Abstract
There are various hormones and growth factors which may modify the intestinal absorption of nutrients, and which might thereby be useful in a therapeutic setting, such as in persons with short bowel syndrome. In partI, we focus first on insulin-like growth factors, epidermal and transferring growth factors, thyroid hormones and glucocorticosteroids. Part II will detail the effects of glucagon-like peptide (GLP)-2 on intestinal absorption and adaptation, and the potential for an additive effect of GLP2 plus steroids.
Keywords: Epidermal growth factor, Glucocortico-steroids, Insulin-like growth factor-I/II, Intestinal growth, Transforming growth factor-α-2, Hepatocyte growth factor, Keratinocyte growth factor
INTRODUCTION
In this review, the hormones and growth factors to be reviewed which alter nutrient absorption include insulin-like growth factor I (IGF-I) and II, EGF, TGF-α, HGF, Erythropoietin and KGF. In Table 1 is outlined the cell types which express those peptides, as well as the cell types in the intestine and elsewhere which express the corresponding receptors. PartIthere are various hormones and growth factors which may modify the intestinal absorption of nutrients, and which might thereby be useful in a therapeutic setting, such as in persons with short bowel syndrome. Part II presented here will detail the effects of glucagon-like peptide (GLP)-2 on intestinal absorption and adaptation, and the potential for an additive effect of GLP2 plus steroids.
Table 1.
Hormones and peptides which affect intestinal nutrient absorption
| Hormones/Peptides | Cell types which express the specific peptide | Cell types in the intestine and elsewhere which express corresponding receptors |
| IGF I& II | Granulosa cells[158] | Non-Islet cell tumors[163] |
| Prostate stromal cells[159] | Hepatoma cells[164] | |
| Neonatal bone marrow-mesenchymal stem cells[160] | Intestinal smooth muscle[165] | |
| Systemic sclerosis lung fibroblasts[161] | Astrocytoma cells[166] | |
| Melanoma Cells (SK-MEL2)[162] | Camel intestinal lamina propria[167] | |
| Camel epithelia of the crypt cells[167] | ||
| Camel villi[167] | ||
| EGF | Rat submandibular glands cells[168] | Keratinocytes[169] |
| HaCaT cells[169] | Urothelial cells[172] | |
| Porcine cumulus cells[170] | Fibromyoblasts[173] | |
| Esophageal epithelial cell[171] | Smooth muscle cells[173] | |
| Fibroblasts[173] | ||
| Colonic epithelial cells[174] | ||
| Murine pancreatic beta cells[175] | ||
| TGF-α | Hypothalamic astrocytes[176] | Pancreatic cancer cells[180] |
| Granulosa cells[177] | Osteoblasts[181] | |
| Ovarian theca cells[177] | Murine conjunctival goblet cells[182] | |
| Endometrial stromal cells[177] | Fundic mucous cells[183] | |
| Eosinophhils[178] | Murine intestinal epithelial cells[184] | |
| Colon cancer cells[179] | ||
| HGF | Lung microvascular endothelial cells[185] | Cardiomyocytes[189] |
| Umbilical vein endothelial cells[185] | Liver progenitor cells[190] | |
| Neutrophils[186] | Glioblastoma cells[191] | |
| Monocytes[187] | Myeloma cells[192] | |
| Adipocytes[188] | Tumor plasma cells[192] | |
| Neuroblasts[193] | ||
| Hepatocellular carcinoma cells[194] | ||
| Epithelial tubular cells[195] | ||
| Antroduodenal G cells[196] | ||
| Rectal enterochromaffin cells[196] | ||
| Pancreatic A and B cells[196] | ||
| Intestinal epithelial cells[196] | ||
| Erythropoietin | Amniotic epithelial cells[197] | Erythroid cells[202] |
| Schwann cells[198] | Endothelial cells[203] | |
| Interstitial fibroblasts[199] | Neuronal cells[203] | |
| Metanephric adenoma cells[200] | Cardiac myocytes[203] | |
| Mesenchymal stromal cells[201] | Vascular smooth muscles[203] | |
| Murine fetal enterocytes[204] | ||
| Intestinal epithelial cells[205] | ||
| KGF | Mucosal and dermal fibroblasts[206] | Alveolar epithelial type II cells[209] |
| Gastric fibroblasts[207] | Breast cancer cells[210] | |
| Endometrial stromal cells[208] | Cholesteatoma cells[211] | |
| Enterochromaffin cells[212] |
Note: All of these are in humans until stated otherwise.
INSULIN-LIKE GROWTH FACTORS (IGF-I, IGF-II)
The insulin-like growth factor family consists of insulin, IGF-I, and IGF-II. The liver is the major site of synthesis for IGF-I, but it is also synthesized in other tissues including the gastrointestinal tract[1,2]. IGF-Iand IGF-II are found in human milk[3], and these peptides are members of a complex mixture of growth factors that the neonate is exposed to during the suckling period.
Intestinal development may involve IGF-Iand IGF-II[4–6]. IGF-Ireceptor (IGF-1R) is detectable in the fetus and the neonate of animals[7,8]. In humans, IGF-I, IGF-II and IGF-IR are also present in fetal intestine[9–11].
Intestinal cells proliferate in vitro when exposed to IGF-I[12–14]. Transgenic mice that over express IGF-Idemonstrate increased small intestinal growth[15], particularly in the muscle layers of the distal small intestine and large intestine[16]. IGF-Ireceptor gene knockout animals have fetal growth retardation, but distinctive effects on the gastrointestinal system have not been found[17]. This may indicate the presence of redundant systems in the gas-trointestinal tract that compensate for the lack of IGF-I.
In vivo studies show variable responses depending on the dose of IGF-Ithat is used. Pharmacological doses of IGF-I, given orally to colostrum-deprived 5 d old piglets, increase electrolyte and nutrient absorption[18]. Neonatal pigs fed formula supplemented with IGF-Ifrom birth to 4 d of age had increased mucosal growth[19]. Parenterally fed 1 d old piglets given enteral IGF-Ihad increased brush border malease (BBM) disaccharidase levels[20]. Houle et al[21] administered IGF-Iorally to neonatal rats, and demonstrated increases in mucosal growth and BBM enzymes. Similarly, Burrin et al[19] showed increases in intestinal weight, protein, and DNA in neonatal pigs fed orogastrically. Alexander and Carey[18] examined the effect of oral IGF-Ion neonatal piglet intestine, and found increases in D-glucose uptake in everted jejunal sleeves, independent of the intestinal mass or surface area. Lane et al[22] used RT-PCR and laser scanning confocal microscopy to show that intragastric IGF-Ior IGF-II increased the expression of the glucose transporters SGLT1 and GLUT2 in suckling rat intestine.
The mechanisms responsible for increased jejunal transport rates observed in tissues treated with orally administered IGF-Imay include the increases in Na+K+-ATPase seen with IGF-Itreatment. The PI3-kinase pathway may also be important, as preincubation with a PI3-kinase inhibitor abolishes the effects of IGF-Ion ion and nutrient transport[23].
Although pharmacological doses of IGF-Ihave clear effects on the neonatal intestine, studies using physiological doses show only limited effects. For example, recent studies with both neonatal pigs[21,24] and calves[25] have shown that the oral administration of physiological concentrations of IGF-Iin formula results in measurable increases in crypt cell proliferation, but there are no demonstrable increases in intestinal mucosal mass or length.
EPIDEMAL GROWTH FACTOR AND TRANSFORMING AND TRANSFORMING GROWTH FACTOR-α (TGF-α)
EGF and TGF-α are two ligands of the EGF receptor that have been postulated to play a role in intestinal ontogeny. Both peptides are present in human breast milk, with TGF-α at a 5-10-fold lower concentration than EGF[26]. Transforming growth factor β (TGFβ) is a family of growth factors, with the three isoforms (TGFβ1, TGFβ2, TGFβ3) having nearly identical biological activities. TGFβ1 and TGFβ2 are present in human milk[27], and mRNA and protein corresponding to the three TGFβ isoforms are found in the intestinal mucosa[28–30]. Small intestinal development, however, is not severely impaired by the targeted disruption of the TGFβ gene[31].
EGF and TGF-α have been isolated in human 15-20 wk gestation fetal intestine, with TGF-α levels as much as 10 times higher than EGF[32]. EGF receptor expression is detectable throughout the GI tract in the human fetus and neonate. EGF receptor is developmentally regulated with expression being low during the human suckling period, and in rodents, unlike humans, EGF receptor expression is delayed until weaning[33].
Oral EGF administration augments gut growth and functional development in neonatal rats and pigs[34–36]. Gene targeting studies have demonstrated that disrupting the expression of the EGF receptor in mice resulted in impaired intestinal development, and death associated with a necrotizing enterocolitis-like disorder[37].
The postnatal development of intestinal transport and the physical composition of the BBM were examined in New Zealand White rabbits receiving EGF (40 mg/kg per day) either intraperitoneally or orogastrically from day 3 to day 17 of life. Intestinal water, Na+ and glucose absorption expressed per cm of intestine were significantly increased in animals receiving EGF by either route. Increased absorption induced by orogastric EGF appeared to be secondary to mucosal hyperplasia. The BBM isolated from EGF-treated animals was significantly more fluid than that of controls. These results suggest that EGF modulates the development of transport function during the postnatal period, both by stimulating mucosal growth and by inducing specific transport processes[38].
Experimental necrotizing enterocolitis (NEC) was induced by exposure of newborn animals to asphyxia and cold stress. The newborn rats were artificially fed either with growth factor-free rat milk substitute or the same formula supplemented with 500 ng/mL of EGF[39]. EGF supplementation of formula reduced the incidence and severity of NEC, as assessed by gross and histological scoring of the ileum. This finding suggests a potential therapeutic approach for the prevention and treatment of NEC.
HEPATOCYTE GROWTH FACTOR, ERYTHROPOIETIN, KERATINOCYTE GROWTH FACTOR AND THYROID HORMONES
Erythropoietin (Epo) is present in breast milk[40], and receptors for Epo are present on intestinal cells[41]. Modest changes in small intestinal length and surface area are observed following subcutaneous Epo injections in artificially fed rat pups. Hepatocyte growth factor (HGF) is also present in breast milk, and both HGF and its receptor are present in fetal intestinal tissue[42,43]. Keratinocyte growth factor (KGF) promotes proliferation and up-regulates SI expression in fetal human small intestine explant culture[44]. It remains to be seen if KGF plays a physiological role in postnatal intestinal development.
The effect of thyroid hormones on the ontogeny of BBM enzymes has been examined. An in vivo study of lactase phlorizin hydrolase (LPH) catalytic activity, synthesis, and degradation was performed in propylthiouracil-induced hypothyroid rat pups, hypothyroid pups injected with thyroxine, and normally weaned rats. T4 regulates LPH ontogeny by posttranslational mechanisms that include altered processing and increased degradation of the BBM lactase enzyme[45]. Monteiro et al[46] examined the role of T4 on the precocious enhancement of GLUT5 in weanling rats. Rat pups were made hypothyroid by giving the dam 0.01% propylthiouracil in the drinking water from day 18 of gestation. The hypothyroid pups and age-matched euthyroid control pups were then fed high-fructose solutions by gavage, twice a day starting at 17 d of age for 3 d. Although serum T4 levels were five times lower in the hypothyroid pups, the mRNA level for the BBM fructose transporter (GLUT5) increased in euthyroid and hypothyroid pups fed high fructose. This result paralleled the increase in fructose uptake. This suggests that during weaning, dietary fructose can precociously enhance intestinal fructose uptake and GLUT5 mRNA expression, independent of developmental increases in serum T4 levels.
GLUCOCORTICOSTEROIDS
Biochemistry
Steroids can be divided into two categories: glucocorti-costeroids (GC), if potency is based on liver glycogen deposition, and mineralocorticoids, if potency is based on sodium retention. The hormones that are secreted in significant amounts are cortisol, corticosterone, and aldosterone. In humans, the major naturally occurring steroid is cortisol, while in rodents the main steroid is corticosterone.
Steroids are synthesized from cholesterol in the inner mitochondrial membrane of the adrenal glands, through a series of reactions including hydroxylations. The release of cortisol from the adrenal glands is regulated by the hypothalamic-pituitary-adrenal axis, and involves the secretion of corticotropin-releasing hormone from the hypothalamus, and the subsequent secretion of adrenocorticotropin hormone (ACTH) from the pituitary gland. The rate-limiting step in steroid synthesis is the transport of cholesterol to the inner mitochondrial membrane by sterol carrier protein 2 (SCP2), steroidogenesis activator protein polypeptide (SAP), peripheral benzodiazepine receptor (PBR), and steroidogenic acute regulatory protein (StAR)[47]. Because corticosteroids are not stored in adrenal tissues, the rate of secretion equals the rate of biosynthesis[48–51].
GC are classified as lipids because they are more soluble in organic solvents than aqueous solvents. Their solubility is affected by the presence of hydroxyl or carbonyl groups[52]. GC enter cells by diffusion across the plasma membrane. About 90% of circulating cortisol is reversibly bound to a plasma protein, the corticosteroid binding globulin (CBG)[48,51,52]. CBG is a high affinity, low capacity plasma protein, while albumin has a low affinity and high capacity to bind steroids[48,51–53]. The bioavailablility of GC in specific tissues is determined by the presence of tissue specific metabolizing enzymes [11-β-hydroxysteroid dehydrogenase (11β-HSD)], CBG levels, the presence of efflux proteins (such as multi-drug resistance protein 1, MDR1), and the expression of the glucocorticosteroid receptors (GR). Other levels of regulation include variations in the receptor protein (isoforms, polymorphisms), alternative receptor dimerization, co-chaperones, levels of hsp, and posttranslational modifications[54].
Approximately 70% of corticosteroid metabolism occurs in the liver, and involves the cytochrome P450 system[55]. Intestinal metabolism is also important, as the intestinal sites of cortisol and cortisone metabolism in humans are saturated before the hepatic sites[56]. The majority of corticosteroids are excreted in the urine, although small amounts are detectable in fecal, biliary and pulmonary CO2 excretions[48,49,57].
Glucocorticosteroid receptors (GR) are 94 kDa proteins found in the cytoplasm of cells from many tissues. When ligand binding occurs, an inhibitory protein is released, the receptor dimerizes, hyperphosphorylation occurs[58–60], and a DNA binding site is exposed (Figure 1). This conformational change, which may unmask nuclear localization signals, allows the GR to translocate to the nucleus. The GR may affect gene transcription and subsequent protein synthesis by interacting with specific nuclear binding sites (referred to as glucocorticosteroid response elements, GRE). This causes either stimulation or, less frequently, inhibition of transcription. Also, the binding of the GR homodimer to the GRE may induce rearrangement of the chromatin, allowing other transcription factors to bind to previously inaccessible DNA[61].
Figure 1.
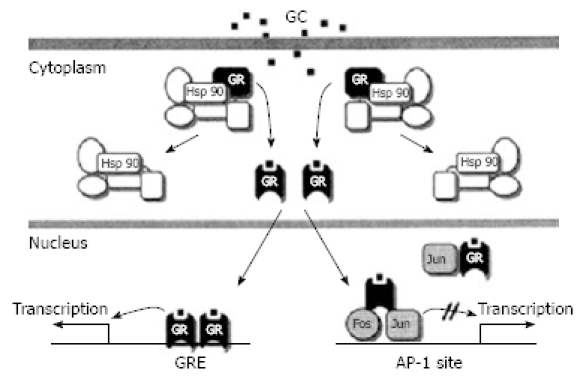
Simplified model of GR-mediated transcriptional modulation. (from Bamberger et al 1996).
The GR may also interact with other transcription factors, such as AP-1, in which case transcription is commonly inhibited[62,63]. There are several other transcription factors that have been linked to the GR, including the p65 subunit of NFκB[64,65].
The unliganded GR may be associated with heat shock proteins (hsp), including hsp56, hsp70 and hsp90. There may be constant cycles of dissociation/reassociation of these components, as well as constant bi-directional shuttling of GR between the cytosol and the nucleus[66,67]. Furthermore, the action of hsp70 and hsp90 may be further regulated, either positively or negatively, by co-chaperones[68–71]. These proteins may also be involved in recruiting transport proteins to the receptor complex, regulating hormone binding to the receptor, and modulating the regulatory function of GR by disassembling transcription complexes[70–73].
Several other factors may influence GR-mediated transcriptional activation. For example, it has been suggested that competition between transcription factors for limited coactivator molecules may lead to gene repression[74,75]. Alternatively, cofactor effects may be restricted to designated compartments, and dynamic cofactor modules may hit promoters in a cyclic way during transcription[72,73,76,77]. In addition to acetylation, methylation and phosphorylation of histones may influence transcriptional control[78–80], as also could phosphorylation of the GR[81].
Some of the effects of GC occur too rapidly to be explained at the genomic level of transcription. Distinct GR forms or a cytosolic subset of GR may interact with signal transduction pathways, which are usually associated with membrane receptor signalling events[82]. Extensive crosstalk between steroid- and growth factor-stimulated signalling pathways occurs, and may impact the functioning of steroid receptors and genes that are regulated by steroids[83].
A number of synthetic GC have been developed with increased potency when compared to the naturally occurring substances. The increased potency, however, comes with an increased potential for adverse effects. The high potency of the synthetic steroid is attributed to its long half life and high affinity for the GR. DEX is commonly used in the research setting due to its’ unique properties. The synthetic DEX differs from the naturally occurring cortisol due to the addition of a double bond between carbon 1 and 2, fluorination of the 9 position, and the addition of a methyl group. Therefore, while most GC in the plasma is bound to the CBG, DEX circulates freely, with blood concentrations unaffected by changes in CBG levels. This is particularly important in studies of development, as CBGs are known to be developmentally regulated[84]. The placental enzyme 11-β hydroxysteroid dehydrogenase type-2 (11β-HSD2) protects the fetus by converting maternal cortisol into inactive cortisone, due to its dehydrogenase activity[85]. However, the unique structure of DEX allows it to cross the placenta by escaping deactivation by 11β-HSD2.
Intestinal maturation
The effect of GC on prenatal intestinal maturation was investigated by Gartner et al[86], using mice lacking a functional glucocorticoid receptor (GR-/). Although a temporal association exists between the prenatal GC surge and corresponding intestinal development, the results of this study suggest that steroids are not mandatory for prenatal intestinal maturation. Histological examination of 18 d old fetal mice failed to demonstrate any effects of the GR-/- phenotype on intestinal morphology, or on measures of functional development (including enterocyte, Goblet and enteroendocrine cell numbers). LPH activity (as measured by X-gel staining) and the number of proliferating cells (as assessed by KI-67 staining) were also unaffected by the absence of GR. Thus, the rodent intestine appears to pass through a period of unresponsiveness to GC during fetal life, prior to becoming responsive in the postnatal period.
The role of GC in postnatal development has been well characterized. Indeed, adrenalectomy during the suckling period retards the functional maturation of the small intestine, while exogenous administration of GC may cause precocious maturation[87–89]. The mechanisms by which GC may influence maturation are not completely understood. There may be direct as well as indirect effects of GC. For example, Schaeffer et al[90]) injected hydrocortisone (50 g/g, sc) into 11 d old rats; they showed that the precocious induction of the BBM enzyme sucrase isomaltose (SI) paralleled decreases in TGF-β and the cytokine IL-1α, and increases in TNF-α. This finding suggests that cytokine levels may mediate the effects of GC on postnatal intestinal maturation.
The development of GC responsiveness was characterized in more detail by Solomon et al[89] using trehalase as a marker of intestinal maturation, they showed three phases of responsiveness to GC in mice: (1) a prenatal phase during which time Dexamethasone (DEX) did not induce maturation; (2) an early postnatal phase (first 2 postnatal weeks) of moderate responsiveness; and (3) a subsequent phase of increasing responsiveness in the third postnatal week. Postnatal increases in responsiveness were not paralleled by increases in GR abundance, but were associated with elevated circulating T4 concentrations. This observation fits with previous work demonstrating a synergistic effect of GC and T4 on BBM enzyme expression[91–93] also previously demonstrated three post-natal phases of responsiveness to GC. Based on the effects on SI mRNA and activity in rats, they identified (1) an early phase (day 10) when activation of the gene occurs; (2) a late phase (day 16) when changes in cell kinetics are observed; and (3) a loss of responsiveness (day 18) to GC. Although the exact timing of the various phases is not consistent between studies, it is clear that steroids have maximal effects in the early postnatal period.
Although some studies have shown that GC are not important in fetal development[86,89], not all researchers would agree with the suggestion that the fetus is unresponsiveness to GC. For example, Buchmiller and colleagues[94] showed that maternal DEX administration produced increases in small intestinal length and a trend towards an increase in LPH and maltase activity in fetal rabbits. The everted sleeve technique was used to demonstrate increases in glucose and proline transport in fetal rabbits exposed to DEX. When the fetal rabbits were subdivided into categories based on uterine position, runt fetuses exposed to DEX exhibited high nutrient uptake rates, surpassing the rates of the favored fetuses. This suggests that the runt intestine may be more responsive to steroid treatment, an observation with interesting implications regarding the potential clinical treatment of low birth weight or growth retarded infants.
The effects of GC on fetal intestine were also examined in a rabbit gastroschisis model. Gastroschisis is a herniation (displacement) of the intestines through a defect on one side of the umbilical cord. Intra-amniotic DEX (0.2 μg/g per day) infusion enhanced intestinal disaccharidase activity and glucose uptake in fetuses with experimentally induced herniation of the small intestine into the amniotic cavity[95]. While this study looks at a specific congenital dysfunction, it clearly demonstrates that the fetal intestine is capable of responding to GC.
What is the effect of GC on the intestine of human neonates? Nanthakumar et al[96]. used human intestinal xenografts to characterize the response of the developing intestine to GC. Responsiveness was determined by lactase activity and cytokine induction after a proinflammatory stimulus. Immature transplants (20 wk) responded to GC, but that this effect was lost in the mature (30 wk) transplants. This suggests that in humans there may also be a brief period of responsiveness in utero to GC.
Costalos et al[97] showed that in humans, maternal DEX administration increased fetal and neonatal plasma gastrin concentrations, while motilin was increased only in neonates, and vasointestinal peptide concentrations were unchanged. The authors speculate that the effects of GC on the gastrointestinal tract may be at least partially mediated by their actions on other GI hormones.
Clearly, there is contradictory evidence regarding the ability of the fetus to respond to steroids. The discrepancies may be due to the use of different indicators (trehalase, SI, nutrient uptake, morphology) of GC responsiveness. Maternal DEX administration may have a direct effect on the fetus, as DEX is known to cross the placenta[98]. However, it is also possible that the maternal administration of DEX has an indirect effect on the fetus, through the modulation of other factors such as cytokines, hormones or growth factors.
There are many other adverse effects of the therapeutic administration of GC, such as glaucoma, poor wound healing, muscle atrophy, hypertension, hyperglycemia/diabetes, withdrawal syndrome, increased risk of infection, GI ulcers/perforation/bleed, thin skin, poorly developed muscle, thin extremities, fat collection in abdomen and upper back (“buffalo hump”), salt and water retention “moon face”, osteoporosis, avascular necrosis, and CNS effects such as increased appetite, insomnia, euphoria and frank psychoses.
GC may also be used to accelerate lung maturation and surfactant production in premature infants, although DEX is not recommended as it is associated with impaired growth and neurodevelopmental delay. GC administration reduces the incidence of NEC in infants: a prospective experiment looking at prenatal or postnatal steroid use demonstrated a reduction in NEC in neonates[99]. Animal models of DEX administration demonstrate increased mucosal maturation, with increased enterocyte and goblet cells, coupled with a thinning of the muscularis[100]. Unfortunately, between early postnatal administrations of DEX is associated with the development of focal small bowel perforation[100,101]. The mechanism associated with the differential effect of GC on intestinal mucosa and muscularis is not fully understood. Although in vitro work on intestinal smooth muscle cells suggests that DEX alters the expression of collagen and other basement membrane elements[102,103], in vivo remodelling of the extracellular matrix by DEX has not been shown.
The tissue specific effects of GC may be the result of a redistribution of growth factors. DEX administration in newborn mice alters IGF-Iimmunolocalization, with increased protein detected in the mucosa, and reduced levels in the muscularis[104]. In situ hybridization analyses for IGF-Itranscripts showed no differences in localization between the groups. In addition to its’ effects on IGF-Iimmunolocalization, DEX (i.p. 1 μg/g, once daily) altered IGF-Ibinding protein composition in the mucosa in newborn mice. This alteration may be responsible for drawing IGF-Ifrom the mesenchyme to the mucosa, and subsequently influencing intestinal maturation[105].
Further support for the involvement of the IGF-Isystem in NEC comes from the work of Burrin et al[106], who showed that in addition to decreases in circulating IGF-Iand alterations in IGF-Ibinding proteins, neonatal pigs treated with DEX (subcutaneous, 1 mg/kg, for 7 d) had increased IGF-Ireceptor mRNA abundance. This increase was only observed in the stomach and ileum, with no effect seen in the jejunum. This corresponds well with the authors’ observation that the effects of DEX are most pronounced in the ileum. The catabolic effects of DEX were also characterized in this study: small intestinal growth, particularly in the ileum, was inhibited due to increases in protein degradation, without significantly affecting protein synthesis.
GC have well documented effects on the ontogeny of BBM hydrolases. Adrenalectomy delays enzyme maturation during the third post-natal week, while exogenous GC administration may induce the precocious appearance of SI activity[107]. In adult animals, however, disaccharidase activities in the small intestine are unaffected by adrenalectomy or by GC administration[108]. The administration of DEX in the neonatal period increases the intestinal uptake of sugars (Drowdowski et al, unpublished observations, 2005).
There may be a syngeristic interaction between GC and T4 on BBM enzyme maturation. This was thought to involve the ability of T4 to increase corticosteroid-binding globulin, which subsequently reduces the clearance of hydrocortisone[109]. However, work by McDonald and Henning[110] demonstrated syngergism between DEX and T4. This indicates that an additional mechanism must be involved since DEX does not bind to CBG. In this study, animals received daily injections of subcutaneous T4 (130 pmol/g body weight) and a non-saturating dose of DEX (0.01 μg/g body weight) from post-natal day 5 to12. The hormones synergistically increased jejunal SI as well as ileal and duodenal alkaline phosphatase, and decreased ileal β-galactosidase activity, but did not affect jejunal or ileal LPH activity. When administered alone, T4 did not affect intestinal maturation, and DEX only partially stimulated maturation, when compared to the combination of hormones. The authors concluded that enzymes that rise post-natally responded to treatment with the combination of the T2 and DEX, while enzymes that decline post-natally show a mixed response.
In suckling rodents, GC regulate the expression of BBM enzymes such as SI-isomaltase and trehalase[107,111,112]. However, following GC administration, it takes 12-24 h to observe increases in the transcription of these genes. This delayed time-course suggests the involvement of secondary response genes[113]. Oesterreicher and Henning[114] identified a region of the trehalase promoter with potential binding sites for several transcription factors. Electromobility shift assays were performed using oligonucleotides from this region, as well as nuclear extracts from jejunum of 8 d old control or DEX-treated (1 g/g body wt) mice. They found that DEX stimulated expression of GATA-4 and GATA-6 proteins. These transcription factors are recognized as being important regulators of intestinal gene expression, and may interact with other transcription factors including those from the Sp family, Cdx, HNF-1 and HNF-4[115–120]. Indeed, GATA factors may allow cooperating transcription factors to bind to the DNA by altering the chromatin structure[119]. Although this study failed to prove that the induction of GATA factors leads to transcriptional activation of the BBM hydrolases, it does increase our understanding of possible mediators of GC effects.
Other molecular signals that may be responsible for the ontogenic changes in intestinal gene expression include a group of transcription factors called CCAAT/enhancer binding proteins (C/EBPs). In an attempt to determine regulatory mechanisms involved in the expression of the C/EBP α, β and σ isoforms, Boudreau et al[120] examined their expression in response to GC in the rat intestinal epithelial crypt-derived cell line IEC-6, using Northern blot, transcription run-on assays, indirect immunofluorescence, Western blot, and electrophoretic mobility shift assays. Whereas C/EBP α expression was not regulated by GC, C/EBP β and σ mRNA and protein levels were rapidly induced. Moreover, C/EBP β- and σ-containing DNA binding complexes were increased by GC as determined by supershift assays, in contrast to C/EBP α containing complexes. Immunofluorescence studies showed cytoplasmic and nuclear localization for C/EBP α. This is in contrast to a restricted nuclear localization for both C/EBP β and C/EBP σ. Differential regulation by GC as well as the different localization of three C/EBP isoforms suggest a role for this class of transcription factors in the control of gene expression in intestinal epithelial cells.
Mechanisms of action
The mechanisms by which GC exert their effects may involve several other factors. Boudreau et al[121] showed that DEX increases c-fos and c-jun, and increased AP-1 DNA-binding capacity in IEC-6 cells. Ras transformation repressed the growth-inhibitory properties of DEX, and inhibited the induction of c-fos protein and mRNA. This suggests that Ras negatively modulates the response of intestinal epithelial cells to GC. Thiesen et al[122] have shown that early response genes may be involved in the effect of GC on the enhancement of intestinal absorption of nutrients that occurs after intestinal resection. This further illustrates the cross-talk that occurs between GC and intracellular signaling pathways.
GC may also exert their effects by influencing cellular proliferation, differentiation and apoptosis. Foligne et al[123] investigated the effects of 10-dbilateral adrenalectomy on morphometry, proliferation and apoptosis in the small intestine of 3 mo old Sprague-Dawley rats. Adrenalectomy led to partial atrophy and disorganization of the epithelium, with an increased number of goblet and Paneth cells. A reduction of crypt cell proliferation was paralleled by a marked increase in apoptosis in the villus.
Several other studies suggest that steroids may increase apoptosis. In vitro studies using rat jejunal epithelial cells (IEC-6) cells show that the locally acting budesonide increases apoptosis, while in vivo studies show that steroids increase apoptosis in intraepithelial lymphocytes[124,125].
There are conflicting reports on the effect of GC on intestinal proliferation. Low to medium doses (10-6-10-11) increase proliferation in IEC cells, while high doses (10-5) inhibit proliferation[126]. Other studies suggest that the effect of steroids on proliferation depends on the developmental stage of the animal[127], Similarly, the location of the cells along the crypt-villous axis is an important factor as DEX reduced proliferation in the upper but not the lower crypt of fetal mouse duodenal explants[128]. This points to an important role of the adrenal glands and GC in the trophic status of the adult small intestinal mucosa. These results also highlight that the atrophy associated with GC is associated with a reduction in proliferation together with an increase in apoptosis. It is possible that some of the negative effects of GC on the intestine could be reduced or prevented by the administration of a trophic agent.
Early exposure to GC has been associated with lasting effects on cardiovascular, endocrine and metabolic systems. For example, prenatal glucocorticiod exposure results in reduced birth weight[129–134], increased blood pressure[135] increased expression of the glucocorticoid receptor in visceral fat[136], impaired coping in adverse situations[137], increases in corticosterone levels[135,137] hyperglycemia/hyperinsulinemia[131,138,139], increased susceptibility of the inner ear to acoustic noise trauma[140] and increases in PEPCK (rate-limiting enzyme in gluconeogenesis) mRNA and activity[141]. Steroids may also influence the development of the retina, although studies have shown both a positive effect on experimentally-induced retinopathy[142,143] and an increased risk of retinopathy of prematurity[144]. Furthermore, neurodevelopmental problems including an increased risk of cerebral palsy[145] have been documented following postnatal steroid administration.
It is not known if early exposure to GC results in lasting effects on intestinal function. However, because steroids are known to influence intestinal transport in adult animals[146], and because steroids influence the ontogeny of the intestine[110,147], it seems plausible that they may also play a role in the programming of intestinal function.
Effects on adult intestine
Does the adult small intestine remain responsive to steroids? Foligne et al[123] attempted to answer this question: three month old Sprague Dawley rats underwent bilateral adrenalectomies, and were sacrificed 10 d after surgery. Adrenalectomy modified maturation and differentiation, particularly in the proximal small intestine. A partial atrophy and disorganization of villous architecture was noted, coupled with decreases in crypt cell proliferation and increases in apoptotic cells in the upper villous region. LPH and SI were increased by adrenalectomy, while aminopeptidase N and intestinal alkaline phosphatase activities were reduced. These results indicate that the adrenal glands and GC play an important role in the trophic status of the small intestine in adult rats.
GC are used to treat various disorders including rheumatoid arthritis, inflammatory bowel disease, systemic lupus erythematosus, allergies and asthma due to their anti-inflammatory effects[148]. Both NFκB and AP-1 are crucial for the induction of many genes involved in inflammation. GC may interfere with the transcriptional activity of these and other factors. Alternatively, GC may also interact with a negative GRE (nGRE) and thereby inhibit transcription. Although the anti-inflammatory effects of GC may be due to negative modulation of pro-inflammatory factors (transrepression), the side effects may be the result of transactivation[149]. This has created interest in developing “dissociating steroids”, in which the desirable transrepression effect is separated from undesirable transactivation effect.
Glucocorticoid resistance may occur as GC reduce GR expression[150]. The importance of GC is clear from the clinical symptoms associated with a deficiency or excess of the hormones. Cortisol deficiency (Addison’s disease) is characterized by postural hypotension, weight loss and hypoglycaemia GC excess (Cushing’s syndrome) is characterized by hypertension, central obesity and glucose intolerance.
Quaroni et al[151] found that exposing IEC cells to GC cause growth arrest, the formation of tight junctions, the appearance of tall slender microvilli, reorganization of the ER and Golgi, and decreased cdk6 as well as p27kip1protein. These results are consistent with the activation of multiple genes important in the functioning of absorptive villous cells, but likely not involved in the induction of cell differentiation.
The role of the mesenchyme in mediating the effects of GR was studied by Simo et al[152] GC treatment of mesenchyme-derived cell populations resulted in an accumulation of laminin at the cell surface, accompanied by enhanced expression of BBM enzymes. This effect was abolished by anti-laminin, suggesting that GC may lead to accelerate laminin organization at the epithelial-mesenchymal interface, leading to epithelial cell differentiation.
Glucocorticosteroids and sugar transport
GC alter sugar transport in a number of other tissues. GLUT4 expression in adipose tissue is diminished in response to DEX, while GLUT4 in muscle is increased[153]. In adipocytes, DEX increased GLUT4 levels at the plasma membrane in the basal state, while GLUT4 translocation in response to insulin was inhibited.
Oral glucocorticosteroids increase intestinal sugar transport. For example, using an in vivo recirculation-perfusion technique in rats, Batt and Peters[154] showed that 7 d of prednisolone increased intestinal galactose absorption per enterocyte, without influencing the intestinal mucosa or cell kinetics. Similarly, short-term pharmacological doses of prednisolone increased digestive/absorptive function, while paradoxically decreasing the epithelial cell population in rats[155]. Long-term (28 d) administration of prednisolone or betamethasone in rats increased the activity of BBM proteins and galactose absorption, but induced atrophy of the mucosa and inhibition of cell turnover[156].
When the proximal jejunum of humans was perfused with glucose (28 mmol), intraluminal (100 mg/L) hydrocortisone increased sodium, water and glucose absorption[157] when compared to controls. Thiesen et al[146] assessed the influence of the glucocorticosteroids budesonide and prednisone on the in vitro uptake of sugars in weaning male rats. The steroids had no effect on the uptake of D-glucose by SGLT1. In contrast, the uptake of D-fructose by GLUT-5 was increased with both budesonide and prednisone. The increases in the uptake of fructose were not due to variations in the weight of the intestinal mucosa, food intake, or in GLUT-5 protein abundance or mRNA expression. This enhanced uptake of fructose was likely regulated by posttranslational processes, such as enhancement of the intrinsic activity of the transporters. There were no steroid-associated changes in mRNA expression of c-myc, c-jun, c-fos, proglucagon, or selected cytokines. However, the abundance of ileal ornithine decarboxylase mRNA was increased with Prednisone.
Thiesen et al[122] further characterized the effect of steroids using a model of intestinal resection. Adult male Sprague Dawley rats underwent transection or resection of 50% of the middle portion of the small intestine. Prednisone had no effect on the in vitro uptake of glucose or fructose in resected animals. In contrast, in resected rats budesonide increased by over 120% the value of the jejunal maximal transport rate (Vmax) for the uptake of glucose, and increased by over 150% ileal uptake of fructose. Changes in SGLT1, GLUT5, GLUT2, and Na+K+-ATPase protein abundance and mRNA expression did not explain the enhancing effect of budesonide. The steroids reduced c-jun, ODC and proglucagon expression. These data suggest that the influence of GC on sugar uptake in resected animals may be achieved by post-translational processes involving signalling with c-jun, ODC, and proglucagon, or perhaps also other as yet unknown signals.
GLUCAGON-LIKE PEPTIDE-2 (GLP2)
Biochemistry
Proglucagon is a 160 amino acid peptide encoded by the glucagon gene, and is present in intestinal L cells and α cells of the islets of Langerhans[213,214]. Proglucagon undergoes post-translational processing in the pancreas liberating glucagon as the main product. In the intestine, several peptide products (collectively referred to as “enteroglucagon”) are produced including glucagon-like peptide-1 (GLP1) and glucagon-like peptide-2 (GLP2) (Figure 2).
Figure 2.

Post-translation processing of proglucagon in the pancreas and the intestinal L-cells. The numbers represent the amino acid at which enzymatic cleavage occurs.
Both GLP1 and GLP2 are secreted from the L cells of the distal small intestine and colon in response to enteral nutrients[215]. Both fatty acids[216] and glucose[217] stimulate secretion from L cells, but protein meals do not increase GLP1 or GLP2 secretion[218,219]. However, amino acid mixtures have been shown to stimulate GLP1 release in humans[220], and meat hydrolysates do stimulate GLP1 secretion from rat intestinal L cells in vitro[221].
The secretion of these peptides is biphasic, with an early peak within 30 min of a meal, followed by a later peak at 60-120 min[219]. It is thought that neuroendocrine pathways may be responsible for the early secretion, as luminal nutrients are not likely to reach the distal L cells within 30 min of ingestion[216,222].
The peptides are degraded in the plasma by dipeptidyl peptidase IV (DPPIV)[223], with a half-life for GLP2 of 7 min[224]. DPPIV-resistant analogs (ALX-600, Teduglutide) with greater potency have been developed for clinical use. These will be discussed in more detail in later sections.
The receptor is a G-protein coupled receptor with 7 transmembrane domains, and is encoded by a single gene localized to chromosome 17p13.3. GLP2R expression is highest in the proximal small intestine, and decreases distally along the longitudinal axis[225]. GLP2R has been localized to intestinal enteroendocrine cells in humans using immunohistochemistry[226], to enteric neurons in mice using reverse transcription polymerase chain reaction (RT-PCR) and immunohistochemistry[227], and to rat enterocytes using 125I-GLP2[228]. Orskov et al[229] found GLP2 receptors mainly on subepithelial myofibroblasts in rat, mouse, and human small and large intestine by immunohistochemistry and in situ hybridization. By double labelling they found that these GLP2 receptor immunoreactive cells also produce smooth muscle actin and keratinocyte growth factor (KGF). KGF antibody abolished the growth promoting effect of GLP2 in the large intestine, but not in the small intestine. This suggests that GLP2 in the gut may act by activating receptors on the subepithelial myofibroblasts, thereby causing the release of growth factors, which in turn stimulate intestinal growth. Therefore, at this time it remains unclear whether the intestinotropic effects of GLP2 are due to direct effects on enterocytes, or are mediated by secondary factors.
Physiological effects
While GLP1 has potent insulinotropic effects[230], GLP2 has been found to be an important intestinotrophic factor. The relationship between enteroglucagon and small bowel growth was first documented by Gleeson et al[231]: a patient with an enteroglucagon-producing tumor exhibited small bowel hyperplasia, and injection of the tumor extract into mice resulted in intestinal growth[232]. Drucker et al[233] demonstrated that GLP2 was the specific agent responsible for this effect. GLP2 appeared to exert a “tissue specific” effect on the gut, as no changes were found in other tissues including spleen, heart, brain and liver.
GLP2 given subcutaneously adult to mice (6-43 g, twice daily, for 10 d) increases small bowel weight and crypt-villous height[234,235]. Intestinal proliferation was increased, and apoptosis was reduced. The effect of GLP2 was not due to changes in food consumption. The effects of GLP2 were sustained, as increases in growth were still evident after three months of administration. The rapid turnover of the epithelium was thought to contribute to the lack of densensitization that was observed with prolonged GLP2 treatment.
In addition to its morphological effects, GLP2 (when administered intravenously or subcutaneously) also increases the activity and expression of BBM enzymes including SI, LPH, maltase-glucoamylase and aminopeptidase N[236–238]. Although the effects on gastric physiology in humans have been conflicting[239,240], inhibition of gastric emptying in pigs has been observed with GLP2 treatment[241]. Reductions in meal-stimulated gastric acid secretion have also been observed with GLP2 administration in humans[242]. GLP2 enhances barrier function in murine intestinal epithelium[243], making it a potential therapeutic for disorders such as inflammatory bowel disease and necrotizing pancreatitis, both of which are characterized by increases in intestinal permeability[243,244].
While the mechanism by which GLP2 stimulates the adaptive response is unknown, its effects on an immediate early gene, PC4/TIS7, have been observed. In postconfluent, quiescent IEC 18 cells in culture, a stable derivative of GLP2, r(gly2)GLP2, increased PC4/TIS7 expression. r(gly2)GLP2 administered intraperitoneally to mice similarly induced increased PC4/TIS7 mRNA compared with vehicle control[245].
A number of studies aimed at establishing the effect of GLP2 on intestinal transport. Cheeseman and Tsang[246] showed that a two hour vascular perfusion of GLP2 (4 h, 400 and 800 pmol/L) in rats increased D-glucose Vmax by about 65% in the baselateral membrane, and by three-fold in the BBM, with a concomitant increase in SGLT1 protein[247]. An anti-GLP antibody abolished the GLP2-induced increase in transport[246].
Not all studies have demonstrated an effect of GLP2 on nutrient transport. For example, Brubaker et al[237] treated mice subcutaneously with GLP2 (2.5 μg) for a 10 d period, and failed to reveal increases in the absorption of glucose or maltose. Curiously, an increased capacity for nutrient digestion was found to be due to increases in BBM enzyme activities, resulting in an apparent uncoupling of digestive capacity from subsequent sugar absorption. It is unclear if there is this discrepancy in the results of these studies on the effect of GLP2 or nutrient uptake.
The rapid trafficking the glucose and fructose transportation contributes to the sugar uptake GLUT2 into the BBM following a glucose infusion of meal[248–250]. GLUT2 protein levels in the BBM increased two-fold when luminal perfusions were increased from 0 mmol/L to 100 mmol/L glucose. One hour vascular infusion of GLP2 doubled the rate of fructose uptake into BBM following luminal fructose perfusion[251]. Fructose absorption in this study was determined by the appearance of fructose in the vascular bed. Western blotting of biotinylated surface-exposed protein showed a doubling of GLUT2 expression in the BBM following GLP2 infusion. Thus, GLP2 may promote the insertion of GLUT2 into the BBM, thereby providing a low affinity/ high capacity route in addition to SGLT1 and GLUTS by which fructose or glucose may be absorbed into the enterocyte.
Ramsanahie et al[252] examined the effect of chronically administered GLP2 on diurnal SGLT1 expression. Rats were treated with [Gly2]GLP2 (twice daily; 1 mg/g body weight) or vehicle (control) for 10 d. GLP2 administration did not alter the diurnal increase in mRNA levels of SGLT1, GLUT2, or GLUT5. However, SGLT1 protein was increased three-fold by GLP2, and in situ hybridization showed that SGLT1 mRNA was distributed along the entire length of the villi. This is in contrast to what was seen in control animals, where SGLT1 was restricted to the mid and upper villus, with less SGLT1 mRNA localized to the villous tip. Also, in contrast to the diffuse staining seen in control animals, immunofluorescence microscopy showed that SGLT1 protein in GLP2 treated animals was preferentially localized to the BBM, with little or no staining in the cytoplasm. This may represent another mechanism by which GLP2 increases intestinal glucose uptake.
The effect of GLP2 on in vivo nutrient absorption may also be attributed to nitric oxide-dependent increases in intestinal blood flow. Infusing GLP2 (500 pmol/kg per hour) for 4 h in TPN-fed piglets led to increased portal-drained visceral (PDV) blood flow rate, intestinal blood volume, and PDV glucose uptake. GLP2 also increased intestinal constitutive nitric oxide synthase (NOS) activity and endothelial NOS protein abundance[253]. Thus, in TPN-fed neonatal pigs, GLP2 acutely stimulates intestinal blood flow and glucose utilization, and that this response is nitric oxide-dependent.
Walsh et al[254] studied GLP2 signalling in isolated rat intestinal mucosal cells expressed mRNA transcripts for the GLP2R, as well as for chromogranin A and β-tubulin III, markers for enteroendocrine and neural cells, respectively. cAMP production in response to [Gly2]GLP2, a degradation-resistant analog of GLP2, was maximal at 10-11 mol/L with reduced cAMP accumulation observed at higher doses. The cAMP response was abolished by pretreatment with 10-6 mol/L GLP2, indicating receptor desensitization. GLP2 treatment of isolated mucosal cells increased 3H-thymidine incorporation, and this was prevented by inhibition of the protein kinase A pathway. In contrast, GLP2 did not affect p44/p42 MAPK phosphorylation or the levels of cytosolic calcium in the mucosal cell preparation. These results provide evidence that activation of the endogenous rat mucosal GLP2 receptor is linked to activation of a cAMP/protein kinase A-dependent, growth-promoting pathway in vitro.
Estall et al[255] examined the mechanisms regulating signaling, internalization, and trafficking of the GLP2R to identify determinants of receptor activation and desensitization. Heterologous cells expressing the transfected rat or human GLP2R exhibited a rapid, dose-dependent, and prolonged desensitization of the GLP2-stimulated cAMP response and a sustained GLP2-induced decrease in levels of cell surface receptor. Surprisingly, inhibitors of clathrin-dependent endocytosis failed to significantly decrease GLP2R internalization, whereas cholesterol sequestration inhibited ligand-induced receptor internalization and potentiated homologous desensitization. The hGLP2R localized to both Triton X-100-soluble and -insoluble (lipid raft) cellular fractions and colocalized transiently with the lipid raft marker caveolin-1. Although GLP2R endocytosis was dependent on lipid raft integrity, the receptor transiently associated with green fluorescent protein tagged-early endosome antigen 1-positive vesicles and inhibitors of endosomal acidification attenuated the reappearance of the GLP2R on the cell surface. This data demonstrates that GLP2R desensitization and raft-dependent trafficking represent distinct and independent cellular mechanisms and provide new evidence implicating the importance of a clathrin- and dynamin-independent, lipid raft-dependent pathway for homologous G protein-coupled receptor internalization.
Intestinal resection
Plasma GLP2 levels rise following intestinal resection in rats[256–258]. When rats were subjected to a 70% midjejunoileal resection or ileal transection, and were maintained with TPN or oral feeding. Resection-induced adaptive growth in TPN- and orally-fed rats was associated with a significant positive correlation between increases in plasma bioactive GLP2 and proglucagon mRNA abundance in the colon of TPN-fed rats and in the ileum of orally fed rats[259]. While these increases were transient in the TPN-fed group, luminal nutrients produced a sustained increase detected at 3 and 7 d post-resection. These data support a significant role for endogenous GLP2 in the adaptive response to mid-small bowel resection in both TPN and orally fed rats.
GLP2 administration in rats increases the adaptive response to massive intestinal resection[260]. Sprague-Dawley rats were divided into two groups, with a 75% mid-jejunum-ileum resection and a sham operated group. Animals were given 0.1 μg/g GLP2 analog (protease resistant human GLP2) or placebo given subcutaneously twice daily for 21 d. Administration of the GLP2 analog was associated with an increase of the mucosal mass in the proximal jejunum and terminal ileum.
Martin et al[261] investigated the effects of GLP2 in a total parenteral nutrition (TPN)-supported model of experimental short bowel syndrome. Juvenile Sprague-Dawley rats underwent a 90% small intestinal resection, and were randomized to three groups: enteral diet and intravenous saline infusion, TPN only, or TPN + 10 μg/kg per hour GLP2. TPN plus GLP2 treatment resulted in increased bowel and body weight, villos height, intestinal mucosal surface area, crypt cell proliferation, and reduced intestinal permeability, as compared with the TPN alone animals. GLP2 increased serum GLP2 levels and intestinal SGLT-1 protein abundance as compared with either TPN or enteral groups. This demonstrates that GLP2 is capable of stimulating intestinal adaptation in the absence of enteral feeding. Because a number of hormones and growth factors have been shown to influence intestinal function, Washizawa et al[262] compared the effects of GLP2, growth hormone (GH) and keratinocye growth factor (KGF) on markers of gut adaptation following massive small bowel resection (MSBR). KGF increased goblet cell numbers and TTF3, a cytoprotective trefoil peptide, in both the small bowel and the colon. While both GH and KGF increased colonic mucosal growth, GLP2 exerted superior trophic effects on jejunal growth. GLP2 also increased the glutathione/glutathione disulfide ratio, resulting in improved mucosal glutathione redox status throughout the bowel. Because of the differential effects of GLP2, GH and KGF on gut adaptation following MSBR, the authors conclude that a combination of these agents may be most beneficial.
Human studies with GLP2 have also been performed: a non-placebo controlled study was conducted in 8 patients with short bowel syndrome (SBS) with an end-enterostomy type of anastomasis (6 had Crohn’s disease and 4 were not receiving HPN)[263]. Treatment with GLP2 (400 μg subcutaneously twice a day for 35 d) increased intestinal absorption of energy, body weight, and lean body mass. Crypt depth and villous height were also increased in 5 and 6 patients, respectively.
The results of more recent studies of GLP2 in SBS have been reviewed[264] and concluded that “Currently, hormonal therapy in short-bowel patients should be considered experimental and it is only recommended in research studies. The optimal duration and concentration requirements for GLP2 to induce beneficial effects on intestinal secretion, motility, morphology and most importantly absorption, are not known. Optimal dosage and administration of this new treatment to short-bowel patients may eventually result in long-term improvements in nutritional status and independence of parenteral nutrition in a larger fraction of short-bowel patients”.
Intestinal development
The topic of the Development of the Infant Intestine: Implications for Nutrition Support and a consideration of trophic factors essential to intestinal development, have recently been considered[265]. A role for GLP2 in the ontogeny of the intestine has been proposed. Lee et al[266] established that proglucagon mRNA was detectable in the rat fetus, and that immunoreactivity increased in the early neonatal period. Prohormone convertases, which are required for the liberation of GLP2 from proglucagon, are also detected in the fetal rat. Lovshin et al[267] detected GLP2R mRNA during fetal and neonatal development in the rat, with levels being higher in the fetal and neonatal gut as compared to adult rats. High levels of GLP2 (1-33) were also detected in the circulation of 13 d old neonatal rats, and GLP2 immunoreactivity was found in the fetal rat intestine. In order to prove that fetal cells were capable of secreting GLP2, fetal rat intestinal cell cultures were studied, and were found to secrete correctly processed GLP2 (1-33). The administration of a degradation resistant GLP2 analog [h (Gly2)-GLP2] to 1 d old rat pups for a period of 10 d resulted in increases in both small bowel weight and length. Thus, the GLP2/GLP2R axis is functional in early life, and that the developing intestine is capable of synthesizing, secreting and responding to GLP2.
A subsequent study done by Petersen et al[236] on premature (92% gestation) TPN fed piglets demonstrated that in addition to increases in maltase mRNA and activity, increases in SI and aminopeptidase N activities were observed. Thus, it appears that some of the effects that GLP2 exerts on intestinal function may be related to gestational age at birth, as the premature intestine was more responsive to exogenous GLP2 than the term neonatal intestine. The authors also noted that GLP2 infused into pig fetuses in vivo passed into the maternal circulation[268]. This suggests that GLP2 may pass through the placenta, and conversely, may expose the fetus to maternal GLP2. This raises the possibility that GLP2 given to pregnant animals may alter the form and function of the offspring.
Signalling events
Even before the GLP2 receptor was cloned, the role of the PI3K pathway in mediating the effects of GLP2 on intestinal sugar transport was studied by Cheeseman et al[247]: in vivo infusions of GLP2 produced an acceleration of sodium-dependent glucose uptake into BBM vesicles, with similar increase in SGLT-1 abundance. The effect of GLP2 could be inhibited by luminal brefeldin A, which blocks protein trafficking from the Golgi to the plasma membrane, or by the PI3K inhibitor, wortmannin. These results indicate that GLP2 is able to induce trafficking of SGLT-1 from an intracellular pool into the BBM and that PI3K may be involved in the intracellular signaling pathway in this response.
Because the GLP2R is not expressed on any intestinal cell lines, in vitro studies on GLP2 receptor signalling have been carried out in transfected heterologous cell types. Work done on transfected baby hamster kidney fibroblasts showed that GLP2 stimulated AP-1 dependent pathways increased cAMP, but did not change intracellular calcium levels[269]. There is decreased apoptosis and reduced caspase-3 activation following GLP2 treatment in vitro[270]. PKA, PI3K and the ERK pathways were not found to be essential for GLP2 inhibition of apoptosis, which was associated with reductions in cytochrome c release and cleavage of poly ADP-ribose polymerase (PARP).
GLP2-treated cells (10 μm, 3 d) demonstrated a greater than 10-fold increase in proliferation in Caco2 cells[271]. This response was inhibited by PI3K and mitogen activated/extracellular signal-regulated kinase (MEK) inhibitors. A significantly greater abundance of the phosphorylated forms of both ERK-1 and ERK-2 was present in cells following GLP2. This suggests that the increase in Caco-2 proliferation in response to GLP2 may be due, at least in part, to the involvement of both the PI 3-kinase and the MAPK pathways.
The limitations of this work, however, centers around the fact that both the authors of this study as well as Yusta et al[270] were unable to show, using Western blotting or RT-PCR, that Caco2 cells express endogenous GLP2R. This suggests that the proliferative effect of GLP2 on Caco2 cells may be mediated by other receptors, such as the EGFR, which mediates GLP1 induced proliferation in pancreatic β cells[272]. Furthermore, the observation that GLP2 is able to induce proliferation in a tumor cell lines, raises a concern regarding the role of GLP2 in promoting tumor growth. Indeed, recent work by Thulesen et al[273] showed that GLP2 promotes the growth of mucosal neoplasms in female C57bl mice whose colonic tumours were experimentally induced by administering a methylating carcinogen.
Koehler et al[274] identified several expressed sequence tags from human cervical carcinoma cDNA libraries that correspond to GLP2R nucleotide sequences. GLP2R mRNA transcripts were detected by RT-PCR in HeLa cervical carcinoma cells and Ca Ski cervical carcinoma cells. GLP2 increased cAMP accumulation and activated ERK1/2 in HeLa cells transiently expressing the cloned human HeLa cell GLP2R cDNA. However, the GLP2R-induced activation of ERK1/2 was not mediated through Gαs, adenylyl cyclase, or transactivation of the epidermal growth factor receptor, but was pertussis toxin sensitive, inhibited by dominant negative Ras, and dependent on betagamma-subunits. GLP2 also induced a significant increase in bromodeoxyuridine incorporation that was blocked by dominant negative Ras. Furthermore, GLP2 inhibited HeLa cell apoptosis induced by LY294002 in a protein kinase A-dependent, but ERK-independent, manner. These findings demonstrate that the HeLa cell GLP2R differentially signals through both G(α)s/cAMP- and G(i)/G(o)-dependent pathways, illustrating for the first time that the GLP2R is capable of coupling to multiple heterotrimeric G proteins defining distinct GLP2R-dependent biological actions.
Finally, the effects of GLP2 may be due to transactivation of other cell surface receptors. For example, GLP1 increases PI3K activity and enhances β-cell proliferation via transactivation of the EGFR[272].
Yusta et al[275] demonstrated that GLP2, in a cycloheximide-insensitive manner, enhanced survival in baby hamster kidney cells stably transfected with the rat GLP2R, reduced mitochondrial cytochrome c efflux, and attenuated the caspase-dependent cleavage of Akt, poly (ADP-ribose) polymerase, and β-catenin following inhibition of phosphatidylinositol 3-kinase (PI3K) by LY294002. The prosurvival effects of GLP2 on LY294002-induced cell death were independent of Akt, p90 (Rsk), or p70 S6 kinase activation; were mimicked by forskolin; and were abrogated by inhibition of protein kinase A (PKA) activity. GLP2 inhibited activation of glycogen synthase kinase-3 (GSK-3) through phosphorylation at Ser (21) in GSK-3α and at Ser (9) in GSK-3β in a PI3K-independent, PKA-dependent manner. GLP2 reduced LY294002-induced mitochondrial association of endogenous Bad and Bax and stimulated phosphorylation of a transfected Bad fusion protein at Ser (155) in a PI3K-independent, but H89-sensitive manner, a modification known to suppress Bad pro-apoptotic activity. These results suggest that GLP2R signaling enhances cell survival independently of PI3K/Akt by inhibiting the activity of a subset of pro-apoptotic downstream targets of Akt in a PKA-dependent manner.
Rocha et al[276] assessed the proliferative actions of GLP2 on the human Caco-2 cell line. GLP2 stimulated proliferation was inhibited in a dose-dependent fashion by both pertussis and cholera toxin (specific G protein inhibitors). This suggests that a G-protein-linked signaling pathway is involved with GLP2 bioactivity in Caco-2 cells. GLP2 stimulated proliferation was also augmented by 2',5'-dideoxyadenosine, which increases adenylate cyclase. Proliferation rates were inversely proportional to changes in intracellular cAMP concentration. These findings suggest that a G-protein linked signaling pathway is involved with GLP2 bioactivity in the intestinal epithelial cell line Caco-2.
Clinical
Burrin et al[277] studied 38 TPN-fed neonatal piglets infused intravenously with either saline or GLP2 at three rates (2.5, 5.0, and 10.0 nmol/kg per day for 7 d). GLP2 infusion dose-dependently increased small intestinal weight, DNA and protein content, and villus height; however, stomach protein synthesis was decreased by GLP2. Intestinal crypt and villus apoptosis decreased and crypt cell number increased linearly with GLP2 infusion rates, whereas cell proliferation and protein synthesis were stimulated only at the high GLP2 dose. The intestinal activities of caspase-3 and -6 and active caspase-3 abundance decreased, yet procaspase-3 abundance increased markedly with increasing infusion rate and plasma concentration of GLP2. The GLP2-dose-dependent suppression of intestinal apoptosis and caspase-3 activity was associated with increased protein kinase B and glycogen-synthase kinase-3 phosphorylation, yet the expression phosphatidylinositol 3-kinase was unaffected by GLP2. Intestinal endothelial nitric oxide synthase mRNA and protein expression was increased, but only at the high GLP2 dose. These authors concluded that the stimulation of intestinal epithelial survival is concentration-dependent at physiological GLP2 concentrations. However, induction of cell proliferation and protein synthesis is a pharmacological response. Moreover, they showed that GLP2 stimulates intestinal cell survival and proliferation in association with induction of protein kinase B and glycogen-synthase kinase-3 phosphorylation and Bcl-2 expression.
Thus, GLP2 increases intestinal growth in premature, TPN-fed pigs by decreasing proteolysis and apoptosis, and enteral nutrition was not required for these effects to occur. The actions of GLP2 are transduced by the GLP2 receptor (GLP2R), which was characterized and cloned by Munroe et al[225]. GLP2R was detected in several rat tissues including the stomach, small bowel, colon, and in small quantities in other tissues such as brain and lung[225]. This raises the possibility that GLP2 may have effects beyond the small intestine. Indeed, Haderslev et al[278] found that GLP2 administration significantly increased spinal bone mineral density in short-bowel patients with no colon, due to beneficial effects of GLP2 on bone resorption[279]. Centrally administered GLP2 increases satiety in rodents[280,281], while peripherally administered GLP2 does not influence gastric emptying, food intake or satiety in humans[238,282].
Although the effects of early exposure to hormones such as GLP2 are unknown, the presence of circulating GLP2 and the detection of the GLP2R in fetal and neonatal rats[267] suggests a role for GLP2 in regulating intestinal development. Drozdowski et al (unpublished observations, 2006) has shown that GLP2 modifies intestinal morphology when given to suckling rats, and enhances sugar uptake into the intestine of rats whose mothers were given GLP2 during pregnancy and lactation. Therefore, it is possible that exposing young animals to GLP2, either directly or via their pregnant and lactating dams, may also have lasting effects on intestinal function.
GLP2 and glucocorticosteroids
The possibility of a growth factor or hormone acting additively or synergistically with a steroid hormone has been demonstrated in various tissues. For example, EGF potentiates the proliferative effects of progesterone and estrogen in the mammary gland[283]. In a review, Lange[83] discusses the cross-talk that occurs between steroid hormone receptors and intracellular signalling pathways. There is clearly evidence of non-genomic and extra-nuclear functions of steroid receptors, including the initiation of signal transduction pathways. Indeed, this type of cross-talk may explain how genes are co-ordinately regulated by mitogenic stimuli in hormone responsive tissues. For example, Migliaccio et al[284] reported MAPK activation by estradiol, and interactions between the progesterone receptor, the estrogen receptor and p60-Src kinase.
A synergistic effect between DEX and GLP2 may occur in the intestine, as GC have a permissive effect on several hormones including catecholamines, thyroid hormones, growth hormone and ACTH[285]. GC mediates a permissive action, mostly for hormones, which act on G-protein coupled receptors, and increases adenylate cyclase[286,287]. This occurs because DEX alters adenylyl cyclase, enhancing the effects of cAMP generating agonists. Therefore, an interaction between DEX and GLP2 is likely, as the GLP2 receptor is a G-protein coupled receptor.
Furthermore, cAMP and PKA, which are activated by GPCRs like GLP2R, may increase the steroid sensitivity of a target cell by increasing the DNA binding ability of the GR for its response elements. In a study by Rangarajan et al[288] using embryonal carcinoma cells lacking cAMP response element binding protein (CREB), activation of PKA increased hormone-dependent trans-activation of the GR. The effect of PKA was related to the DNA binding domain of the GR, as deletion of the amino-terminal or the ligand-binding domain did not alter PKA’s effect. However, the absence of a consensus PKA phosphorylation site within the GR DNA binding domain led the authors to suggest that the GR is not a direct substrate for phosphorylation by PKA. Instead, they propose a multi-step process involving other cellular kinases and phosphatases that may interact with the GR.
As discussed previously, there are several pieces of evidence that suggest that GLP2 may exert its effect via the PI3K pathway. Steroid receptors may interact with this pathway as Simoncini et al[82] showed that the estrogen receptor binds to p85 subunit of PI3K.
Although there are no reports of the effect of DEX on PI3K in the intestine, an association has been observed in other tissues. For example, Saad et al[289] showed that DEX induced a 69% increase in the level of PI 3-kinase in adipocytes as determined by immunoblotting. Conversely, Buren et al[290] demonstrated that DEX decreases PI3K, PKB, insulin-stimulated PKB phosphorylation and glucose transport in isolated rat adipocytes, without changes in GLUT4. These results suggest that glucocorticoids, independently of the surrounding glucose and insulin concentration, impair glucose transport capacity in fat cells. Finally, Krasil’nikov et al[291] showed that prolonged exposure to DEX increased PI3K in Rous sarcoma virus-transformed hamster fibroblasts. Certainly in other tissues, such as adipose and muscle, PI3K is involved in regulating insulin-stimulated glucose uptake[292]. In the intestine, EGF stimulated increases in intestinal glucose transport in rabbits is abolished by the PI3K inhibitor LY294002[293]. Similarly, IGF-Iassociated increases in jejunal glucose uptake and Na+K+-ATPase activity are abolished by wortmannin, another PI3K inhibitor[23]. So clearly this pathway plays an important role in transducing signals from hormones and growth factors to the proteins involved in sugar transport. Indeed, preliminary studies in the young rat show that GLP2 and DEX may stimulate the intestinal uptake of glucose, and this is essential with increased abundance of Akt and mTOR, part of the PI3K pathway.
Peer reviewer: Martin Anlauf, MD, Department of Pathology, University of Kiel, Michaelisstrasse 11, 24105 Kiel, Germany
S- Editor Cheng JX L- Editor Alpini GD E- Editor Ma WH
References
- 1.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 2.Yang H, Ney DM. Insulin-like growth factor-I (IGF-I) responses in rats maintained with intravenous or intragastric infusion of total parenteral nutrition solutions containing medium- or long-chain triglyceride emulsions. Am J Clin Nutr. 1994;59:1403–1408. doi: 10.1093/ajcn/59.6.1403. [DOI] [PubMed] [Google Scholar]
- 3.Donovan SM, Hintz RL, Rosenfeld RG. Insulin-like growth factors I and II and their binding proteins in human milk: effect of heat treatment on IGF and IGF binding protein stability. J Pediatr Gastroenterol Nutr. 1991;13:242–253. doi: 10.1097/00005176-199110000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Beck F, Samani NJ, Penschow JD, Thorley B, Tregear GW, Coghlan JP. Histochemical localization of IGF-I and -II mRNA in the developing rat embryo. Development. 1987;101:175–184. doi: 10.1242/dev.101.1.175. [DOI] [PubMed] [Google Scholar]
- 5.Dvorak B, Stephana AL, Holubec H, Williams CS, Philipps AF, Koldovsky O. Insulin-like growth factor-I (IGF-I) mRNA in the small intestine of suckling and adult rats. FEBS Lett. 1996;388:155–160. doi: 10.1016/0014-5793(96)00495-4. [DOI] [PubMed] [Google Scholar]
- 6.Orlowski CC, Brown AL, Ooi GT, Yang YW, Tseng LY, Rechler MM. Tissue, developmental, and metabolic regulation of messenger ribonucleic acid encoding a rat insulin-like growth factor-binding protein. Endocrinology. 1990;126:644–652. doi: 10.1210/endo-126-1-644. [DOI] [PubMed] [Google Scholar]
- 7.Schober DA, Simmen FA, Hadsell DL, Baumrucker CR. Perinatal expression of type I IGF receptors in porcine small intestine. Endocrinology. 1990;126:1125–1132. doi: 10.1210/endo-126-2-1125. [DOI] [PubMed] [Google Scholar]
- 8.Young GP, Taranto TM, Jonas HA, Cox AJ, Hogg A, Werther GA. Insulin-like growth factors and the developing and mature rat small intestine: receptors and biological actions. Digestion. 1990;46 Suppl 2:240–252. doi: 10.1159/000200392. [DOI] [PubMed] [Google Scholar]
- 9.Hormi K, Lehy T. Developmental expression of transforming growth factor-alpha and epidermal growth factor receptor proteins in the human pancreas and digestive tract. Cell Tissue Res. 1994;278:439–450. doi: 10.1007/BF00331362. [DOI] [PubMed] [Google Scholar]
- 10.Menard D, Pothier P. Radioautographic localization of epidermal growth factor receptors in human fetal gut. Gastroenterology. 1991;101:640–649. doi: 10.1016/0016-5085(91)90520-u. [DOI] [PubMed] [Google Scholar]
- 11.Han VK, Lund PK, Lee DC, D'Ercole AJ. Expression of somatomedin/insulin-like growth factor messenger ribonucleic acids in the human fetus: identification, characterization, and tissue distribution. J Clin Endocrinol Metab. 1988;66:422–429. doi: 10.1210/jcem-66-2-422. [DOI] [PubMed] [Google Scholar]
- 12.Conteas CN, McMorrow B, Luk GD. Modulation of epidermal growth factor-induced cell proliferation and receptor binding by insulin in cultured intestinal epithelial cells. Biochem Biophys Res Commun. 1989;161:414–419. doi: 10.1016/0006-291x(89)92614-4. [DOI] [PubMed] [Google Scholar]
- 13.Kurokowa M, Lynch K, Podolsky DK. Effects of growth factors on an intestinal epithelial cell line: transforming growth factor beta inhibits proliferation and stimulates differentiation. Biochem Biophys Res Commun. 1987;142:775–782. doi: 10.1016/0006-291x(87)91481-1. [DOI] [PubMed] [Google Scholar]
- 14.Duncan MD, Korman LY, Bass BL. Epidermal growth factor primes intestinal epithelial cells for proliferative effect of insulin-like growth factor I. Dig Dis Sci. 1994;39:2197–2201. doi: 10.1007/BF02090371. [DOI] [PubMed] [Google Scholar]
- 15.Ohneda K, Ulshen MH, Fuller CR, D'Ercole AJ, Lund PK. Enhanced growth of small bowel in transgenic mice expressing human insulin-like growth factor I. Gastroenterology. 1997;112:444–454. doi: 10.1053/gast.1997.v112.pm9024298. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Niu W, Nikiforov Y, Naito S, Chernausek S, Witte D, LeRoith D, Strauch A, Fagin JA. Targeted overexpression of IGF-I evokes distinct patterns of organ remodeling in smooth muscle cell tissue beds of transgenic mice. J Clin Invest. 1997;100:1425–1439. doi: 10.1172/JCI119663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 18.Alexander AN, Carey HV. Oral IGF-I enhances nutrient and electrolyte absorption in neonatal piglet intestine. Am J Physiol. 1999;277:G619–G625. doi: 10.1152/ajpgi.1999.277.3.G619. [DOI] [PubMed] [Google Scholar]
- 19.Burrin DG, Wester TJ, Davis TA, Amick S, Heath JP. Orally administered IGF-I increases intestinal mucosal growth in formula-fed neonatal pigs. Am J Physiol. 1996;270:R1085–R1091. doi: 10.1152/ajpregu.1996.270.5.R1085. [DOI] [PubMed] [Google Scholar]
- 20.Park YK, Monaco MH, Donovan SM. Enteral insulin-like growth factor-I augments intestinal disaccharidase activity in piglets receiving total parenteral nutrition. J Pediatr Gastroenterol Nutr. 1999;29:198–206. doi: 10.1097/00005176-199908000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Houle VM, Schroeder EA, Odle J, Donovan SM. Small intestinal disaccharidase activity and ileal villus height are increased in piglets consuming formula containing recombinant human insulin-like growth factor-I. Pediatr Res. 1997;42:78–86. doi: 10.1203/00006450-199707000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Lane RH, Dvorak B, MacLennan NK, Dvorakova K, Halpern MD, Pham TD, Philipps AF. IGF alters jejunal glucose transporter expression and serum glucose levels in immature rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1450–R1460. doi: 10.1152/ajpregu.00172.2002. [DOI] [PubMed] [Google Scholar]
- 23.Alexander AN, Carey HV. Involvement of PI 3-kinase in IGF-I stimulation of jejunal Na+-K+-ATPase activity and nutrient absorption. Am J Physiol Gastrointest Liver Physiol. 2001;280:G222–G228. doi: 10.1152/ajpgi.2001.280.2.G222. [DOI] [PubMed] [Google Scholar]
- 24.Xu RJ, Mellor DJ, Birtles MJ, Breier BH, Gluckman PD. Effects of oral IGF-I or IGF-II on digestive organ growth in newborn piglets. Biol Neonate. 1994;66:280–287. doi: 10.1159/000244118. [DOI] [PubMed] [Google Scholar]
- 25.Baumrucker CR, Hadsell DL, Blum JW. Effects of dietary insulin-like growth factor I on growth and insulin-like growth factor receptors in neonatal calf intestine. J Anim Sci. 1994;72:428–433. doi: 10.2527/1994.722428x. [DOI] [PubMed] [Google Scholar]
- 26.Okada M, Ohmura E, Kamiya Y, Murakami H, Onoda N, Iwashita M, Wakai K, Tsushima T, Shizume K. Transforming growth factor (TGF)-alpha in human milk. Life Sci. 1991;48:1151–1156. doi: 10.1016/0024-3205(91)90452-h. [DOI] [PubMed] [Google Scholar]
- 27.Saito S, Yoshida M, Ichijo M, Ishizaka S, Tsujii T. Transforming growth factor-beta (TGF-beta) in human milk. Clin Exp Immunol. 1993;94:220–224. doi: 10.1111/j.1365-2249.1993.tb06004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas DM, Nasim MM, Gullick WJ, Alison MR. Immunoreactivity of transforming growth factor alpha in the normal adult gastrointestinal tract. Gut. 1992;33:628–631. doi: 10.1136/gut.33.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnard JA, Beauchamp RD, Coffey RJ, Moses HL. Regulation of intestinal epithelial cell growth by transforming growth factor type beta. Proc Natl Acad Sci USA. 1989;86:1578–1582. doi: 10.1073/pnas.86.5.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnard JA, Warwick GJ, Gold LI. Localization of transforming growth factor beta isoforms in the normal murine small intestine and colon. Gastroenterology. 1993;105:67–73. doi: 10.1016/0016-5085(93)90011-z. [DOI] [PubMed] [Google Scholar]
- 31.Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- 32.Miettinen PJ, Perheentupa J, Otonkoski T, Lahteenmaki A, Panula P. EGF- and TGF-alpha-like peptides in human fetal gut. Pediatr Res. 1989;26:25–30. doi: 10.1203/00006450-198907000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Chailler P, Menard D. Ontogeny of EGF receptors in the human gut. Front Biosci. 1999;4:D87–D101. doi: 10.2741/chailler. [DOI] [PubMed] [Google Scholar]
- 34.Berseth CL. Enhancement of intestinal growth in neonatal rats by epidermal growth factor in milk. Am J Physiol. 1987;253:G662–G665. doi: 10.1152/ajpgi.1987.253.5.G662. [DOI] [PubMed] [Google Scholar]
- 35.Jaeger LA, Lamar CH, Cline TR, Cardona CJ. Effect of orally administered epidermal growth factor on the jejunal mucosa of weaned pigs. Am J Vet Res. 1990;51:471–474. [PubMed] [Google Scholar]
- 36.Zijlstra RT, Odle J, Hall WF, Petschow BW, Gelberg HB, Litov RE. Effect of orally administered epidermal growth factor on intestinal recovery of neonatal pigs infected with rotavirus. J Pediatr Gastroenterol Nutr. 1994;19:382–390. doi: 10.1097/00005176-199411000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, Derynck R. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 38.Opleta-Madsen K, Meddings JB, Gall DG. Epidermal growth factor and postnatal development of intestinal transport and membrane structure. Pediatr Res. 1991;30:342–350. doi: 10.1203/00006450-199110000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol. 2002;282:G156–G164. doi: 10.1152/ajpgi.00196.2001. [DOI] [PubMed] [Google Scholar]
- 40.Kling PJ, Sullivan TM, Roberts RA, Philipps AF, Koldovsky O. Human milk as a potential enteral source of erythropoietin. Pediatr Res. 1998;43:216–221. doi: 10.1203/00006450-199802000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Juul SE, Joyce AE, Zhao Y, Ledbetter DJ. Why is erythro-poietin present in human milk? Studies of erythropoietin receptors on enterocytes of human and rat neonates. Pediatr Res. 1999;46:263–268. doi: 10.1203/00006450-199909000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Kermorgant S, Walker F, Hormi K, Dessirier V, Lewin MJ, Lehy T. Developmental expression and functionality of hepatocyte growth factor and c-Met in human fetal digestive tissues. Gastroenterology. 1997;112:1635–1647. doi: 10.1016/s0016-5085(97)70046-5. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Harvey C, Rousset M, Swallow DM. Expression of human intestinal mRNA transcripts during development: analysis by a semiquantitative RNA polymerase chain reaction method. Pediatr Res. 1994;36:514–521. doi: 10.1203/00006450-199410000-00018. [DOI] [PubMed] [Google Scholar]
- 44.Chailler P, Basque JR, Corriveau L, Menard D. Functional characterization of the keratinocyte growth factor system in human fetal gastrointestinal tract. Pediatr Res. 2000;48:504–510. doi: 10.1203/00006450-200010000-00014. [DOI] [PubMed] [Google Scholar]
- 45.Liu T, Reisenauer AM, Castillo RO. Ontogeny of intestinal lactase: posttranslational regulation by thyroxine. Am J Physiol. 1992;263:G538–G543. doi: 10.1152/ajpgi.1992.263.4.G538. [DOI] [PubMed] [Google Scholar]
- 46.Monteiro IM, Jiang L, Ferraris RP. Dietary modulation of intestinal fructose transport and GLUT5 mRNA expression in hypothyroid rat pups. J Pediatr Gastroenterol Nutr. 1999;29:563–570. doi: 10.1097/00005176-199911000-00017. [DOI] [PubMed] [Google Scholar]
- 47.Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17:221–244. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- 48.Haynes RCJ, Murad F. Andrenocorticotropic Hormone; Adrenocortical Steroids and their Synthetic Analogs; Inhibitors of Adrenocortical Biosynthesis. In: AG Gilman, LS Goodman., editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. Macmillan Publishing: New York; 1985. pp. 1459–1489. [Google Scholar]
- 49.Haynes RCJ, Larner J. Adrenocorticotropic Hormone; Adrenocortical Steroids and their Synthetic Analogs; Inhibitors of Adrenocortical Biosynthesis. In: AG Gilman, LS Goodman., editors. The Pharmacological Basis of Therapeutics. Macmillan Publishing: New York; 1975. pp. 1472–1506. [Google Scholar]
- 50.Simpson ER, Mason JI. Molecular Aspects of the Biosynthesis of Adrenal Steroids. In: GN Gill., editor. Pharmacology of Adrenal Corticol Hormones. Pergamon Press: New York; 1979. pp. 1–33. [Google Scholar]
- 51.Bowman WC, Rand MJ. The Endocrine System and Drugs Affecting Endocrine Function. In: of Pharmacology Textbook., editor. Blackwell Scientific Publications: Oxford; 1980. pp. 29–43. [Google Scholar]
- 52.Myles AB, Daly JR. Adrenocortical Steroids and their Metabolism. In: and ACTH Treatment Corticosteroid., editor. Edward Arnold: London; 1974. pp. 1–9. [Google Scholar]
- 53.Girdwood RH, Petrie JC. Endocrine System and Metabolic Diseases. In: RH Girdwood, JC Petrie., editors. Textbook of Medical Treatment. Churchill Livingstone: New York; 1987. pp. 497–499. [Google Scholar]
- 54.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 55.Brattsand R. Overview of newer glucocorticosteroids preparations for inflammatory bowel disease. Can J Gastroenterology. 1990;4:407–414. [Google Scholar]
- 56.Barr WH, Chung M, Shukur M. Intestinal Drug Metabolism-Presystemic and Systemic Mechanisms and Implications. In: A Modern View Pharmacokinetics., editor. Plenum Press: New York; 1984. pp. 426–429. [Google Scholar]
- 57.Sutherland VC. Hormones. In: VC Sutherland., editor. A Synopsis of Pharmacology. Suanders: Philadelphia; 1970. pp. 381–391. [Google Scholar]
- 58.Hu LM, Bodwell J, Hu JM, Orti E, Munck A. Glucocorticoid receptors in ATP-depleted cells. Dephosphorylation, loss of hormone binding, HSP90 dissociation, and ATP-dependent cycling. J Biol Chem. 1994;269:6571–6577. [PubMed] [Google Scholar]
- 59.Orti E, Bodwell JE, Munck A. Phosphorylation of steroid hormone receptors. Endocr Rev. 1992;13:105–128. doi: 10.1210/edrv-13-1-105. [DOI] [PubMed] [Google Scholar]
- 60.Bodwell JE, Hu LM, Hu JM, Orti E, Munck A. Glucocorticoid receptors: ATP-dependent cycling and hormone-dependent hyperphosphorylation. J Steroid Biochem Mol Biol. 1993;47:31–38. doi: 10.1016/0960-0760(93)90054-z. [DOI] [PubMed] [Google Scholar]
- 61.Li S, Rosen JM. Glucocorticoid regulation of rat whey acidic protein gene expression involves hormone-induced alterations of chromatin structure in the distal promoter region. Mol Endocrinol. 1994;8:1328–1335. doi: 10.1210/mend.8.10.7854350. [DOI] [PubMed] [Google Scholar]
- 62.Jonat C, Rahmsdorf HJ, Park KK, Cato AC, Gebel S, Ponta H, Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorti-coid hormone. Cell. 1990;62:1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- 63.Yang-Yen HF, Chambard JC, Sun YL, Smeal T, Schmidt TJ, Drouin J, Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990;62:1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- 64.Ray A, Prefontaine KE. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci USA. 1994;91:752–756. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caldenhoven E, Liden J, Wissink S, Van de Stolpe A, Raaijmakers J, Koenderman L, Okret S, Gustafsson JA, Van der Saag PT. Negative cross-talk between RelA and the glucocorticoid receptor: a possible mechanism for the antiinflammatory action of glucocorticoids. Mol Endocrinol. 1995;9:401–412. doi: 10.1210/mend.9.4.7659084. [DOI] [PubMed] [Google Scholar]
- 66.Pratt WB. The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem. 1993;268:21455–21458. [PubMed] [Google Scholar]
- 67.Madan AP, DeFranco DB. Bidirectional transport of glucocorticoid receptors across the nuclear envelope. Proc Natl Acad Sci USA. 1993;90:3588–3592. doi: 10.1073/pnas.90.8.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 69.DeFranco DB, Csermely P. Steroid receptor and molecular chaperone encounters in the nucleus. Sci STKE. 2000;2000:PE1. doi: 10.1126/stke.2000.42.pe1. [DOI] [PubMed] [Google Scholar]
- 70.Cato AC, Mink S. BAG-1 family of cochaperones in the modulation of nuclear receptor action. J Steroid Biochem Mol Biol. 2001;78:379–388. doi: 10.1016/s0960-0760(01)00114-5. [DOI] [PubMed] [Google Scholar]
- 71.Davies TH, Ning YM, Sanchez ER. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem. 2002;277:4597–4600. doi: 10.1074/jbc.C100531200. [DOI] [PubMed] [Google Scholar]
- 72.Freeman BC, Yamamoto KR. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science. 2002;296:2232–2235. doi: 10.1126/science.1073051. [DOI] [PubMed] [Google Scholar]
- 73.Morimoto RI. Dynamic remodeling of transcription complexes by molecular chaperones. Cell. 2002;110:281–284. doi: 10.1016/s0092-8674(02)00860-7. [DOI] [PubMed] [Google Scholar]
- 74.Gerritsen ME, Williams AJ, Neish AS, Moore S, Shi Y, Collins T. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci USA. 1997;94:2927–2932. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheppard KA, Rose DW, Haque ZK, Kurokawa R, McInerney E, Westin S, Thanos D, Rosenfeld MG, Glass CK, Collins T. Transcriptional activation by NF-kappaB requires multiple coactivators. Mol Cell Biol. 1999;19:6367–6378. doi: 10.1128/mcb.19.9.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tansey WP. Transcriptional activation: risky business. Genes Dev. 2001;15:1045–1050. doi: 10.1101/gad.896501. [DOI] [PubMed] [Google Scholar]
- 77.Kino T, Chrousos GP. Tumor necrosis factor alpha receptor- and Fas-associated FLASH inhibit transcriptional activity of the glucocorticoid receptor by binding to and interfering with its interaction with p160 type nuclear receptor coactivators. J Biol Chem. 2003;278:3023–3029. doi: 10.1074/jbc.M209234200. [DOI] [PubMed] [Google Scholar]
- 78.Cheung WL, Briggs SD, Allis CD. Acetylation and chromosomal functions. Curr Opin Cell Biol. 2000;12:326–333. doi: 10.1016/s0955-0674(00)00096-x. [DOI] [PubMed] [Google Scholar]
- 79.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 80.Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NC, Schreiber SL, Mellor J, Kouzarides T. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419:407–411. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- 81.Rochette-Egly C. Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cell Signal. 2003;15:355–366. doi: 10.1016/s0898-6568(02)00115-8. [DOI] [PubMed] [Google Scholar]
- 82.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lange CA. Making sense of cross-talk between steroid hormone receptors and intracellular signaling pathways: who will have the last word? Mol Endocrinol. 2004;18:269–278. doi: 10.1210/me.2003-0331. [DOI] [PubMed] [Google Scholar]
- 84.D'Agostino J, Henning SJ. Hormonal control of postnatal development of corticosteroid-binding globulin. Am J Physiol. 1981;240:E402–E406. doi: 10.1152/ajpendo.1981.240.4.E402. [DOI] [PubMed] [Google Scholar]
- 85.Godfrey KM. The role of the placenta in fetal programming-a review. Placenta. 2002;23 Suppl A:S20–S27. doi: 10.1053/plac.2002.0773. [DOI] [PubMed] [Google Scholar]
- 86.Gartner H, Graul MC, Oesterreicher TJ, Finegold MJ, Henning SJ. Development of the fetal intestine in mice lacking the glucocorticoid receptor (GR) J Cell Physiol. 2003;194:80–87. doi: 10.1002/jcp.10189. [DOI] [PubMed] [Google Scholar]
- 87.Moog F, Birkenmeier EH, Glazier HS. Leucylnaphthylamidase in the small intestine of the mouse: normal development and influence of cortisone and antibiotics. Dev Biol. 1971;25:398–419. doi: 10.1016/0012-1606(71)90039-x. [DOI] [PubMed] [Google Scholar]
- 88.Galand G. Brush border membrane sucrase-isomaltase, maltase-glucoamylase and trehalase in mammals. Comparative development, effects of glucocorticoids, molecular mechanisms, and phylogenetic implications. Comp Biochem Physiol B. 1989;94:1–11. doi: 10.1016/0305-0491(89)90002-3. [DOI] [PubMed] [Google Scholar]
- 89.Solomon NS, Gartner H, Oesterreicher TJ, Henning SJ. Development of glucocorticoid-responsiveness in mouse intestine. Pediatr Res. 2001;49:782–788. doi: 10.1203/00006450-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 90.Schaeffer C, Diab-Assef M, Plateroti M, Laurent-Huck F, Reimund JM, Kedinger M, Foltzer-Jourdainne C. Cytokine gene expression during postnatal small intestinal development: regulation by glucocorticoids. Gut. 2000;47:192–198. doi: 10.1136/gut.47.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yeh KY, Yeh M, Holt PR. Thyroxine and cortisone cooperate to modulate postnatal intestinal enzyme differentiation in the rat. Am J Physiol. 1991;260:G371–G378. doi: 10.1152/ajpgi.1991.260.3.G371. [DOI] [PubMed] [Google Scholar]
- 92.Leeper LL, McDonald MC, Heath JP, Henning SJ. Sucrase-isomaltase ontogeny: synergism between glucocorticoids and thyroxine reflects increased mRNA and no change in cell migration. Biochem Biophys Res Commun. 1998;246:765–770. doi: 10.1006/bbrc.1998.8707. [DOI] [PubMed] [Google Scholar]
- 93.Nanthakumar NN, Henning SJ. Ontogeny of sucrase-isomaltase gene expression in rat intestine: responsiveness to glucocorticoids. Am J Physiol. 1993;264:G306–G311. doi: 10.1152/ajpgi.1993.264.2.G306. [DOI] [PubMed] [Google Scholar]
- 94.Buchmiller TL, Shaw KS, Lam ML, Stokes R, Diamond JS, Fonkalsrud EW. Effect of prenatal dexamethasone administration: fetal rabbit intestinal nutrient uptake and disaccharidase development. J Surg Res. 1994;57:274–279. doi: 10.1006/jsre.1994.1144. [DOI] [PubMed] [Google Scholar]
- 95.Guo W, Swaniker F, Fonkalsrud EW, Vo K, Karamanoukian R. Effect of intraamniotic dexamethasone administration on intestinal absorption in a rabbit gastroschisis model. J Pediatr Surg. 1995;30:983–986; discussion 986-987. doi: 10.1016/0022-3468(95)90326-7. [DOI] [PubMed] [Google Scholar]
- 96.Nanthakumar NN, Young C, Ko JS, Meng D, Chen J, Buie T, Walker WA. Glucocorticoid responsiveness in developing human intestine: possible role in prevention of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G85–G92. doi: 10.1152/ajpgi.00169.2004. [DOI] [PubMed] [Google Scholar]
- 97.Costalos C, Gounaris A, Sevastiadou S, Hatzistamatiou Z, Theodoraki M, Alexiou EN, Constandellou E. The effect of antenatal corticosteroids on gut peptides of preterm infants-a matched group comparison: corticosteroids and gut development. Early Hum Dev. 2003;74:83–88. doi: 10.1016/s0378-3782(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 98.Reece EA, Hobbins JC, Mahoney MJ, Petrie RH. Handbook of medicine of the fetus and mother. Philadelphia: Lippincott; 1995. p. 533. [Google Scholar]
- 99.Halac E, Halac J, Begue EF, Casanas JM, Indiveri DR, Petit JF, Figueroa MJ, Olmas JM, Rodriguez LA, Obregon RJ. Prenatal and postnatal corticosteroid therapy to prevent neonatal necrotizing enterocolitis: a controlled trial. J Pediatr. 1990;117:132–138. doi: 10.1016/s0022-3476(05)72461-6. [DOI] [PubMed] [Google Scholar]
- 100.Gordon P, Rutledge J, Sawin R, Thomas S, Woodrum D. Early postnatal dexamethasone increases the risk of focal small bowel perforation in extremely low birth weight infants. J Perinatol. 1999;19:573–577. doi: 10.1038/sj.jp.7200269. [DOI] [PubMed] [Google Scholar]
- 101.Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran S, Donovan EF, Oh W, Bauer CR, Saha S, et al. Adverse effects of early dexamethasone in extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 2001;344:95–101. doi: 10.1056/NEJM200101113440203. [DOI] [PubMed] [Google Scholar]
- 102.Walsh MJ, LeLeiko NS, Sterling KM Jr. Regulation of types I, III, and IV procollagen mRNA synthesis in glucocorticoid-mediated intestinal development. J Biol Chem. 1987;262:10814–10818. [PubMed] [Google Scholar]
- 103.Graham MF, Willey A, Adams J, Diegelmann RF. Cortico-steroids increase procollagen gene expression, synthesis, and secretion by human intestinal smooth muscle cells. Gastroenterology. 1995;109:1454–1461. doi: 10.1016/0016-5085(95)90630-4. [DOI] [PubMed] [Google Scholar]
- 104.Gordon PV, Price WA, Stiles AD. Dexamethasone administration to newborn mice alters mucosal and muscular morphology in the ileum and modulates IGF-I localization. Pediatr Res. 2001;49:93–100. doi: 10.1203/00006450-200101000-00020. [DOI] [PubMed] [Google Scholar]
- 105.Gordon PV, Moats-Staats BM, Stiles AD, Price WA. Dexamethasone changes the composition of insulin-like growth factor binding proteins in the newborn mouse ileum. J Pediatr Gastroenterol Nutr. 2002;35:532–538. doi: 10.1097/00005176-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 106.Burrin DG, Fiorotto ML, Hadsell DL. Transgenic hypersecretion of des(1-3) human insulin-like growth factor I in mouse milk has limited effects on the gastrointestinal tract in suckling pups. J Nutr. 1999;129:51–56. doi: 10.1093/jn/129.1.51. [DOI] [PubMed] [Google Scholar]
- 107.Henning SJ, Rubin DC, Shulman RJ. Ontogeny of the intestinal mucosa. In: LR Johnson., editor. Physiology of the gastrointestinal tract. 3rd ed. Raven Press: New York; 1994. pp. 571–599. [Google Scholar]
- 108.Deren JJ, Broitman SA, Zamcheck N. Effect of diet upon intestinal disaccharidases and disaccharide absorption. J Clin Invest. 1967;46:186–195. doi: 10.1172/JCI105521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith CL, Hammond GL. Hormonal regulation of corticosteroid-binding globulin biosynthesis in the male rat. Endocrinology. 1992;130:2245–2251. doi: 10.1210/endo.130.4.1547738. [DOI] [PubMed] [Google Scholar]
- 110.McDonald MC, Henning SJ. Synergistic effects of thyroxine and dexamethasone on enzyme ontogeny in rat small intestine. Pediatr Res. 1992;32:306–311. doi: 10.1203/00006450-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 111.Leeper LL, Henning SJ. Development and tissue distribution of sucrase-isomaltase mRNA in rats. Am J Physiol. 1990;258:G52–G58. doi: 10.1152/ajpgi.1990.258.1.G52. [DOI] [PubMed] [Google Scholar]
- 112.Oesterreicher TJ, Nanthakumar NN, Winston JH, Henning SJ. Rat trehalase: cDNA cloning and mRNA expression in adult rat tissues and during intestinal ontogeny. Am J Physiol. 1998;274:R1220–R1227. doi: 10.1152/ajpregu.1998.274.5.R1220. [DOI] [PubMed] [Google Scholar]
- 113.Dean DM, Sanders MM. Ten years after: reclassification of steroid-responsive genes. Mol Endocrinol. 1996;10:1489–1495. doi: 10.1210/mend.10.12.8961259. [DOI] [PubMed] [Google Scholar]
- 114.Oesterreicher TJ, Henning SJ. Rapid induction of GATA transcription factors in developing mouse intestine following glucocorticoid administration. Am J Physiol Gastrointest Liver Physiol. 2004;286:G947–G953. doi: 10.1152/ajpgi.00470.2003. [DOI] [PubMed] [Google Scholar]
- 115.Boudreau F, Rings EH, van Wering HM, Kim RK, Swain GP, Krasinski SD, Moffett J, Grand RJ, Suh ER, Traber PG. Hepatocyte nuclear factor-1 alpha, GATA-4, and caudal related homeodomain protein Cdx2 interact functionally to modulate intestinal gene transcription. Implication for the developmental regulation of the sucrase-isomaltase gene. J Biol Chem. 2002;277:31909–31917. doi: 10.1074/jbc.M204622200. [DOI] [PubMed] [Google Scholar]
- 116.Divine JK, McCaul SP, Simon TC. HNF-1alpha and endodermal transcription factors cooperatively activate Fabpl: MODY3 mutations abrogate cooperativity. Am J Physiol Gastrointest Liver Physiol. 2003;285:G62–G72. doi: 10.1152/ajpgi.00074.2003. [DOI] [PubMed] [Google Scholar]
- 117.Dusing MR, Florence EA, Wiginton DA. High-level activation by a duodenum-specific enhancer requires functional GATA binding sites. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1053–G1065. doi: 10.1152/ajpgi.00483.2002. [DOI] [PubMed] [Google Scholar]
- 118.Krasinski SD, Van Wering HM, Tannemaat MR, Grand RJ. Differential activation of intestinal gene promoters: functional interactions between GATA-5 and HNF-1 alpha. Am J Physiol Gastrointest Liver Physiol. 2001;281:G69–G84. doi: 10.1152/ajpgi.2001.281.1.G69. [DOI] [PubMed] [Google Scholar]
- 119.Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell. 2002;9:279–289. doi: 10.1016/s1097-2765(02)00459-8. [DOI] [PubMed] [Google Scholar]
- 120.Boudreau F, Blais S, Asselin C. Regulation of CCAAT/enhancer binding protein isoforms by serum and glucocorticoids in the rat intestinal epithelial crypt cell line IEC-6. Exp Cell Res. 1996;222:1–9. doi: 10.1006/excr.1996.0001. [DOI] [PubMed] [Google Scholar]
- 121.Boudreau F, Zannoni S, Pelletier N, Bardati T, Yu SJ, Asselin C. Negative regulation of glucocorticoid-dependent induction of c-fos by ras in intestinal epithelial cells. Mol Cell Biochem. 1999;195:99–111. doi: 10.1023/a:1006987313013. [DOI] [PubMed] [Google Scholar]
- 122.Thiesen A, Wild GE, Tappenden KA, Drozdowski L, Keelan M, Thomson BK, McBurney MI, Clandinin MT, Thomson AB. The locally acting glucocorticosteroid budesonide enhances intestinal sugar uptake following intestinal resection in rats. Gut. 2003;52:252–259. doi: 10.1136/gut.52.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Foligne B, Aissaoui S, Senegas-Balas F, Cayuela C, Bernard P, Antoine JM, Balas D. Changes in cell proliferation and differentiation of adult rat small intestine epithelium after adrenalectomy: kinetic, biochemical, and morphological studies. Dig Dis Sci. 2001;46:1236–1246. doi: 10.1023/a:1010611228730. [DOI] [PubMed] [Google Scholar]
- 124.Murosaki S, Inagaki-Ohara K, Kusaka H, Ikeda H, Yoshikai Y. Apoptosis of intestinal intraepithelial lymphocytes induced by exogenous and endogenous glucocorticoids. Microbiol Immunol. 1997;41:139–148. doi: 10.1111/j.1348-0421.1997.tb01179.x. [DOI] [PubMed] [Google Scholar]
- 125.Ruiz-Santana S, Lopez A, Torres S, Rey A, Losada A, Latasa L, Manzano JL, Diaz-Chico BN. Prevention of dexamethasone-induced lymphocytic apoptosis in the intestine and in Peyer patches by enteral nutrition. JPEN J Parenter Enteral Nutr. 2001;25:338–345. doi: 10.1177/0148607101025006338. [DOI] [PubMed] [Google Scholar]
- 126.Goke MN, Schneider M, Beil W, Manns MP. Differential glucocorticoid effects on repair mechanisms and NF-kappaB activity in the intestinal epithelium. Regul Pept. 2002;105:203–214. doi: 10.1016/s0167-0115(02)00023-x. [DOI] [PubMed] [Google Scholar]
- 127.Sauter SN, Roffler B, Philipona C, Morel C, Rome V, Guilloteau P, Blum JW, Hammon HM. Intestinal development in neonatal calves: effects of glucocorticoids and dependence of colostrum feeding. Biol Neonate. 2004;85:94–104. doi: 10.1159/000074965. [DOI] [PubMed] [Google Scholar]
- 128.Beaulieu JF, Calvert R. Hormonal regulation of epithelial cell proliferation in the fetal mouse duodenum in vitro. Anat Rec. 1987;217:250–255. doi: 10.1002/ar.1092170305. [DOI] [PubMed] [Google Scholar]
- 129.Reinisch JM, Simon NG, Karow WG, Gandelman R. Prenatal exposure to prednisone in humans and animals retards intrauterine growth. Science. 1978;202:436–438. doi: 10.1126/science.705336. [DOI] [PubMed] [Google Scholar]
- 130.Ikegami M, Jobe AH, Newnham J, Polk DH, Willet KE, Sly P. Repetitive prenatal glucocorticoids improve lung function and decrease growth in preterm lambs. Am J Respir Crit Care Med. 1997;156:178–184. doi: 10.1164/ajrccm.156.1.9612036. [DOI] [PubMed] [Google Scholar]
- 131.Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest. 1998;101:2174–2181. doi: 10.1172/JCI1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol. 1999;180:114–121. doi: 10.1016/s0002-9378(99)70160-2. [DOI] [PubMed] [Google Scholar]
- 133.Newnham JP, Moss TJ. Antenatal glucocorticoids and growth: single versus multiple doses in animal and human studies. Semin Neonatol. 2001;6:285–292. doi: 10.1053/siny.2001.0064. [DOI] [PubMed] [Google Scholar]
- 134.Bloom SL, Sheffield JS, McIntire DD, Leveno KJ. Antenatal dexamethasone and decreased birth weight. Obstet Gynecol. 2001;97:485–490. doi: 10.1016/s0029-7844(00)01206-0. [DOI] [PubMed] [Google Scholar]
- 135.Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- 136.Cleasby ME, Kelly PA, Walker BR, Seckl JR. Programming of rat muscle and fat metabolism by in utero overexposure to glucocorticoids. Endocrinology. 2003;144:999–1007. doi: 10.1210/en.2002-220559. [DOI] [PubMed] [Google Scholar]
- 137.Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104:71–79. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- 138.Lindsay RS, Lindsay RM, Waddell BJ, Seckl JR. Prenatal glucocorticoid exposure leads to offspring hyperglycaemia in the rat: studies with the 11 beta-hydroxysteroid dehydro-genase inhibitor carbenoxolone. Diabetologia. 1996;39:1299–1305. doi: 10.1007/s001250050573. [DOI] [PubMed] [Google Scholar]
- 139.Sugden MC, Langdown ML, Munns MJ, Holness MJ. Maternal glucocorticoid treatment modulates placental leptin and leptin receptor expression and materno-fetal leptin physiology during late pregnancy, and elicits hypertension associated with hyperleptinaemia in the early-growth-retarded adult offspring. Eur J Endocrinol. 2001;145:529–539. doi: 10.1530/eje.0.1450529. [DOI] [PubMed] [Google Scholar]
- 140.Canlon B, Erichsen S, Nemlander E, Chen M, Hossain A, Celsi G, Ceccatelli S. Alterations in the intrauterine environment by glucocorticoids modifies the developmental programme of the auditory system. Eur J Neurosci. 2003;17:2035–2041. doi: 10.1046/j.1460-9568.2003.02641.x. [DOI] [PubMed] [Google Scholar]
- 141.Drake AJ, Walker BR, Seckl JR. Intergenerational conse-quences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R34–R38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- 142.Rotschild T, Nandgaonkar BN, Yu K, Higgins RD. Dexamethasone reduces oxygen induced retinopathy in a mouse model. Pediatr Res. 1999;46:94–100. doi: 10.1203/00006450-199907000-00016. [DOI] [PubMed] [Google Scholar]
- 143.Yossuck P, Yan Y, Tadesse M, Higgins RD. Dexamethasone alters TNF-alpha expression in retinopathy. Mol Genet Metab. 2001;72:164–167. doi: 10.1006/mgme.2000.3124. [DOI] [PubMed] [Google Scholar]
- 144.Haroon Parupia MF, Dhanireddy R. Association of postnatal dexamethasone use and fungal sepsis in the development of severe retinopathy of prematurity and progression to laser therapy in extremely low-birth-weight infants. J Perinatol. 2001;21:242–247. doi: 10.1038/sj.jp.7200531. [DOI] [PubMed] [Google Scholar]
- 145.Shinwell ES, Karplus M, Reich D, Weintraub Z, Blazer S, Bader D, Yurman S, Dolfin T, Kogan A, Dollberg S, et al. Early postnatal dexamethasone treatment and increased incidence of cerebral palsy. Arch Dis Child Fetal Neonatal Ed. 2000;83:F177–F181. doi: 10.1136/fn.83.3.F177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thiesen A, Wild GE, Keelan M, Clandinin MT, Thomson AB. Locally and systemically active glucocorticosteroids modify intestinal absorption of sugars in rats. J Appl Physiol. 2003;94:583–590. doi: 10.1152/japplphysiol.00134.2002. [DOI] [PubMed] [Google Scholar]
- 147.Marti A, Fernandez-Otero MP. Prostaglandin E2 accelerates enzymatic and morphological maturation of the small intestine in suckling rats. Biol Neonate. 1994;65:119–125. doi: 10.1159/000244037. [DOI] [PubMed] [Google Scholar]
- 148.Xu W, Chen H, Du K, Asahara H, Tini M, Emerson BM, Montminy M, Evans RM. A transcriptional switch mediated by cofactor methylation. Science. 2001;294:2507–2511. doi: 10.1126/science.1065961. [DOI] [PubMed] [Google Scholar]
- 149.Nishioka K, Reinberg D. Transcription. Switching partners in a regulatory tango. Science. 2001;294:2497–2498. doi: 10.1126/science.1067622. [DOI] [PubMed] [Google Scholar]
- 150.Okret S, Poellinger L, Dong Y, Gustafsson JA. Down-regulation of glucocorticoid receptor mRNA by glucoco-rticoid hormones and recognition by the receptor of a specific binding sequence within a receptor cDNA clone. Proc Natl Acad Sci USA. 1986;83:5899–5903. doi: 10.1073/pnas.83.16.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Quaroni A, Tian JQ, Goke M, Podolsky DK. Glucocorticoids have pleiotropic effects on small intestinal crypt cells. Am J Physiol. 1999;277:G1027–G1040. doi: 10.1152/ajpgi.1999.277.5.G1027. [DOI] [PubMed] [Google Scholar]
- 152.Simo P, Simon-Assmann P, Arnold C, Kedinger M. Mesenchyme-mediated effect of dexamethasone on laminin in cocultures of embryonic gut epithelial cells and mesenchyme-derived cells. J Cell Sci. 1992;101(Pt 1):161–171. doi: 10.1242/jcs.101.1.161. [DOI] [PubMed] [Google Scholar]
- 153.Coderre L, Vallega GA, Pilch PF, Chipkin SR. In vivo effects of dexamethasone and sucrose on glucose transport (GLUT-4) protein tissue distribution. Am J Physiol. 1996;271:E643–E648. doi: 10.1152/ajpendo.1996.271.4.E643. [DOI] [PubMed] [Google Scholar]
- 154.Batt RM, Peters TJ. Effects of prednisolone on the small intestinal mucosa of the rat. Clin Sci Mol Med. 1976;50:511–523. doi: 10.1042/cs0500511. [DOI] [PubMed] [Google Scholar]
- 155.Scott J, Batt RM, Maddison YE, Peters TJ. Differential effect of glucocorticoids on structure and function of adult rat jejunum. Am J Physiol. 1981;241:G306–G312. doi: 10.1152/ajpgi.1981.241.4.G306. [DOI] [PubMed] [Google Scholar]
- 156.Batt RM, Scott J. Response of the small intestinal mucosa to oral glucocorticoids. Scand J Gastroenterol Suppl. 1982;74:75–88. [PubMed] [Google Scholar]
- 157.Crake T, Crisp AJ, Shearing M, Record CO, Sandle GI. Effect of intraluminal hydrocortisone on solute and water absorption in the human jejunum. Clin Sci (Lond) 1984;67:105–110. doi: 10.1042/cs0670105. [DOI] [PubMed] [Google Scholar]
- 158.Bao B, Thomas MG, Griffith MK, Burghardt RC, Williams GL. Steroidogenic activity, insulin-like growth factor-I production, and proliferation of granulosa and theca cells obtained from dominant preovulatory and nonovulatory follicles during the bovine estrous cycle: effects of low-density and high-density lipoproteins. Biol Reprod. 1995;53:1271–1279. doi: 10.1095/biolreprod53.6.1271. [DOI] [PubMed] [Google Scholar]
- 159.Kawada M, Inoue H, Masuda T, Ikeda D. Insulin-like growth factor I secreted from prostate stromal cells mediates tumor-stromal cell interactions of prostate cancer. Cancer Res. 2006;66:4419–4425. doi: 10.1158/0008-5472.CAN-05-4239. [DOI] [PubMed] [Google Scholar]
- 160.Markel TA, Wang M, Crisostomo PR, Manukyan MC, Poynter JA, Meldrum DR. Neonatal stem cells exhibit specific characteristics in function, proliferation, and cellular signaling that distinguish them from their adult counterparts. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1491–R1497. doi: 10.1152/ajpregu.00031.2008. [DOI] [PubMed] [Google Scholar]
- 161.Hsu E, Feghali-Bostwick CA. Insulin-like growth factor-II is increased in systemic sclerosis-associated pulmonary fibrosis and contributes to the fibrotic process via Jun N-terminal kinase- and phosphatidylinositol-3 kinase-dependent pathways. Am J Pathol. 2008;172:1580–1590. doi: 10.2353/ajpath.2008.071021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee SK, Kang JS, Jung da J, Hur DY, Kim JE, Hahm E, Bae S, Kim HW, Kim D, Cho BJ, et al. Vitamin C suppresses proliferation of the human melanoma cell SK-MEL-2 through the inhibition of cyclooxygenase-2 (COX-2) expression and the modulation of insulin-like growth factor II (IGF-II) production. J Cell Physiol. 2008;216:180–188. doi: 10.1002/jcp.21391. [DOI] [PubMed] [Google Scholar]
- 163.Chung JO, Hong SI, Cho DH, Lee JH, Chung DJ, Chung MY. Hypoglycemia associated with the production of insulin-like growth factor II in a pancreatic islet cell tumor: a case report. Endocr J. 2008;55:607–612. doi: 10.1507/endocrj.k07e-153. [DOI] [PubMed] [Google Scholar]
- 164.Kim DG, Lee DY, Cho BH, You KR, Kim MY, Ahn DS. Down-regulation of insulin-like growth factor binding proteins and growth modulation in hepatoma cells by retinoic acid. Hepatology. 1999;29:1091–1098. doi: 10.1002/hep.510290414. [DOI] [PubMed] [Google Scholar]
- 165.Kuemmerle JF, Murthy KS. Coupling of the insulin-like growth factor-I receptor tyrosine kinase to Gi2 in human intestinal smooth muscle: Gbetagamma -dependent mitogen-activated protein kinase activation and growth. J Biol Chem. 2001;276:7187–7194. doi: 10.1074/jbc.M011145200. [DOI] [PubMed] [Google Scholar]
- 166.Rorive S, Berton A, D'haene N, Takacs CN, Debeir O, Decaestecker C, Salmon I. Matrix metalloproteinase-9 interplays with the IGFBP2-IGFII complex to promote cell growth and motility in astrocytomas. Glia. 2008;56:1679–1690. doi: 10.1002/glia.20719. [DOI] [PubMed] [Google Scholar]
- 167.Al Haj Ali M, Mensah-Brown E, Chandranath SI, Adeghate E, Adem A. Distribution of insulin like growth factor-1 (IGF-1) and its receptor in the intestines of the one-humped camel (Camelus dromedarius) Growth Factors. 2003;21:131–137. doi: 10.1080/08977190310001637233. [DOI] [PubMed] [Google Scholar]
- 168.Moreira A, Napimoga MH, Benatti BB, Silva GA, Rocha-Rodrigues DB, Clemente-Napimoga JT, Vieira GA, Alves JB. Morphological changes and EGF expression in the granular convoluted tubule cells of submandibular glands of Trypanosoma cruzi infected rats. Tissue Cell. 2008;40:293–298. doi: 10.1016/j.tice.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 169.Nakai K, Yoneda K, Igarashi J, Moriue T, Kosaka H, Kubota Y. Angiotensin II enhances EGF receptor expression levels via ROS formation in HaCaT cells. J Dermatol Sci. 2008;51:181–189. doi: 10.1016/j.jdermsci.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 170.Kawashima I, Okazaki T, Noma N, Nishibori M, Yamashita Y, Shimada M. Sequential exposure of porcine cumulus cells to FSH and/or LH is critical for appropriate expression of steroidogenic and ovulation-related genes that impact oocyte maturation in vivo and in vitro. Reproduction. 2008;136:9–21. doi: 10.1530/REP-08-0074. [DOI] [PubMed] [Google Scholar]
- 171.Majewski M, Jaworski T, Sarosiek I, Sostarich S, Roeser K, Edlavitch SA, Kralstein J, Wallner G, McCallum RW, Sarosiek J. Significant enhancement of esophageal pre-epithelial defense by tegaserod: implications for an esophagoprotective effect. Clin Gastroenterol Hepatol. 2007;5:430–438. doi: 10.1016/j.cgh.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 172.Varley CL, Southgate J. Effects of PPAR agonists on proliferation and differentiation in human urothelium. Exp Toxicol Pathol. 2008;60:435–441. doi: 10.1016/j.etp.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 173.Ingram JL, Bonner JC. EGF and PDGF receptor tyrosine kinases as therapeutic targets for chronic lung diseases. Curr Mol Med. 2006;6:409–421. doi: 10.2174/156652406777435426. [DOI] [PubMed] [Google Scholar]
- 174.Zhao D, Zhan Y, Zeng H, Koon HW, Moyer MP, Pothoulakis C. Neurotensin stimulates expression of early growth response gene-1 and EGF receptor through MAP kinase activation in human colonic epithelial cells. Int J Cancer. 2007;120:1652–1656. doi: 10.1002/ijc.22407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Miettinen P, Ormio P, Hakonen E, Banerjee M, Otonkoski T. EGF receptor in pancreatic beta-cell mass regulation. Biochem Soc Trans. 2008;36:280–285. doi: 10.1042/BST0360280. [DOI] [PubMed] [Google Scholar]
- 176.Ma YJ, Berg-von der Emde K, Moholt-Siebert M, Hill DF, Ojeda SR. Region-specific regulation of transforming growth factor alpha (TGF alpha) gene expression in astrocytes of the neuroendocrine brain. J Neurosci. 1994;14:5644–5651. doi: 10.1523/JNEUROSCI.14-09-05644.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mason HD, Carr L, Leake R, Franks S. Production of transforming growth factor-alpha by normal and polycystic ovaries. J Clin Endocrinol Metab. 1995;80:2053–2056. doi: 10.1210/jcem.80.7.7608254. [DOI] [PubMed] [Google Scholar]
- 178.Wong DT, Weller PF, Galli SJ, Elovic A, Rand TH, Gallagher GT, Chiang T, Chou MY, Matossian K, McBride J. Human eosinophils express transforming growth factor alpha. J Exp Med. 1990;172:673–681. doi: 10.1084/jem.172.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kuwai T, Nakamura T, Sasaki T, Kim SJ, Fan D, Villares GJ, Zigler M, Wang H, Bar-Eli M, Kerbel RS, et al. Phosphorylated epidermal growth factor receptor on tumor-associated endothelial cells is a primary target for therapy with tyrosine kinase inhibitors. Neoplasia. 2008;10:489–500. doi: 10.1593/neo.08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Smith JJ, Derynck R, Korc M. Production of transforming growth factor alpha in human pancreatic cancer cells: evidence for a superagonist autocrine cycle. Proc Natl Acad Sci USA. 1987;84:7567–7570. doi: 10.1073/pnas.84.21.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Angelucci A, Garofalo S, Speca S, Bovadilla A, Gravina GL, Muzi P, Vicentini C, Bologna M. Arachidonic acid modulates the crosstalk between prostate carcinoma and bone stromal cells. Endocr Relat Cancer. 2008;15:91–100. doi: 10.1677/ERC-07-0100. [DOI] [PubMed] [Google Scholar]
- 182.Gu J, Chen L, Shatos MA, Rios JD, Gulati A, Hodges RR, Dartt DA. Presence of EGF growth factor ligands and their effects on cultured rat conjunctival goblet cell proliferation. Exp Eye Res. 2008;86:322–334. doi: 10.1016/j.exer.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Coffey RJ, Washington MK, Corless CL, Heinrich MC. Menetrier disease and gastrointestinal stromal tumors: hyperproliferative disorders of the stomach. J Clin Invest. 2007;117:70–80. doi: 10.1172/JCI30491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Xian CJ, Cool JC, Howarth GS, Read LC. Effects of TGF-alpha gene knockout on epithelial cell kinetics and repair of methotrexate-induced damage in mouse small intestine. J Cell Physiol. 2002;191:105–115. doi: 10.1002/jcp.10079. [DOI] [PubMed] [Google Scholar]
- 185.Morisako T, Takahashi K, Kishi K, Kiguchi T, Mikami R, Kobayashi K, Yagyu H, Nakamura H, Matsuoka KT. Production of hepatocyte growth factor from human lung microvascular endothelial cells induced by interleukin-1beta. Exp Lung Res. 2001;27:675–688. doi: 10.1080/019021401317138478. [DOI] [PubMed] [Google Scholar]
- 186.Wislez M, Rabbe N, Marchal J, Milleron B, Crestani B, Mayaud C, Antoine M, Soler P, Cadranel J. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Res. 2003;63:1405–1412. [PubMed] [Google Scholar]
- 187.Zhu Y, Hojo Y, Ikeda U, Shimada K. Production of hepatocyte growth factor during acute myocardial infarction. Heart. 2000;83:450–455. doi: 10.1136/heart.83.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bell LN, Ward JL, Degawa-Yamauchi M, Bovenkerk JE, Jones R, Cacucci BM, Gupta CE, Sheridan C, Sheridan K, Shankar SS, et al. Adipose tissue production of hepatocyte growth factor contributes to elevated serum HGF in obesity. Am J Physiol Endocrinol Metab. 2006;291:E843–E848. doi: 10.1152/ajpendo.00174.2006. [DOI] [PubMed] [Google Scholar]
- 189.Gude NA, Emmanuel G, Wu W, Cottage CT, Fischer K, Quijada P, Muraski JA, Alvarez R, Rubio M, Schaefer E, et al. Activation of Notch-mediated protective signaling in the myocardium. Circ Res. 2008;102:1025–1035. doi: 10.1161/CIRCRESAHA.107.164749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.del Castillo G, Factor VM, Fernandez M, Alvarez-Barrientos A, Fabregat I, Thorgeirsson SS, Sanchez A. Deletion of the Met tyrosine kinase in liver progenitor oval cells increases sensitivity to apoptosis in vitro. Am J Pathol. 2008;172:1238–1247. doi: 10.2353/ajpath.2008.070793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wondergem R, Ecay TW, Mahieu F, Owsianik G, Nilius B. HGF/SF and menthol increase human glioblastoma cell calcium and migration. Biochem Biophys Res Commun. 2008;372:210–215. doi: 10.1016/j.bbrc.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 192.Zdzisinska B, Bojarska-Junak A, Dmoszynska A, Kandefer-Szerszen M. Abnormal cytokine production by bone marrow stromal cells of multiple myeloma patients in response to RPMI8226 myeloma cells. Arch Immunol Ther Exp (Warsz) 2008;56:207–221. doi: 10.1007/s00005-008-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Garzotto D, Giacobini P, Crepaldi T, Fasolo A, De Marchis S. Hepatocyte growth factor regulates migration of olfactory interneuron precursors in the rostral migratory stream through Met-Grb2 coupling. J Neurosci. 2008;28:5901–5909. doi: 10.1523/JNEUROSCI.1083-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nussbaum T, Samarin J, Ehemann V, Bissinger M, Ryschich E, Khamidjanov A, Yu X, Gretz N, Schirmacher P, Breuhahn K. Autocrine insulin-like growth factor-II stimulation of tumor cell migration is a progression step in human hepatocarcinogenesis. Hepatology. 2008;48:146–156. doi: 10.1002/hep.22297. [DOI] [PubMed] [Google Scholar]
- 195.Rampino T, Gregorini M, Guidetti C, Broggini M, Marchini S, Bonomi R, Maggio M, Roscini E, Soccio G, Tiboldo R, et al. KCNA1 and TRPC6 ion channels and NHE1 exchanger operate the biological outcome of HGF/scatter factor in renal tubular cells. Growth Factors. 2007;25:382–391. doi: 10.1080/08977190801892184. [DOI] [PubMed] [Google Scholar]
- 196.La Rosa S, Uccella S, Capella C, Erba S, Sessa F. Localization of Hepatocyte Growth Factor and Its Receptor met in Endocrine Cells and Related Tumors of the Gut and Pancreas: An Immunohistochemical Study. Endocr Pathol. 2000;11:315–329. doi: 10.1385/ep:11:4:315. [DOI] [PubMed] [Google Scholar]
- 197.Ogawa A, Terada S, Sakuragawa N, Masuda S, Nagao M, Miki M. Progesterone, but not 17beta-estradiol, up-regulates erythropoietin (EPO) production in human amniotic epithelial cells. J Biosci Bioeng. 2003;96:448–453. doi: 10.1016/S1389-1723(03)70130-3. [DOI] [PubMed] [Google Scholar]
- 198.Campana WM. Schwann cells: activated peripheral glia and their role in neuropathic pain. Brain Behav Immun. 2007;21:522–527. doi: 10.1016/j.bbi.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Thedieck C, Kalbacher H, Kuczyk M, Muller GA, Muller CA, Klein G. Cadherin-9 is a novel cell surface marker for the heterogeneous pool of renal fibroblasts. PLoS ONE. 2007;2:e657. doi: 10.1371/journal.pone.0000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yoshioka K, Miyakawa A, Ohno Y, Namiki K, Horiguchi Y, Murai M, Mukai M, Tachibana M. Production of erythropoietin and multiple cytokines by metanephric adenoma results in erythrocytosis. Pathol Int. 2007;57:529–536. doi: 10.1111/j.1440-1827.2007.02136.x. [DOI] [PubMed] [Google Scholar]
- 201.Kucic T, Copland IB, Cuerquis J, Coutu DL, Chalifour LE, Gagnon RF, Galipeau J. Mesenchymal stromal cells genetically engineered to overexpress IGF-I enhance cell-based gene therapy of renal failure-induced anemia. Am J Physiol Renal Physiol. 2008;295:F488–F496. doi: 10.1152/ajprenal.00044.2008. [DOI] [PubMed] [Google Scholar]
- 202.Bose C, Udupa KB. Erythropoietin enhancement of rat pancreatic tumor cell proliferation requires the activation of ERK and JNK signals. Am J Physiol Cell Physiol. 2008;295:C394–C405. doi: 10.1152/ajpcell.00423.2007. [DOI] [PubMed] [Google Scholar]
- 203.Paschos N, Lykissas MG, Beris AE. The role of erythropoietin as an inhibitor of tissue ischemia. Int J Biol Sci. 2008;4:161–168. doi: 10.7150/ijbs.4.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Juul SE, Ledbetter DJ, Joyce AE, Dame C, Christensen RD, Zhao Y, DeMarco V. Erythropoietin acts as a trophic factor in neonatal rat intestine. Gut. 2001;49:182–189. doi: 10.1136/gut.49.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nair RR, Warner BB, Warner BW. Role of epidermal growth factor and other growth factors in the prevention of necrotizing enterocolitis. Semin Perinatol. 2008;32:107–113. doi: 10.1053/j.semperi.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 206.Shannon DB, McKeown ST, Lundy FT, Irwin CR. Phenotypic differences between oral and skin fibroblasts in wound contraction and growth factor expression. Wound Repair Regen. 2006;14:172–178. doi: 10.1111/j.1743-6109.2006.00107.x. [DOI] [PubMed] [Google Scholar]
- 207.Yashiro M, Nakazawa K, Tendo M, Kosaka K, Shinto O, Hirakawa K. Selective cyclooxygenase-2 inhibitor downregulates the paracrine epithelial-mesenchymal interactions of growth in scirrhous gastric carcinoma. Int J Cancer. 2007;120:686–693. doi: 10.1002/ijc.22329. [DOI] [PubMed] [Google Scholar]
- 208.Nasu K, Arima K, Fujisawa K, Nishida M, Kai K, Miyakawa I. Secretion of keratinocyte growth factor by cultured human endometrial stromal cells is induced through a cyclic adenosine monophosphate-dependent pathway. Fertil Steril. 2002;77:392–395. doi: 10.1016/s0015-0282(01)02975-2. [DOI] [PubMed] [Google Scholar]
- 209.Grau V, Wilker S, Lips KS, Hartmann P, Rose F, Padberg W, Fehrenbach H, Wessler I, Kummer W. Administration of keratinocyte growth factor down-regulates the pulmonary capacity of acetylcholine production. Int J Biochem Cell Biol. 2007;39:1955–1963. doi: 10.1016/j.biocel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 210.Zang XP, Pento JT, Tari AM. Wilms’ tumor 1 protein and focal adhesion kinase mediate keratinocyte growth factor signaling in breast cancer cells. Anticancer Res. 2008;28:133–137. [PubMed] [Google Scholar]
- 211.Barbara M, Raffa S, Mure C, Manni V, Ronchetti F, Monini S, Torrisi MR. Keratinocyte growth factor receptor (KGF-R) in cholesteatoma tissue. Acta Otolaryngol. 2008;128:360–364. doi: 10.1080/00016480701785004. [DOI] [PubMed] [Google Scholar]
- 212.Watanabe M, Ishiwata T, Nishigai K, Moriyama Y, Asano G. Overexpression of keratinocyte growth factor in cancer cells and enterochromaffin cells in human colorectal cancer. Pathol Int. 2000;50:363–372. doi: 10.1046/j.1440-1827.2000.01054.x. [DOI] [PubMed] [Google Scholar]
- 213.Bell GI, Santerre RF, Mullenbach GT. Hamster preproglu-cagon contains the sequence of glucagon and two related peptides. Nature. 1983;302:716–718. doi: 10.1038/302716a0. [DOI] [PubMed] [Google Scholar]
- 214.Holst JJ, Orskov C. Glucagon and other proglucagon derived peptides. In: JH Walsh, GJ Dockray., editors. Cut Peptides: Biochemistry and Physiology. Raven: New York; 1994. pp. 305–340. [Google Scholar]
- 215.Eissele R, Goke R, Willemer S, Harthus HP, Vermeer H, Arnold R, Goke B. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest. 1992;22:283–291. doi: 10.1111/j.1365-2362.1992.tb01464.x. [DOI] [PubMed] [Google Scholar]
- 216.Roberge JN, Brubaker PL. Secretion of proglucagon-derived peptides in response to intestinal luminal nutrients. Endocrinology. 1991;128:3169–3174. doi: 10.1210/endo-128-6-3169. [DOI] [PubMed] [Google Scholar]
- 217.Roberge JN, Brubaker PL. Regulation of intestinal proglucagon-derived peptide secretion by glucose-dependent insulinotropic peptide in a novel enteroendocrine loop. Endocrinology. 1993;133:233–240. doi: 10.1210/endo.133.1.8319572. [DOI] [PubMed] [Google Scholar]
- 218.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol. 1993;138:159–166. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- 219.Xiao Q, Boushey RP, Drucker DJ, Brubaker PL. Secretion of the intestinotropic hormone glucagon-like peptide 2 is differentially regulated by nutrients in humans. Gastroenterology. 1999;117:99–105. doi: 10.1016/s0016-5085(99)70555-x. [DOI] [PubMed] [Google Scholar]
- 220.Herrmann C, Goke R, Richter G, Fehmann HC, Arnold R, Goke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56:117–126. doi: 10.1159/000201231. [DOI] [PubMed] [Google Scholar]
- 221.Reimer RA, Darimont C, Gremlich S, Nicolas-Metral V, Ruegg UT, Mace K. A human cellular model for studying the regulation of glucagon-like peptide-1 secretion. Endocrinology. 2001;142:4522–4528. doi: 10.1210/endo.142.10.8415. [DOI] [PubMed] [Google Scholar]
- 222.Ferraris RP, Yasharpour S, Lloyd KC, Mirzayan R, Diamond JM. Luminal glucose concentrations in the gut under normal conditions. Am J Physiol. 1990;259:G822–G837. doi: 10.1152/ajpgi.1990.259.5.G822. [DOI] [PubMed] [Google Scholar]
- 223.Tavares W, Drucker DJ, Brubaker PL. Enzymatic- and renal-dependent catabolism of the intestinotropic hormone glucagon-like peptide-2 in rats. Am J Physiol Endocrinol Metab. 2000;278:E134–E139. doi: 10.1152/ajpendo.2000.278.1.E134. [DOI] [PubMed] [Google Scholar]
- 224.Hartmann B, Harr MB, Jeppesen PB, Wojdemann M, Deacon CF, Mortensen PB, Holst JJ. In vivo and in vitro degradation of glucagon-like peptide-2 in humans. J Clin Endocrinol Metab. 2000;85:2884–2888. doi: 10.1210/jcem.85.8.6717. [DOI] [PubMed] [Google Scholar]
- 225.Munroe DG, Gupta AK, Kooshesh F, Vyas TB, Rizkalla G, Wang H, Demchyshyn L, Yang ZJ, Kamboj RK, Chen H, et al. Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc Natl Acad Sci USA. 1999;96:1569–1573. doi: 10.1073/pnas.96.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yusta B, Huang L, Munroe D, Wolff G, Fantaske R, Sharma S, Demchyshyn L, Asa SL, Drucker DJ. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology. 2000;119:744–755. doi: 10.1053/gast.2000.16489. [DOI] [PubMed] [Google Scholar]
- 227.Bjerknes M, Cheng H. Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc Natl Acad Sci USA. 2001;98:12497–12502. doi: 10.1073/pnas.211278098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Thulesen J, Hartmann B, Orskov C, Jeppesen PB, Holst JJ, Poulsen SS. Potential targets for glucagon-like peptide 2 (GLP-2) in the rat: distribution and binding of i.v. injected (125)I-GLP-2. Peptides. 2000;21:1511–1517. doi: 10.1016/s0196-9781(00)00305-3. [DOI] [PubMed] [Google Scholar]
- 229.Orskov C, Hartmann B, Poulsen SS, Thulesen J, Hare KJ, Holst JJ. GLP-2 stimulates colonic growth via KGF, released by subepithelial myofibroblasts with GLP-2 receptors. Regul Pept. 2005;124:105–112. doi: 10.1016/j.regpep.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 230.Fehmann HC, Habener JF. Insulinotropic hormone glucagon-like peptide-I(7-37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma beta TC-1 cells. Endocrinology. 1992;130:159–166. doi: 10.1210/endo.130.1.1309325. [DOI] [PubMed] [Google Scholar]
- 231.Gleeson MH, Bloom SR, Polak JM, Henry K, Dowling RH. An endocrine tumour in kidney affecting small bowel structure, motility, and function. Gut. 1970;11:1060. [PubMed] [Google Scholar]
- 232.Bloom SR, Polak JM. The hormonal pattern of intestinal adaptation. A major role for enteroglucagon. Scand J Gastroenterol Suppl. 1982;74:93–103. [PubMed] [Google Scholar]
- 233.Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA. 1996;93:7911–7916. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Drucker DJ, DeForest L, Brubaker PL. Intestinal response to growth factors administered alone or in combination with human [Gly2]glucagon-like peptide 2. Am J Physiol. 1997;273:G1252–G1262. doi: 10.1152/ajpgi.1997.273.6.G1252. [DOI] [PubMed] [Google Scholar]
- 235.Tsai CH, Hill M, Asa SL, Brubaker PL, Drucker DJ. Intestinal growth-promoting properties of glucagon-like peptide-2 in mice. Am J Physiol. 1997;273:E77–E84. doi: 10.1152/ajpendo.1997.273.1.E77. [DOI] [PubMed] [Google Scholar]
- 236.Petersen YM, Elnif J, Schmidt M, Sangild PT. Glucagon-like peptide 2 enhances maltase-glucoamylase and sucrase-isomaltase gene expression and activity in parenterally fed premature neonatal piglets. Pediatr Res. 2002;52:498–503. doi: 10.1203/00006450-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 237.Brubaker PL, Izzo A, Hill M, Drucker DJ. Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. Am J Physiol. 1997;272:E1050–E1058. doi: 10.1152/ajpendo.1997.272.6.E1050. [DOI] [PubMed] [Google Scholar]
- 238.Kitchen PA, Fitzgerald AJ, Goodlad RA, Barley NF, Ghatei MA, Legon S, Bloom SR, Price A, Walters JR, Forbes A. Glucagon-like peptide-2 increases sucrase-isomaltase but not caudal-related homeobox protein-2 gene expression. Am J Physiol Gastrointest Liver Physiol. 2000;278:G425–G428. doi: 10.1152/ajpgi.2000.278.3.G425. [DOI] [PubMed] [Google Scholar]
- 239.Schmidt PT, Naslund E, Gryback P, Jacobsson H, Hartmann B, Holst JJ, Hellstrom PM. Peripheral administration of GLP-2 to humans has no effect on gastric emptying or satiety. Regul Pept. 2003;116:21–25. doi: 10.1016/s0167-0115(03)00175-7. [DOI] [PubMed] [Google Scholar]
- 240.Nagell CF, Wettergren A, Pedersen JF, Mortensen D, Holst JJ. Glucagon-like peptide-2 inhibits antral emptying in man, but is not as potent as glucagon-like peptide-1. Scand J Gastroenterol. 2004;39:353–358. doi: 10.1080/00365520410004424. [DOI] [PubMed] [Google Scholar]
- 241.Wojdemann M, Wettergren A, Hartmann B, Holst JJ. Glucagon-like peptide-2 inhibits centrally induced antral motility in pigs. Scand J Gastroenterol. 1998;33:828–832. doi: 10.1080/00365529850171486. [DOI] [PubMed] [Google Scholar]
- 242.Wojdemann M, Wettergren A, Hartmann B, Hilsted L, Holst JJ. Inhibition of sham feeding-stimulated human gastric acid secretion by glucagon-like peptide-2. J Clin Endocrinol Metab. 1999;84:2513–2517. doi: 10.1210/jcem.84.7.5840. [DOI] [PubMed] [Google Scholar]
- 243.Benjamin MA, McKay DM, Yang PC, Cameron H, Perdue MH. Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut. 2000;47:112–119. doi: 10.1136/gut.47.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kouris GJ, Liu Q, Rossi H, Djuricin G, Gattuso P, Nathan C, Weinstein RA, Prinz RA. The effect of glucagon-like peptide 2 on intestinal permeability and bacterial translocation in acute necrotizing pancreatitis. Am J Surg. 2001;181:571–575. doi: 10.1016/s0002-9610(01)00635-3. [DOI] [PubMed] [Google Scholar]
- 245.Swietlicki E, Iordanov H, Fritsch C, Yi L, Levin MS, Rubin DC. Growth factor regulation of PC4/TIS7, an immediate early gene expressed during gut adaptation after resection. JPEN J Parenter Enteral Nutr. 2003;27:123–131. doi: 10.1177/0148607103027002123. [DOI] [PubMed] [Google Scholar]
- 246.Cheeseman CI, Tsang R. The effect of GIP and glucagon-like peptides on intestinal basolateral membrane hexose transport. Am J Physiol. 1996;271:G477–G482. doi: 10.1152/ajpgi.1996.271.3.G477. [DOI] [PubMed] [Google Scholar]
- 247.Cheeseman CI. Upregulation of SGLT-1 transport activity in rat jejunum induced by GLP-2 infusion in vivo. Am J Physiol. 1997;273:R1965–R1971. doi: 10.1152/ajpregu.1997.273.6.R1965. [DOI] [PubMed] [Google Scholar]
- 248.Helliwell PA, Richardson M, Affleck J, Kellett GL. Regulation of GLUT5, GLUT2 and intestinal brush-border fructose absorption by the extracellular signal-regulated kinase, p38 mitogen-activated kinase and phosphatidylinositol 3-kinase intracellular signalling pathways: implications for adaptation to diabetes. Biochem J. 2000;350 Pt 1:163–169. [PMC free article] [PubMed] [Google Scholar]
- 249.Helliwell PA, Richardson M, Affleck J, Kellett GL. Stimulation of fructose transport across the intestinal brush-border membrane by PMA is mediated by GLUT2 and dynamically regulated by protein kinase C. Biochem J. 2000;350 Pt 1:149–154. [PMC free article] [PubMed] [Google Scholar]
- 250.Kellett GL, Helliwell PA. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J. 2000;350 Pt 1:155–162. [PMC free article] [PubMed] [Google Scholar]
- 251.Au A, Gupta A, Schembri P, Cheeseman CI. Rapid insertion of GLUT2 into the rat jejunal brush-border membrane promoted by glucagon-like peptide 2. Biochem J. 2002;367:247–254. doi: 10.1042/BJ20020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ramsanahie AP, Berger UV, Zinner MJ, Whang EE, Rhoads DB, Ashley SW. Effect of glucagon-like peptide-2 (GLP-2) on diurnal SGLT1 expression. Dig Dis Sci. 2004;49:1731–1737. doi: 10.1007/s10620-004-9561-8. [DOI] [PubMed] [Google Scholar]
- 253.Guan X, Stoll B, Lu X, Tappenden KA, Holst JJ, Hartmann B, Burrin DG. GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets 1. Gastroenterology. 2003;125:136–147. doi: 10.1016/s0016-5085(03)00667-x. [DOI] [PubMed] [Google Scholar]
- 254.Walsh NA, Yusta B, DaCambra MP, Anini Y, Drucker DJ, Brubaker PL. Glucagon-like peptide-2 receptor activation in the rat intestinal mucosa. Endocrinology. 2003;144:4385–4392. doi: 10.1210/en.2003-0309. [DOI] [PubMed] [Google Scholar]
- 255.Estall JL, Yusta B, Drucker DJ. Lipid raft-dependent glucagon-like peptide-2 receptor trafficking occurs independently of agonist-induced desensitization. Mol Biol Cell. 2004;15:3673–3687. doi: 10.1091/mbc.E03-11-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Topstad D, Martin G, Sigalet D. Systemic GLP-2 levels do not limit adaptation after distal intestinal resection. J Pediatr Surg. 2001;36:750–754. doi: 10.1053/jpsu.2001.22952. [DOI] [PubMed] [Google Scholar]
- 257.Thulesen J, Hartmann B, Kissow H, Jeppesen PB, Orskov C, Holst JJ, Poulsen SS. Intestinal growth adaptation and glucagon-like peptide 2 in rats with ileal--jejunal transposition or small bowel resection. Dig Dis Sci. 2001;46:379–388. doi: 10.1023/a:1005572832571. [DOI] [PubMed] [Google Scholar]
- 258.Ljungmann K, Hartmann B, Kissmeyer-Nielsen P, Flyvbjerg A, Holst JJ, Laurberg S. Time-dependent intestinal adaptation and GLP-2 alterations after small bowel resection in rats. Am J Physiol Gastrointest Liver Physiol. 2001;281:G779–G785. doi: 10.1152/ajpgi.2001.281.3.G779. [DOI] [PubMed] [Google Scholar]
- 259.Dahly EM, Guo Z, Ney DM. IGF-I augments resection-induced mucosal hyperplasia by altering enterocyte kinetics. Am J Physiol Regul Integr Comp Physiol. 2003;285:R800–R808. doi: 10.1152/ajpregu.00014.2003. [DOI] [PubMed] [Google Scholar]
- 260.Scott RB, Kirk D, MacNaughton WK, Meddings JB. GLP-2 augments the adaptive response to massive intestinal resection in rat. Am J Physiol. 1998;275:G911–G921. doi: 10.1152/ajpgi.1998.275.5.G911. [DOI] [PubMed] [Google Scholar]
- 261.Martin GR, Wallace LE, Sigalet DL. Glucagon-like peptide-2 induces intestinal adaptation in parenterally fed rats with short bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2004;286:G964–G972. doi: 10.1152/ajpgi.00509.2003. [DOI] [PubMed] [Google Scholar]
- 262.Washizawa N, Gu LH, Gu L, Openo KP, Jones DP, Ziegler TR. Comparative effects of glucagon-like peptide-2 (GLP-2), growth hormone (GH), and keratinocyte growth factor (KGF) on markers of gut adaptation after massive small bowel resection in rats. JPEN J Parenter Enteral Nutr. 2004;28:399–409. doi: 10.1177/0148607104028006399. [DOI] [PubMed] [Google Scholar]
- 263.Jeppesen PB, Hartmann B, Thulesen J, Graff J, Lohmann J, Hansen BS, Tofteng F, Poulsen SS, Madsen JL, Holst JJ, et al. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology. 2001;120:806–815. doi: 10.1053/gast.2001.22555. [DOI] [PubMed] [Google Scholar]
- 264.Jeppesen PB, Mortensen PB. Experimental approaches: dietary and hormone therapy. Best Pract Res Clin Gastroenterol. 2003;17:1041–1054. doi: 10.1016/s1521-6918(03)00080-5. [DOI] [PubMed] [Google Scholar]
- 265.Commare CE, Tappenden KA. Development of the infant intestine: implications for nutrition support. Nutr Clin Pract. 2007;22:159–173. doi: 10.1177/0115426507022002159. [DOI] [PubMed] [Google Scholar]
- 266.Lee YC, Brubaker PL, Drucker DJ. Developmental and tissue-specific regulation of proglucagon gene expression. Endocrinology. 1990;127:2217–2222. doi: 10.1210/endo-127-5-2217. [DOI] [PubMed] [Google Scholar]
- 267.Lovshin J, Yusta B, Iliopoulos I, Migirdicyan A, Dableh L, Brubaker PL, Drucker DJ. Ontogeny of the glucagon-like peptide-2 receptor axis in the developing rat intestine. Endocrinology. 2000;141:4194–4201. doi: 10.1210/endo.141.11.7773. [DOI] [PubMed] [Google Scholar]
- 268.Petersen YM, Burrin DG, Sangild PT. GLP-2 has differential effects on small intestine growth and function in fetal and neonatal pigs. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1986–R1993. doi: 10.1152/ajpregu.2001.281.6.R1986. [DOI] [PubMed] [Google Scholar]
- 269.Yusta B, Somwar R, Wang F, Munroe D, Grinstein S, Klip A, Drucker DJ. Identification of glucagon-like peptide-2 (GLP-2)-activated signaling pathways in baby hamster kidney fibroblasts expressing the rat GLP-2 receptor. J Biol Chem. 1999;274:30459–30467. doi: 10.1074/jbc.274.43.30459. [DOI] [PubMed] [Google Scholar]
- 270.Yusta B, Boushey RP, Drucker DJ. The glucagon-like peptide-2 receptor mediates direct inhibition of cellular apoptosis via a cAMP-dependent protein kinase-independent pathway. J Biol Chem. 2000;275:35345–35352. doi: 10.1074/jbc.M005510200. [DOI] [PubMed] [Google Scholar]
- 271.Jasleen J, Shimoda N, Shen ER, Tavakkolizadeh A, Whang EE, Jacobs DO, Zinner MJ, Ashley SW. Signaling mechanisms of glucagon-like peptide 2-induced intestinal epithelial cell proliferation. J Surg Res. 2000;90:13–18. doi: 10.1006/jsre.2000.5818. [DOI] [PubMed] [Google Scholar]
- 272.Buteau J, Foisy S, Joly E, Prentki M. Glucagon-like peptide 1 induces pancreatic beta-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes. 2003;52:124–132. doi: 10.2337/diabetes.52.1.124. [DOI] [PubMed] [Google Scholar]
- 273.Thulesen J, Hartmann B, Hare KJ, Kissow H, Orskov C, Holst JJ, Poulsen SS. Glucagon-like peptide 2 (GLP-2) accelerates the growth of colonic neoplasms in mice. Gut. 2004;53:1145–1150. doi: 10.1136/gut.2003.035212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Koehler JA, Yusta B, Drucker DJ. The HeLa cell glucagon-like peptide-2 receptor is coupled to regulation of apoptosis and ERK1/2 activation through divergent signaling pathways. Mol Endocrinol. 2005;19:459–473. doi: 10.1210/me.2004-0196. [DOI] [PubMed] [Google Scholar]
- 275.Yusta B, Estall J, Drucker DJ. Glucagon-like peptide-2 receptor activation engages bad and glycogen synthase kinase-3 in a protein kinase A-dependent manner and prevents apoptosis following inhibition of phosphatidylinositol 3-kinase. J Biol Chem. 2002;277:24896–24906. doi: 10.1074/jbc.M201358200. [DOI] [PubMed] [Google Scholar]
- 276.Rocha FG, Shen KR, Jasleen J, Tavakkolizadeh A, Zinner MJ, Whang EE, Ashley SW. Glucagon-like peptide-2: divergent signaling pathways. J Surg Res. 2004;121:5–12. doi: 10.1016/j.jss.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 277.Burrin DG, Stoll B, Guan X, Cui L, Chang X, Holst JJ. Glucagon-like peptide 2 dose-dependently activates intestinal cell survival and proliferation in neonatal piglets. Endocrinology. 2005;146:22–32. doi: 10.1210/en.2004-1119. [DOI] [PubMed] [Google Scholar]
- 278.Haderslev KV, Jeppesen PB, Hartmann B, Thulesen J, Sorensen HA, Graff J, Hansen BS, Tofteng F, Poulsen SS, Madsen JL, et al. Short-term administration of glucagon-like peptide-2. Effects on bone mineral density and markers of bone turnover in short-bowel patients with no colon. Scand J Gastroenterol. 2002;37:392–398. doi: 10.1080/003655202317316006. [DOI] [PubMed] [Google Scholar]
- 279.Henriksen DB, Alexandersen P, Byrjalsen I, Hartmann B, Bone HG, Christiansen C, Holst JJ. Reduction of nocturnal rise in bone resorption by subcutaneous GLP-2. Bone. 2004;34:140–147. doi: 10.1016/j.bone.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 280.Tang-Christensen M, Larsen PJ, Thulesen J, Romer J, Vrang N. The proglucagon-derived peptide, glucagon-like peptide-2, is a neurotransmitter involved in the regulation of food intake. Nat Med. 2000;6:802–807. doi: 10.1038/77535. [DOI] [PubMed] [Google Scholar]
- 281.Lovshin J, Estall J, Yusta B, Brown TJ, Drucker DJ. Glucagon-like peptide (GLP)-2 action in the murine central nervous system is enhanced by elimination of GLP-1 receptor signaling. J Biol Chem. 2001;276:21489–21499. doi: 10.1074/jbc.M009382200. [DOI] [PubMed] [Google Scholar]
- 282.Sorensen LB, Flint A, Raben A, Hartmann B, Holst JJ, Astrup A. No effect of physiological concentrations of glucagon-like peptide-2 on appetite and energy intake in normal weight subjects. Int J Obes Relat Metab Disord. 2003;27:450–456. doi: 10.1038/sj.ijo.0802247. [DOI] [PubMed] [Google Scholar]
- 283.Haslam SZ, Counterman LJ, Nummy KA. Effects of epidermal growth factor, estrogen, and progestin on DNA synthesis in mammary cells in vivo are determined by the developmental state of the gland. J Cell Physiol. 1993;155:72–78. doi: 10.1002/jcp.1041550110. [DOI] [PubMed] [Google Scholar]
- 284.Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 285.Baxter JD. Glucocorticoid hormone action. Pharmacol Ther [B] 1976;2:605–669. doi: 10.1016/0306-039x(76)90010-6. [DOI] [PubMed] [Google Scholar]
- 286.Michel MC, Knapp J, Ratjen H. Sensitization by dexamethasone of lymphocyte cyclic AMP formation: evidence for increased function of the adenylyl cyclase catalyst. Br J Pharmacol. 1994;113:240–246. doi: 10.1111/j.1476-5381.1994.tb16200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Meier CA. Regulation of gene expression by nuclear hormone receptors. J Recept Signal Transduct Res. 1997;17:319–335. doi: 10.3109/10799899709036612. [DOI] [PubMed] [Google Scholar]
- 288.Rangarajan PN, Umesono K, Evans RM. Modulation of glucocorticoid receptor function by protein kinase A. Mol Endocrinol. 1992;6:1451–1457. doi: 10.1210/mend.6.9.1435789. [DOI] [PubMed] [Google Scholar]
- 289.Saad MJ, Folli F, Araki E, Hashimoto N, Csermely P, Kahn CR. Regulation of insulin receptor, insulin receptor substrate-1 and phosphatidylinositol 3-kinase in 3T3-F442A adipocytes. Effects of differentiation, insulin, and dexamethasone. Mol Endocrinol. 1994;8:545–557. doi: 10.1210/mend.8.5.7520127. [DOI] [PubMed] [Google Scholar]
- 290.Buren J, Liu HX, Jensen J, Eriksson JW. Dexamethasone impairs insulin signalling and glucose transport by depletion of insulin receptor substrate-1, phosphatidylinositol 3-kinase and protein kinase B in primary cultured rat adipocytes. Eur J Endocrinol. 2002;146:419–429. doi: 10.1530/eje.0.1460419. [DOI] [PubMed] [Google Scholar]
- 291.Krasil'nikov MA, Shatskaya VA, Stavrovskaya AA, Erohina M, Gershtein ES, Adler VV. The role of phosphatidylinositol 3-kinase in the regulation of cell response to steroid hormones. Biochim Biophys Acta. 1999;1450:434–443. doi: 10.1016/s0167-4889(99)00066-x. [DOI] [PubMed] [Google Scholar]
- 292.Furtado LM, Somwar R, Sweeney G, Niu W, Klip A. Activation of the glucose transporter GLUT4 by insulin. Biochem Cell Biol. 2002;80:569–578. doi: 10.1139/o02-156. [DOI] [PubMed] [Google Scholar]
- 293.Millar GA, Hardin JA, Johnson LR, Gall DG. The role of PI 3-kinase in EGF-stimulated jejunal glucose transport. Can J Physiol Pharmacol. 2002;80:77–84. doi: 10.1139/y02-012. [DOI] [PubMed] [Google Scholar]


