’s capsule which subsequently invaded into the liver. A reported tumor was also speculated to originate from mesothelial cells of the Glisson’s capsule, although there was no evidence[10]. From a standpoint of p53 immunoreactivity, a few cells showed faint p53 immunoreactivity in papillary epithelioid components, whereas most tumor cells showed strong p53 immunoreacitivity in microcystic and sarcomatoid components. Therefore, it is conceivable that the mesothelioma of papillary epithelioid type initially arose on the surface of the liver and tumor cells showing microcystic and sarcomatoid patterns invaded and grew into the liver with more aggressive features originating from p53 mutation in the present case. Since this tumor was located on the hepatic capsule just under the diaphragm and compressed, it may be easier for tumor cells to grow into hepatic parenchyma than spreading in the surface of the liver, and therefore adhesion did not occur between the diaphragm and hepatic capsule. Taken together, a localized mesothelioma of the liver may arise as a localized mesothelioma of the Glisson’s capsule (peritoneum) and may subsequently show an intrahepatic nodular growth.
The differential diagnosis of primary malignant mesothelioma of the liver includes primary liver cancers and metastases from pleural malignant mesothelioma. In particular, it is important but may be difficult to distinguish from intrahepatic cholangiocarcinoma. Since both malignant mesothelioma of epithelioid type and cholangiocarcinoma show a tubular and papillary structure[3,20], a similar panel of immunohistochemical markers may be useful in the differential diagnosis between pleural mesothelioma and pulmonary adenocarcinoma, which is sometimes difficult[3,21–23]. There is no single absolute marker for mesothelioma, so far[3]; therefore, a combination of two or more positive immunohistochemical mesothelial markers (CK5/6, calretinin and Wilms tumor gene-1 (WT1)) with negative epithelial (adenocarcinoma) markers (CEA, CD15, BerEP4, B72.3, BG8, MOC31) is recommended for a diagnosis of pleural mesothelioma[3,21,23]. Since both malignant mesothelioma and cholangiocarcinoma show immunoreacitivity for CK7, CK19, polyclonal cytokeratin, EMA (a glycosylated form of MUC1) and unglycosylated form of MUC1 detected by DF3, these markers are not helpful for differential diagnosis. Recently, D2-40 was reported to be a sensitive marker for cells of mesothelial origin, and useful in the differential diagnosis of epithelioid malignant mesothelioma vs adenocarcinoma[22]. In previous reports[9,10,12], the diagnosis of primary hepatic mesotheliomas has been made based on positive immunoreactivity for calretinin[9,10,12], HBME-1[9], D2-40[10], thrombomodulin[10], vimentin[10,12] and cytokeratins[9,10,12] and negative immunoreactivity for CEA[9,10], LeuM1 (Lewis X)[9], CD34[9,10,12], CA19-9[10] and Ber-EP4[10]. There have been no reports regarding immunoreactivity for calretinin and D2-40 in intrahepatic cholangiocarcinoma, so far. In our preliminary study, cytoplasmic, but not nuclear calretinin immunoreactivity was detected in some intrahepatic cholangiocarcinomas and D2-40 imunoreactivity was not detected in any intrahepatic cholangiocarcinomas examined. Since the expression of CEA and LeuM1 (Lewis X) is less frequent in poorly differentiated intrahepatic cholangiocarcinoma[24] and the specificity of HBME-1 for mesothelioma is rather low (45%)[21], a combination of calretinin, HBME-1, CEA and LeuM1 may not be enough for a definite diagnosis of mesothelioma in the previously reported primary hepatic mesothelioma arising in a patient with cirrhosis and chronic hepatitis C[9]. The possibility of cholangiocarcinoma associated with cirrhosis and chronic hepatitis C[25] should be excluded in the reported case[9].
In the present case, tumor cells, especially of epithelioid type, showed distinct immunoreactivity for mesothelial markers (WT-1, calretinin, D2-40, mesothelin, thrombomodulin) and no immunoreactivity for epithelial (adenocarcinoma) markers (CEA, CD15, BerEP4, BG8, MOC31). Markers suggesting other types of tumor such as CD34, CD31, HepPar1 were totally negative; therefore the tumor fulfills the immunohistochemical diagnostic criteria for mesothelioma. However, it was quite difficult to reach a final diagnosis from a needle biopsy specimen including only the sarcomatoid component with immunoreactivity for cytokeratin and vimentin, and faint and focal immunoreactivity for calretinin and D2-40. It is reported that intrahepatic cholangiocarcinoma shows only occasional sarcomatous change[20,26]. In this type, tumor cells show immunoreactivity for both epithelial markers (cytokeratins and EMA) and vimentin[20,26]. Clinical information about asbestos exposure was important to raise the possibility of mesothelioma.
In summary, we presented for the first time a localized malignant biphasic mesothelioma arising in the liver in a 66-year-old man with a history of asbestos exposure. A panel of immunohistochemical markers to distinguish pleural mesothelioma from pulmonary mesothelioma was useful for diagnosis. Malignant mesothelioma should be included in the differential diagnosis of primary hepatic tumor, especially in patients with a history of asbestosis exposure. Because of unusual localization of the tumor, a very careful histological and immunohistochemical examination was required to reach the final diagnosis in the present case. Accumulation of more cases similar to the present case is important to characterize the features of mesothelioma of the liver, including the biological behavior and the prognosis.
Peer reviewer: Dr. Yukihiro Shimizu, Kyoto Katsura Hospital, 17 Yamada-Hirao, Nishikyo, Kyoto 615-8256, Japan
S- Editor Li LF L- Editor Logan S E- Editor Zheng XM
References
- 1.Maitra A, Kumar V. The lung and upper respiratory tract. In: V Kumar, R Cotran, S Robbins., editors. Robbins Basic Pathology. 7th ed. Saunders: Philadelphia; 2002. pp. 453–509. [Google Scholar]
- 2.Flemming P, Becker T, Klempnauer J, Högemann D, Kreft A, Kreipe HH. Benign cystic mesothelioma of the liver. Am J Surg Pathol. 2002;26:1523–1527. doi: 10.1097/00000478-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Churg A, Roggli V, Galateau-Salle F, Cagle P, Gibbs A, Hasleton P, Henderson D, Vignaud J. Mesothelioma. In: W Travis, E Brambilla, H Muller-Hermelink, C Harris., editors. Pathology & Genetics Tumours of the Lung, Pleura, Thymus and Heart. IARC: Lyon; 2004. pp. 125–136. [Google Scholar]
- 4.Marubayashi S, Ohdan H, Asahara T, Ikeda M, Hinoi T, Fukuma K, Maeda T, Oshiro Y, Shimamoto F, Dohi K. Malignant mesothelioma originating in the hepatic falciform ligament: report of a case. Surg Today. 1998;28:929–931. doi: 10.1007/s005950050254. [DOI] [PubMed] [Google Scholar]
- 5.Bierhoff E, Pfeifer U. Malignant mesothelioma arising from a benign mediastinal mesothelial cyst. Gen Diagn Pathol. 1996;142:59–62. [PubMed] [Google Scholar]
- 6.Alvarez-Fernandez E, Escalona-Zapata J. Intrapulmonary mesotheliomas: their identification by tissue culture. Virchows Arch A Pathol Anat Histol. 1982;395:331–343. doi: 10.1007/BF00429358. [DOI] [PubMed] [Google Scholar]
- 7.Attanoos RL, Gibbs AR. Primary malignant gonadal mesotheliomas and asbestos. Histopathology. 2000;37:150–159. doi: 10.1046/j.1365-2559.2000.00942.x. [DOI] [PubMed] [Google Scholar]
- 8.Allen TC, Cagle PT, Churg AM, Colby TV, Gibbs AR, Hammar SP, Corson JM, Grimes MM, Ordonez NG, Roggli V. Localized malignant mesothelioma. Am J Surg Pathol. 2005;29:866–873. doi: 10.1097/01.pas.0000165529.78945.dc. [DOI] [PubMed] [Google Scholar]
- 9.Imura J, Ichikawa K, Takeda J, Iwasaki Y, Tomita S, Kubota K, Fujimori T. Localized malignant mesothelioma of the epithelial type occurring as a primary hepatic neoplasm: a case report with review of the literature. APMIS. 2002;110:789–794. doi: 10.1034/j.1600-0463.2002.t01-1-1101102.x. [DOI] [PubMed] [Google Scholar]
- 10.Gütgemann I, Standop J, Fischer HP. Primary intrahepatic malignant mesothelioma of epithelioid type. Virchows Arch. 2006;448:655–658. doi: 10.1007/s00428-006-0175-8. [DOI] [PubMed] [Google Scholar]
- 11.DeStephano DB, Wesley JR, Heidelberger KP, Hutchinson RJ, Blane CE, Coran AG. Primitive cystic hepatic neoplasm of infancy with mesothelial differentiation: report of a case. Pediatr Pathol. 1985;4:291–302. doi: 10.3109/15513818509026902. [DOI] [PubMed] [Google Scholar]
- 12.Leonardou P, Semelka RC, Kanematsu M, Braga L, Woosley JT. Primary malignant mesothelioma of the liver: MR imaging findings. Magn Reson Imaging. 2003;21:1091–1093. doi: 10.1016/s0730-725x(03)00197-8. [DOI] [PubMed] [Google Scholar]
- 13.Barnoud R, Arvieux C, Pasquier D, Pasquier B, Letoublon C. Solitary fibrous tumour of the liver with CD34 expression. Histopathology. 1996;28:551–554. doi: 10.1046/j.1365-2559.1996.d01-468.x. [DOI] [PubMed] [Google Scholar]
- 14.Kasano Y, Tanimura H, Tabuse K, Nagai Y, Mori K, Minami K. Giant fibrous mesothelioma of the liver. Am J Gastroenterol. 1991;86:379–380. [PubMed] [Google Scholar]
- 15.Kottke-Marchant K, Hart WR, Broughan T. Localized fibrous tumor (localized fibrous mesothelioma) of the liver. Cancer. 1989;64:1096–1102. doi: 10.1002/1097-0142(19890901)64:5<1096::aid-cncr2820640521>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Moran CA, Ishak KG, Goodman ZD. Solitary fibrous tumor of the liver: a clinicopathologic and immunohistochemical study of nine cases. Ann Diagn Pathol. 1998;2:19–24. doi: 10.1016/s1092-9134(98)80031-2. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz S, Kirimlioglu V, Ertas E, Hilmioglu F, Yildirim B, Katz D, Mizrak B. Giant solitary fibrous tumor of the liver with metastasis to the skeletal system successfully treated with trisegmentectomy. Dig Dis Sci. 2000;45:168–174. doi: 10.1023/a:1005438116772. [DOI] [PubMed] [Google Scholar]
- 18.Goodlad JR, Fletcher CD. Solitary fibrous tumour arising at unusual sites: analysis of a series. Histopathology. 1991;19:515–522. doi: 10.1111/j.1365-2559.1991.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki M, Ikeda H, Sato Y, Nakanuma Y. Decreased expression of Bmi1 is closely associated with cellular senescence in small bile ducts in primary biliary cirrhosis. Am J Pathol. 2006;169:831–845. doi: 10.2353/ajpath.2006.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton SR, Aaltonen LA. Endocrine tumours of the stomach. In: Health Orga-nization Classification of Tumours World, et al., editors. Pathology and Genetics of Tumours of the Digestive System. Vol. 169. IARC Press: Lyon; 2000. [Google Scholar]
- 21.Yaziji H, Battifora H, Barry TS, Hwang HC, Bacchi CE, McIntosh MW, Kussick SJ, Gown AM. Evaluation of 12 antibodies for distinguishing epithelioid mesothelioma from adenocarcinoma: identification of a three-antibody immunohistochemical panel with maximal sensitivity and specificity. Mod Pathol. 2006;19:514–523. doi: 10.1038/modpathol.3800534. [DOI] [PubMed] [Google Scholar]
- 22.Chu PG, Schwarz RE, Lau SK, Yen Y, Weiss LM. Immunohistochemical staining in the diagnosis of pancreatobiliary and ampulla of Vater adenocarcinoma: application of CDX2, CK17, MUC1, and MUC2. Am J Surg Pathol. 2005;29:359–367. doi: 10.1097/01.pas.0000149708.12335.6a. [DOI] [PubMed] [Google Scholar]
- 23.Ordóñez NG. What are the current best immuno-histochemical markers for the diagnosis of epithelioid mesothelioma? A review and update. Hum Pathol. 2007;38:1–16. doi: 10.1016/j.humpath.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Minato H, Nakanuma Y, Terada T. Expression of blood group-related antigens in cholangiocarcinoma in relation to non-neoplastic bile ducts. Histopathology. 1996;28:411–419. doi: 10.1046/j.1365-2559.1996.343384.x. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki M, Tsuneyama K, Ishikawa A, Nakanuma Y. Intrahepatic cholangiocarcinoma in cirrhosis presents granulocyte and granulocyte-macrophage colony-stimulating factor. Hum Pathol. 2003;34:1337–1344. doi: 10.1016/j.humpath.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki M, Nakanuma Y, Nagai Y, Nonomura A. Intrahepatic cholangiocarcinoma with sarcomatous transformation: an autopsy case. J Clin Gastroenterol. 1991;13:220–225. doi: 10.1097/00004836-199104000-00022. [DOI] [PubMed] [Google Scholar]


