Abstract
Midkine (MK) is a heparin-binding growth factor with its gene first identified in embryonal carcinoma cells at early stages of retinoic acid-induced differentiation. MK is frequently and highly expressed in a variety of human carcinomas. Furthermore, the blood MK level is frequently elevated with advance of human carcinomas, decreased after surgical removal of the tumors. Thus, it is expected to become a promising marker for evaluating the progress of carcinomas. There is mounting evidence that MK plays a significant role in carcinogenesis-related activities, such as proliferation, migration, anti-apoptosis, mitogenesis, transforming, and angiogenesis. In addition, siRNA and anti-sense oligonucleotides for MK have yielded great effects in anti-tumor activities. Therefore, MK appears to be a potential candidate molecular target of therapy for human carcinomas. In this paper, we review MK targeting at nucleoli in different tumor cells and its role in carcinogenesis to deepen our understanding of the mechanism of MK involved in carcinogenesis.
Keywords: Midkine, Nuclear localization, Carcinogenesis
INTRODUCTION
Midkine (MK), also known as MDK, FLJ27379, and NEGF2, is a 13-kDa heparin-binding growth factor with a high affinity for heparin, which shares a 50% homology in amino acid sequence with pleiotrophin (PTN), another member of MK family. Previous findings indicate that MK could be internalized depending on lipoprotein receptor-related protein (LRP), a low-density lipoprotein (LDL) receptor-related protein, and is further transported to nuclei depending on nucleolin. In our study, MK could be translocated to and accumulated in nuclei and nucleoli of different kinds of tumor cell[1]. Furthermore, K79R81, K86K87, and C-terminal tail of MK constitute the nuclear localization determinants of MK. The C-terminal tail is the key element controlling MK nucleoli accumulation though the N-terminal tail. K79R81 and K86K87 also contribute to this process. Immunogold-labeling electron microscopy can investigate the exact location of MK mainly localized in granular component (GC), dense fibrillar component (DFC) and the border between DFC and FC[1]. The phenomenon of nuclear targeting of MK is related to the promotion of cell survival and anti-apoptotic activity[2].
MK is detectable in various carcinomas such as breast, prostate, grastric, colon, hepatocellular and urinary bladder carcinomas, neuroblastoma, glioma and Wilms’ tumor, exhibiting a proto-oncogene function[3–6]. However, its expression is low or undetectable in adult normal tissues. MK is deeply involved in cancer progression, onset of inflammatory diseases and repair of injured tissues. MK suppresses tumor growth both in vitro and in vivo and it is believed that MK is a new therapeutic target for curing tumors.
MK TRANSLOCATED TO AND ACCUMULATED IN NUCLEI
It has been reported that MK localizes in the nuclei[2,7], nucleoli[8], or cytosol[9], which is inconsistent with the intracellular localization of MK. Human MK exclusively localizes in nuclei and nucleoli in HepG2, DU145, and MCF7 cells by using GFP as a tracking molecule. GFP protein is stable in vivo and fused to the C or N terminus of many cellular and extracellular proteins without loss of activity, permitting tagging of proteins for gene regulation analysis, protein localization, or specific organelle labeling, and thus can be used as a perfect positive control to detect the information of MK. We cloned two MK gene fragments with or without signal peptide and separately inserted them into pEGFP-N2 plasmids (enhanced green fluorescent protein N-terminal protein fusion vector). Then, two recombinant plasmids pEGFP-MKS (EGFP fused with MK with signal peptide) and pEGFP-MKN (EGFP fused with MK without signal peptide) were obtained. The results showed that 24 h after transfection, both fusion proteins of MKS-GFP and MKN-GFP in the two cell lines, HepG2 and DU145, were localized exclusively in the nuclei and accumulated in the nucleoli, while GFP control was detected diffusely over the entire cell body except in nucleoli, indicating that signal peptide in pEGFP-MKS does not participate in nuclear and nucleolar localization (Figure 1). Similar results were obtained in MCF7 cell line (data not shown).
Figure 1.
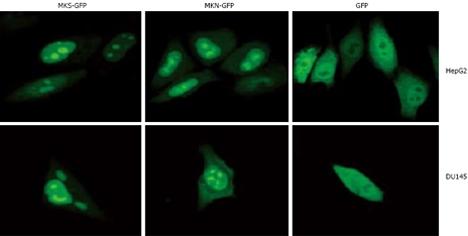
Subcellular localization analysis of MKS-GFP and MKN-GFP fusion proteins in carcinoma cell lines. MKS-GFP and MKN-GFP fusion proteins were localized to nuclei and accumulated in nucleoli, but rarely localized to cytoplasm. The high light “spots” in the center of nuclei are nucleoli. HepG2 cells (upper panel) and DU145 cells (bottom panel) are transfected with pEGFP-N2, and diffuse freely in the whole cell except in nucleoli.
MK IS INTERNALIZED IN A LRP-DEPENDENT MANNER AND PROTEASOMAL DEGRADATION
Lipoprotein receptor-related protein (LRP), also known as a low-density lipoprotein (LDL), is a midkine-binding protein[10]. LRP belongs to the LDL receptor family, which includes five prototype members: LDL receptor, ApoE receptor2, very low-density lipoprotein (VLDL) receptor, LRP, and LRP2/megalin. The major functions of these receptors are to endocytose and deliver their ligands to lysosomes for degradation or catabolism[11,12]. MK is not internalized in LRP-deficient cells, whereas transfection of a LRP expression vector can restore MK internalization and subsequent nuclear translocation, suggesting that LRP binds to heparin-binding growth factor, MK, and mediates nuclear targeting by MK. Internalized MK in cytoplasm binds to nucleolin, a nucleocytoplasmic shuttle protein and tanslocates to nuclei. When the nuclear localization signal located next to the acidic stretches is deleted, the mutant nucleolin not only accumulates in cytoplasm but also suppresses the nuclear translocation of MK. With respect to nuclear targeting by MK, 37-kDa laminin-binding protein precursor (LBP) binds to MK and is cotranslocated with MK into nuclei[7]. It is possible that MK uses both nucleolin and LBP precursor as shuttle proteins, revealing a novel role of LRP in intracellular signaling by its ligand, and the importance of nucleolin and LBP in the process of nuclear target of MK (Figure 2).
Figure 2.
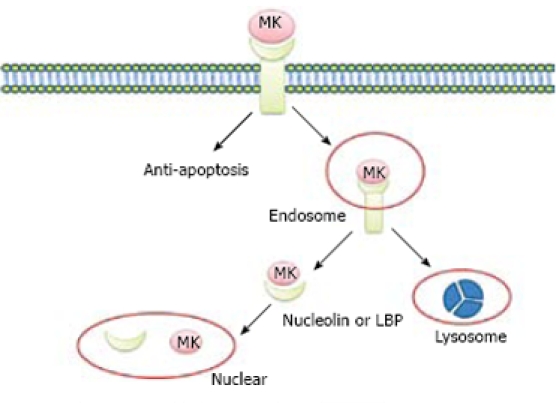
Nuclear targeting by growth factor MK. MK was endocytosed into cell cytosol through binding to the receptor of LRP, and then tanslocated to nuclei in the presence of nucleulin or LBP. At the same time, the internalized MK is degraded by proteasome to “off” the signals from the cell surface receptors.
It is widely held that growth factor signaling is terminated by lysosomal degradation of its activated receptor and endocytosed growth factor is transported to lysosome. Nuclear targeting is another important pathway through which signals of growth factors are mediated. The nuclear targeting pathway is down-regulated by the proteasome system. Degradation of endocytosed MK is suppressed by both proteasome and lysosome inhibitors. By contrast, proteasome inhibitor rather than lysosome inhibitor accelerates the nuclear accumulation of MK. Expression vector of less signal sequence MK in cytosol, can be constructed because endocytosed MK may be translocated to cytosol from cellular compartments before entering nuclei. Cytosol-produced MK undergoes proteasomal degradation, accumulates in nuclei as endocytosed MK, and is polyubiquitinated with its nuclear accumulation increased by proteasome inhibitors. MK molecule can be further dissected by cytosol-produced MK to investigate its role in degradation and trafficking. The N terminal half-domain of MK is more significantly susceptible to proteasomal degradation, whereas the C terminal half-domain is sufficient to locate nuclei. These data highlight the desensitization of nuclear targeting by growth factor MK and indicate that the proteasome system plays a critical role in desensitization of nuclear targeting. MK is prone to proteasomal degradation[13]. Because “off” signaling is essential for life, it is reasonable that nuclear targeting growth factor MK is prone to degradation by proteasome (Figure 2).
CONFORMATIONAL DETERMINANTS OF INTRACELLULAR LOCALIZATION OF MK
Nuclei orchestrate the running of cells and is a highly dynamic organelle containing dynamic compartments created by relatively immobile binding or assembly sites[14]. In order to function properly, nuclei should rely on a constant flow of molecules between cytosol and nuclei. Many proteins can be translocated into nuclei by the presence of import signals such as nuclear localization signal or sequence (NLS) which can be specifically recognized by receptors on nuclear pore complex[15], or certain shuttling proteins such as nucleolin[16]. After transported into nuclei, proteins can be docked at different subnuclear compartments. In subnuclear structures such as nucleoli, proteins may accumulate in a steady-state compartment mediated by nucleolar localization sequence (NoLS) or domain which might interact with local high-affinity binding sites[17]. We have identified the conformational determinants for MK nuclear and nucleolar localization[18], showing that K79R81, K86K87, and C-terminal tail of MK constitute the nuclear localization determinant, and play an important role in nuclear localization.
DISTRIBUTION OF MK IN NUCLEOLI
At ultra-structure level, nucleoli include three components: fibrillar center (FC), dense fibrillar component (DFC), and granular component (GC)[19,20]. Although MK tanslocated to nucleoli and accumulated in nucleoli, its exact location in nucleoli is still unclear. Immunogold-labeling electron microscopy shows that MK is mainly localized in GC, DFC and the border between DFC and FC (data unpublished). Because each component is corresponding to a special biological function, the nascent transcripts appear in the junction region between FC and DFC, accumulate in DFC and continue intranucleolar migration of rRNA towards GC[21,22], suggesting that the role of MK in nucleoli may be related to the control of rRNA gene transcription, pre-rRNA processing, and nascent ribosome subunit assembly, the downstream elements to control cell proliferation.
INVOLVEMENT OF MK IN CARCINOGENESIS
MK exhibits various cancer-related activities and is involved in carcinogenesis and cancer advancement. MK exerts its fibrinolytic activity by enhancing the level of plasminogen activator in endothelial cells[23], and transforms NIH3T3 cells[24]. MK promotes migration of neutrophils, osteoblastic, osteosarcoma cells, neural cells, and macrophages, which is always dependent on the receptors: N-syndecan and PTPζ[25]. MK mediates cell survival and growth mainly through P13-kinase and extracellular signal-regulated kinase (ERK) in intracellular signaling[26]. MK exerts its anti-apoptotic activity to rescue Wilms’ tumor cells from cisplatin[27]. MK also induces a strong angiogenic response in rabbit cornea, when its cDNA is transfected[28]. Resistance to cytotoxic agents is a major limitation for its use in treatment of human cancers. MK has been recently identified as a mediator of intercellular transfer of drug resistance[29] (Figure 3).
Figure 3.
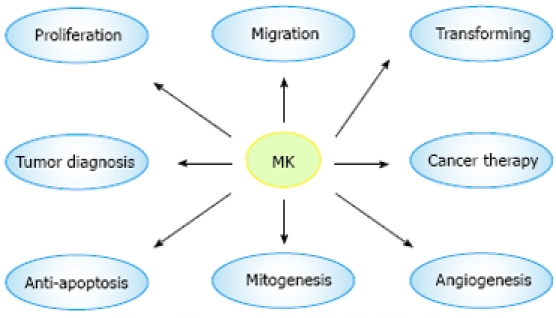
Biological function and medical application of MK. The most attractive feature of MK is its involvement in carcinogenesis, which plays a great role in proliferation, migration, anti-apoptosis, mitogenesis, transformation, and angiogenesis. Furthermore, MK is expected to be a target molecule of diagnosis of and therapy for tumor.
MEDICAL APPLICATION OFMKIN CANCER THERAPY
MK is frequently over-expressed in most carcinomas[30]. Serum and urinary MK levels are becoming prognostic and diagnostic markers of various tumors. Furthermore, MK demonstrates many activities that are significantly correlated to carcinogenesis, indicating that MK is a candidate target of therapy for carcinomas.
MOLECULAR TARGET
Anti-sense ODNs have a huge potential as agents to turn off the expression of specific proteins in vitro and in vivo. Indeed, anti-sense MK ODNs show anti-tumor activities in various carcinoma cells. Moreover, they also suppress the growth of pregrown tumors in nude mice via atelocollagen-mediated gene transfer, and exert their inhibitory effect on mitosis of cancer cells[31–33]. Thus, abolition of MK production or disruption of its signaling pathway is a good treatment modality for human carcinomas.
GENE TARGET
Currently, studies on gene therapy for cancer are carried out. The most difficult aspect of developing an in vivo approach to cancer is correctly targeting cancer cells. Many vectors target tumors for gene delivery, including viral and synthetic vectors (liposome and emulsion), which show promise in targeting cancer cells. It is essential to use a strong and tissue-specific promoter region if a suicide gene is to be expressed selectively in cancer cells. Compared with cytomegalovirus (CMV) promoter, MK promoter exhibits a stronger expression in Wilms’ tumor cells than CMV promoter[34]. Furthermore, adenovirus containing CMV promoter-thymidine kinase gene can cause severe side effect to liver, while the MK promoter-thymidine kinase gene does not have such a side effect. Therefore, a suicide gene under the control of MK promoter is a highly potential strategy for the treatment of carcinomas[35].
CONCLUSION AND PERSPECTIVES
In this review, we have described that MK can be translocated to nucleoli, where it accumulates in different tumor cells. A growing body of evidence indicates that nuclear targeting plays an indispensable role in the biological activities of growth factors. For example, nuclear localization of fibroblast growth factor 1 and Schwannoma-derived growth factor is necessary for their mitogenic activity[36,37]. Increased ribosomal gene transcription and cell proliferation are closely correlated to the nuclear translocation of fibroblast growth factor-2[38]. It is, thus, important to make clear that signals from tumor cell surface receptors and ligands translocated to nuclei play a role in the biological activities of MK.
MK is comparatively ubiquitous in many kinds of tumor, whereas its expression is usually low or undetectable in normal adult tissue. Serum MK levels are also elevated in patients with various carcinomas, such as gastric, colon, hepatocellular, and lung carcinomas[27]. These observations indicate that MK should be considered indispensable to the development and enlargement of tumors. Further analyses of MK mechanisms related to carcinogenesis should shed light on therapies for tumors and cancers. To further establish blood MK as a tumor marker, prospective studies on blood levels and prognosis will provide useful information. Further study should focus on the application of MK in gene therapy for cancer.
Supported by The National Natural Science Foundation of China, No. 30772534; Science and Technology Program Fund of Zhejiang Province, No. 2006C33028 and the Huzhou Natural Science Foundation, No. 2004SZX07-11
Peer reviewer: Dr. Jean Rosenbaum, Inserm E362, Universite Victor Segalen Bordeaux 2, Bordeaux 33076, France
S- Editor Li LF L- Editor Wang XL E- Editor Ma WH
References
- 1.Dai LC, Shao JZ, Min LS, Xiao YT, Xiang LX, Ma ZH. Midkine accumulated in nucleolus of HepG2 cells involved in rRNA transcription. World J Gastroenterol. 2008;14:6249–6253. doi: 10.3748/wjg.14.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shibata Y, Muramatsu T, Hirai M, Inui T, Kimura T, Saito H, McCormick LM, Bu G, Kadomatsu K. Nuclear targeting by the growth factor midkine. Mol Cell Biol. 2002;22:6788–6796. doi: 10.1128/MCB.22.19.6788-6796.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem. 2002;132:359–371. doi: 10.1093/oxfordjournals.jbchem.a003231. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawara A, Milbrandt J, Muramatsu T, Deuel TF, Zhao H, Cnaan A, Brodeur GM. Differential expression of pleiotrophin and midkine in advanced neuroblastomas. Cancer Res. 1995;55:1792–1797. [PubMed] [Google Scholar]
- 5.Tsutsui J, Kadomatsu K, Matsubara S, Nakagawara A, Hamanoue M, Takao S, Shimazu H, Ohi Y, Muramatsu T. A new family of heparin-binding growth/differentiation factors: increased midkine expression in Wilms’ tumor and other human carcinomas. Cancer Res. 1993;53:1281–1285. [PubMed] [Google Scholar]
- 6.Garver RI Jr, Chan CS, Milner PG. Reciprocal expression of pleiotrophin and midkine in normal versus malignant lung tissues. Am J Respir Cell Mol Biol. 1993;9:463–466. doi: 10.1165/ajrcmb/9.5.463. [DOI] [PubMed] [Google Scholar]
- 7.Salama RH, Muramatsu H, Zou K, Inui T, Kimura T, Muramatsu T. Midkine binds to 37-kDa laminin binding protein precursor, leading to nuclear transport of the complex. Exp Cell Res. 2001;270:13–20. doi: 10.1006/excr.2001.5341. [DOI] [PubMed] [Google Scholar]
- 8.Callebaut C, Nisole S, Briand JP, Krust B, Hovanessian AG. Inhibition of HIV infection by the cytokine midkine. Virology. 2001;281:248–264. doi: 10.1006/viro.2000.0767. [DOI] [PubMed] [Google Scholar]
- 9.Kato M, Maeta H, Kato S, Shinozawa T, Terada T. Immuno-histochemical and in situ hybridization analyses of midkine expression in thyroid papillary carcinoma. Mod Pathol. 2000;13:1060–1065. doi: 10.1038/modpathol.3880195. [DOI] [PubMed] [Google Scholar]
- 10.Muramatsu H, Zou K, Sakaguchi N, Ikematsu S, Sakuma S, Muramatsu T. LDL receptor-related protein as a component of the midkine receptor. Biochem Biophys Res Commun. 2000;270:936–941. doi: 10.1006/bbrc.2000.2549. [DOI] [PubMed] [Google Scholar]
- 11.Hussain MM, Strickland DK, Bakillah A. The mammalian low-density lipoprotein receptor family. Annu Rev Nutr. 1999;19:141–172. doi: 10.1146/annurev.nutr.19.1.141. [DOI] [PubMed] [Google Scholar]
- 12.Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki N, Shibata Y, Urano T, Murohara T, Muramatsu T, Kadomatsu K. Proteasomal degradation of the nuclear targeting growth factor midkine. J Biol Chem. 2004;279:17785–17791. doi: 10.1074/jbc.M310772200. [DOI] [PubMed] [Google Scholar]
- 14.Carmo-Fonseca M. The contribution of nuclear compartme-ntalization to gene regulation. Cell. 2002;108:513–521. doi: 10.1016/s0092-8674(02)00650-5. [DOI] [PubMed] [Google Scholar]
- 15.Jans DA, Xiao CY, Lam MH. Nuclear targeting signal recognition: a key control point in nuclear transport? Bioessays. 2000;22:532–544. doi: 10.1002/(SICI)1521-1878(200006)22:6<532::AID-BIES6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 16.Jans DA, Hassan G. Nuclear targeting by growth factors, cytokines, and their receptors: a role in signaling? Bioessays. 1998;20:400–411. doi: 10.1002/(SICI)1521-1878(199805)20:5<400::AID-BIES7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 17.Misteli T. Protein dynamics: implications for nuclear architecture and gene expression. Science. 2001;291:843–847. doi: 10.1126/science.291.5505.843. [DOI] [PubMed] [Google Scholar]
- 18.Dai L, Xu D, Yao X, Lu Y, Xu Z. Conformational determinants of the intracellular localization of midkine. Biochem Biophys Res Commun. 2005;330:310–317. doi: 10.1016/j.bbrc.2005.02.155. [DOI] [PubMed] [Google Scholar]
- 19.Cmarko D, Verschure PJ, Rothblum LI, Hernandez-Verdun D, Amalric F, van Driel R, Fakan S. Ultrastructural analysis of nucleolar transcription in cells microinjected with 5-bromo-UTP. Histochem Cell Biol. 2000;113:181–187. doi: 10.1007/s004180050437. [DOI] [PubMed] [Google Scholar]
- 20.Shaw PJ, Jordan EG. The nucleolus. Annu Rev Cell Dev Biol. 1995;11:93–121. doi: 10.1146/annurev.cb.11.110195.000521. [DOI] [PubMed] [Google Scholar]
- 21.Fromont-Racine M, Senger B, Saveanu C, Fasiolo F. Ribosome assembly in eukaryotes. Gene. 2003;313:17–42. doi: 10.1016/s0378-1119(03)00629-2. [DOI] [PubMed] [Google Scholar]
- 22.Fatica A, Tollervey D. Making ribosomes. Curr Opin Cell Biol. 2002;14:313–318. doi: 10.1016/s0955-0674(02)00336-8. [DOI] [PubMed] [Google Scholar]
- 23.Kojima S, Muramatsu H, Amanuma H, Muramatsu T. Midkine enhances fibrinolytic activity of bovine endothelial cells. J Biol Chem. 1995;270:9590–9596. doi: 10.1074/jbc.270.16.9590. [DOI] [PubMed] [Google Scholar]
- 24.Kadomatsu K, Hagihara M, Akhter S, Fan QW, Muramatsu H, Muramatsu T. Midkine induces the transformation of NIH3T3 cells. Br J Cancer. 1997;75:354–359. doi: 10.1038/bjc.1997.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeda N, Noda M. Involvement of receptor-like protein tyrosine phosphatase zeta/RPTPbeta and its ligand pleiotrophin/heparin-binding growth-associated molecule (HB-GAM) in neuronal migration. J Cell Biol. 1998;142:203–216. doi: 10.1083/jcb.142.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi M, Ikematsu S, Maeda N, Ichihara-Tanaka K, Sakuma S, Noda M, Muramatsu T, Kadomatsu K. Haptotactic migration induced by midkine. Involvement of protein-tyrosine phosphatase zeta. Mitogen-activated protein kinase, and phosphatidylinositol 3-kinase. J Biol Chem. 2001;276:15868–15875. doi: 10.1074/jbc.m005911200. [DOI] [PubMed] [Google Scholar]
- 27.Qi M, Ikematsu S, Ichihara-Tanaka K, Sakuma S, Muramatsu T, Kadomatsu K. Midkine rescues Wilms’ tumor cells from cisplatin-induced apoptosis: regulation of Bcl-2 expression by Midkine. J Biochem. 2000;127:269–277. doi: 10.1093/oxfordjournals.jbchem.a022604. [DOI] [PubMed] [Google Scholar]
- 28.Laaroubi K, Delbe J, Vacherot F, Desgranges P, Tardieu M, Jaye M, Barritault D, Courty J. Mitogenic and in vitro angiogenic activity of human recombinant heparin affin regulatory peptide. Growth Factors. 1994;10:89–98. doi: 10.3109/08977199409010982. [DOI] [PubMed] [Google Scholar]
- 29.Mirkin BL, Clark S, Zheng X, Chu F, White BD, Greene M, Rebbaa A. Identification of midkine as a mediator for intercellular transfer of drug resistance. Oncogene. 2005;24:4965–4974. doi: 10.1038/sj.onc.1208671. [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Sook-Kim M, Ratovitski E. Midkine promotes tetraspanin-integrin interaction and induces FAK-Stat1alpha pathway contributing to migration/invasiveness of human head and neck squamous cell carcinoma cells. Biochem Biophys Res Commun. 2008;377:474–478. doi: 10.1016/j.bbrc.2008.09.138. [DOI] [PubMed] [Google Scholar]
- 31.Takei Y, Kadomatsu K, Matsuo S, Itoh H, Nakazawa K, Kubota S, Muramatsu T. Antisense oligodeoxynucleotide targeted to Midkine, a heparin-binding growth factor, suppresses tumorigenicity of mouse rectal carcinoma cells. Cancer Res. 2001;61:8486–8491. [PubMed] [Google Scholar]
- 32.Dai LC, Wang X, Yao X, Lu YL, Ping JL, He JF. Antisense oligonucleotide targeting midkine suppresses in vivo angiogenesis. World J Gastroenterol. 2007;13:1208–1213. doi: 10.3748/wjg.v13.i8.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai LC, Wang X, Yao X, Lu YL, Ping JL, He JF. Enhanced therapeutic effects of combined chemotherapeutic drugs and midkine antisense oligonucleotides for hepatocellular carcinoma. World J Gastroenterol. 2007;13:1989–1994. doi: 10.3748/wjg.v13.i13.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adachi Y, Reynolds PN, Yamamoto M, Grizzle WE, Overturf K, Matsubara S, Muramatsu T, Curiel DT. Midkine promoter-based adenoviral vector gene delivery for pediatric solid tumors. Cancer Res. 2000;60:4305–4310. [PubMed] [Google Scholar]
- 35.Miyauchi M, Yoshida Y, Tada Y, Narita M, Maeda T, Bahar R, Kadomatsu K, Muramatsu T, Matsubara S, Nakagawara A, et al. Expression of herpes simplex virus-thymidine kinase gene controlled by a promoter region of the midkine gene confers selective cytotoxicity to ganciclovir in human carcinoma cells. Int J Cancer. 2001;91:723–727. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1112>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 36.Imamura T, Engleka K, Zhan X, Tokita Y, Forough R, Roeder D, Jackson A, Maier JA, Hla T, Maciag T. Recovery of mitogenic activity of a growth factor mutant with a nuclear translocation sequence. Science. 1990;249:1567–1570. doi: 10.1126/science.1699274. [DOI] [PubMed] [Google Scholar]
- 37.Kimura H. Schwannoma-derived growth factor must be transported into the nucleus to exert its mitogenic activity. Proc Natl Acad Sci USA. 1993;90:2165–2169. doi: 10.1073/pnas.90.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baldin V, Roman AM, Bosc-Bierne I, Amalric F, Bouche G. Translocation of bFGF to the nucleus is G1 phase cell cycle specific in bovine aortic endothelial cells. EMBO J. 1990;9:1511–1517. doi: 10.1002/j.1460-2075.1990.tb08269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


