Abstract
AIM: To assess the role of the 13C-methacetin breath test (MBT) in patients with acute liver disease.
METHODS: Fifteen patients with severe acute liver disease from diverse etiologies were followed-up with 13C-MBT during the acute phase of their illnesses (range 3-116 d after treatment). Patients fasted for 8 h and ingested 75 mg of methacetin prior to the MBT. We compared results from standard clinical assessment, serum liver enzymes, synthetic function, and breath test scores.
RESULTS: Thirteen patients recovered and two patients died. In patients that recovered, MBT parameters improved in parallel with improvements in lab results. Evidence of consistent improvement began on day 3 for MBT parameters and between days 7 and 9 for blood tests. Later convergence to normality occurred at an average of 9 d for MBT parameters and from 13 to 28 d for blood tests. In both patients that died, MBT parameters remained low despite fluctuating laboratory values.
CONCLUSION: The 13C-MBT provides a rapid, non-invasive assessment of liver function in acute severe liver disease of diverse etiologies. The results of this pilot clinical trial suggest that the MBT may offer greater sensitivity than standard clinical tests for managing patients with severe acute liver disease.
Keywords: Acute liver disease, Methacetin, Breath test, Fulminant hepatitis
INTRODUCTION
Acute liver disorders, including severe acute liver disease, acute exacerbation of chronic liver disease, and fulminant hepatic failure comprise a major cause of morbidity and mortality from liver disease[1–3]. Decision-making in the treatment of patients with severe acute liver disease focuses on the early identification of patients that require liver transplantation. This identification is currently based on several clinical and laboratory parameters[4,5], including clinical assessments, serum liver enzymes, synthetic tests, and serum ammonia levels; however, these parameters lack accuracy in assessing liver function[4,6]. In addition, frequent assessment of liver function is important in the follow-up and management of patients with severe acute liver diseases[2,7–9].
Fulminant hepatic failure (FHF) is a medical emergency that affects approximately 2000 individuals each year in the United States, and is characterized by severe and sudden liver cell dysfunction resulting in coagulation disorders and hepatic encephalopathy in patients without previous liver disease[10,11]. FHF does not follow a homogenous course. The overall survival rates for patients with FHF are approximately 10%-30%[11]. Therefore, frequent assessment of liver function is critical. Given the significant morbidity and mortality associated with acute liver disease and FHF, there is considerable urgency in the early assessment of the patients’ clinical situation and disease severity. This early assessment affects patient placement (intensive care unit versus ward), initiation of supportive therapies, and listing for liver transplantation. Current predictors of survival in patients with FHF are far from optimal[12,13]. Thus, it is often problematic to make decisions concerning medical treatment and time of transplantation based on clinical and laboratory assessments[14–16]. A therapeutic dilemma arises from the need to provide expedient transplants to patients with a failing liver while avoiding unnecessary transplantations in patients that are likely to recover spontaneously[17–19].
Breath tests have been used for several decades in patients with acute and chronic liver disorders[20–22]. These tests are based on measuring exhaled metabolites of labeled substrates that have been ingested and metabolized by the liver. The amount and rate of appearance of the metabolite in the exhaled breath represent liver enzymatic activity, and a decline may serve as a measure of hepatic injury. The ideal substrate would be metabolized solely by the liver and, therefore, selectively reflect liver metabolic function. 13C-phenylalanine (PheBT) and 13C-galactose (GBT) breath tests provide liver-specific substrates that reflect the activities of two enzymes localized to the hepatocellular cytosol[23]. Both tests have been shown to accurately predict the severity of liver cirrhosis, and correlate well with the Child-Turcotte-Pugh score (CTP). The 13C-caffeine breath test can detect chronic hepatitis B virus (HBV)-related fibrosis and has been used to monitor improvement in liver function in response to long-term lamivudine therapy[24]. Additional substrates include indocyanine green and aminopyrine; however, their use is limited due to a dependence on portal flow rate and low first-pass metabolism, respectively. Though each substrate measures only one metabolic pathway, studies have shown that each reflected overall hepatic metabolism[25–27].
Methacetin is rapidly metabolized by cytochrome P450 and lacks toxicity in small doses; thus, it is a good substrate for evaluating cytochrome P450 enzyme activity[28]. In healthy liver cells 13C-methacetin is rapidly absorbed and, with a single O-dealkylation step, cytochrome P450 1A2 breaks it down into acetaminophen and 13CO2. Like phenacetin, methacetin undergoes extensive first-pass clearance and any remaining labeled methacetin and metabolites are excreted in the urine[29]. In patients with histologically-proven chronic liver diseases, the rate of O-dealkylation of methacetin, assessed by the MBT, accurately reflects the degree of liver damage[30]. After oral administration of 13C-methacetin, the recovery of 13CO2 in the exhaled air over 30-min was significantly reduced in patients with chronic hepatitis or liver cirrhosis compared to controls. The non-invasive MBT reliably distinguished between early cirrhotic (Child A) and non-cirrhotic patients with 95.0% sensitivity and 96.7% specificity[31]. Although the MBT test is potentially useful, it has not been integrated into everyday clinical practice[32].
A major drawback in using traditional breath tests is the cumbersome method of isotopic ratio mass spectrometry. This method requires prolonged testing and analysis, imposes patient inconvenience, and delivers limited data points. In contrast, the BreathID® continuous online 13C-methacetin breath test (MBT, Exalenz, Israel) is based on the measurement of 12CO2 and 13CO2 concentrations by molecular correlation spectroscopy (MCSTM) that can detect variations less than 1/1000 in the 13CO2/12CO2 ratio[33,34]. A recent study showed its effectiveness in assessing liver unction in patients with chronic liver disorders[35].
The aim of this study was to assess the role of the MBT for evaluation and follow-up in patients with severe acute liver disease. The data suggest that the MBT correlated with standard parameters and may serve as a more sensitive decision-making tool than conventional laboratory tests for follow-up of patients with severe acute liver disease.
MATERIALS AND METHODS
Patients
Between August, 2005, and September, 2007, all consenting adult patients with severe acute liver disease were recruited to the study group. Inclusion criteria consisted of age ≥ 18 years, and an acute elevation of transaminases ≥ 10 × ULN or elevation of bilirubin ≥ 10 × ULN with or without an elevated INR ≥ 1.5. All patients gave written informed consent to their participation in the study. Patients with significant encephalopathy that were unable to give informed consent were not recruited. The study was conducted in strict adherence to the principles of the Declaration of Helsinki, and was approved by the Institutional Review Board (IRB) committees and the Israel Ministry of Health Committee for Human Clinical Trials.
Biochemical analysis
All patients underwent a biochemical work-up which included a complete blood count and the activities of: aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-glutamyltranspeptidase (GGTP), lactate dehydrogenase (LDH), albumin, total bilirubin, and prothrombin. Routine biochemical tests were performed with commercially available kits. Coagulation tests included INR and serum levels of coagulation factors 5 and 7. In cases where several blood tests were drawn in one day, statistical calculations were based either on the set of tests taken closest to the time of the breath test or the earliest and most complete set of tests for each date. Obstruction was ruled out in patients with elevated bilirubin.
Noninvasive evaluation of liver injury by breath test
Following an 8 h overnight fast, in the morning, patients were connected to the breath testing unit via a nasal cannula (IDcircuitTM) and received 75 mg of N-(4-methoxy-13C-phenyl) acetamide (Methacetin, Isotec, USA) dissolved in 150 mL of water. As a solution, its absorption is not affected by gastric motility. Breath samples were automatically collected by the BreathID device (Exalenz, Israel) before and for 60 min after the labeled substrate was administered to the patient. The 13CO2/12CO2 ratio in the breath samples was determined every 2-3 min. During the test period patients continued fasting and remained at rest to eliminate any variability in CO2 production due to the ingestion of food or physical activity. Number of tests performed was not constant.
Analysis of breath test data
Results obtained from the device were expressed as percentage of administered dose of 13C recovery (PDR) and the cumulative percentage of 13C recovery over time (CPDR) at 20, 30 and 60 min after ingestion of methacetin, respectively, as well as the PDRpeak, and peak time. PDR refers to the rate at which the 13C substrate is metabolized and exhaled expressed as percent per hour. PDR is based on the change in 13C/12C ratio for each patient, taking into account their specific parameters affecting overall CO2, normalizing the results for any weight, height, dose and substrate type and purity[36,37]. CPDR is the numeric integral of PDR, and describes the total percent of substrate metabolized at any given accumulated time. Data are expressed in units of percent. The BreathID® device plots the PDR and CPDR in real time and the PDR peak value is then calculated.
Statistical analysis
The correlation of the Breath test parameters and blood test values was calculated using Pearson Correlation. A model of recovery based on exponential function [1-const.*exp (time_from_baseline/time_constant)] for MBT returning to normal metabolic function and [1+const.*exp (time_from_baseline/time_constant)] for blood test value decrease and return to normality was used to extract the time to recovery. Statistical analysis was performed only for patients who had at least 3 breath tests and 3 sets of blood tests over at least three different days. Generalized Reduced Gradient (GRG2) with nonlinear optimization was used to determine the functions constants.
Normal values
Normal values were determined using a group of 100 healthy volunteers (57 males and 43 females). These healthy controls were screened by medical history, physical examination, routine liver function tests, and abdominal ultrasound. All healthy volunteers had blood test results within normal limits. None had a history of active or previous liver disease, alcohol abuse, drug abuse, and none were taking medications. Based on the analysis of MBT results from 100 normal volunteers, a PDR Peak value of 30% h was considered to be normal. The average PDR Peak value was 35% ± 9 % h in healthy volunteers, with a minimum of 20% h and a maximum of 60% h. Because the blood test results were considered normal within a range of ± 30% of normal, we decided to set a similar range for normal PDR peak values. Furthermore, the normal ranges for blood tests typically take into account that a high value is critical, while the low value is less relevant. Therefore, the normal range is expressed as anything below the normal value + 30% (Table 1). Similarly, the PDR peak had critical significance at low values, but high values were less relevant. Therefore, we set the normal range as anything above 21% h (Table 1).
Table 1.
Definition of normality for breath test parameters and blood tests
| Units | Normal values | ± 30% of normal | |
| Breath test | %/h | > 30 | > 21 |
| AL | U/L | < 53 | < 69 |
| AST | U/L | < 60 | < 78 |
| INR | U/L | 1.00 | < 1.30 |
| BIL | μmol/L | < 17 | < 22 |
RESULTS
Patient population
Fifteen adult patients had severe acute liver disease of diverse etiologies, including autoimmune hepatitis (n = 5), acute hepatitis A virus (HAV) (n = 2), acute HBV (n = 2), drug-induced liver injury (n = 3), neoplastic disease (n = 1), Wilson’s disease (n = 1), and pregnancy associated liver disease (n = 1). The demographic and clinical characteristics are summarized in Tables 2 and 3. In two patients a grade 2 hepatic encephalopathy developed.
Table 2.
Liver disease etiology and outcome
| Etiology | Number of patients | Number recovered | Number deceased |
| Autoimmune hepatitis | 5 | 4 | 1 |
| Drug induced liver injury | 3 | 3 | 0 |
| Acute HBV | 2 | 2 | 0 |
| Acute HAV | 2 | 2 | 0 |
| Wilson’s disease | 1 | 1 | 0 |
| Pregnancy associated | 1 | 1 | 0 |
| Space occupying lesion | 1 | 0 | 1 |
Table 3.
Liver tests parameters (mean ± SD)
| Parameter | Female | Male | Overall |
| No. of patients | 8 | 7 | 15 |
| Weight (kg) | 70.13 ± 20.03 (52-115) | 82 ± 19.59 (55-115) | 75.67 ± 20.07 (52-115) |
| Height (cm) | 163.75 ± 6.69 (155-176) | 175.57 ± 5.32 (167-184) | 169.27 ± 8.47 (155-184) |
| BMI (kg/m2) | 25.91 ± 5.69 (19.95-37.13) | 26.53 ± 5.86 (18.59-35.89) | 26.20 ± 5.57 (18.59-37.13) |
| Age (yr) | 33.50 ± 17.04 (18-61) | 31.14 ± 14.02 (14-53) | 32.40 ± 15.19 (14-61) |
| ALT (IU) | 1162.38 ± 1926.69 (45-5795) | 2807.86 ± 2468.82 (58-7535) | 1930.27 ± 2278.22 (45-7535) |
| AST (IU) | 996.88 ± 1306.56 (50-3928) | 1475.71 ± 1104.09 (96-3294) | 1220.33 ± 1198.8 (50-3928) |
| Bilirubin (μmol/L) | 193.50 ± 207.39 (9-565) | 232.86 ± 194.49 (41-599) | 211.87 ± 195.27 (9-599) |
| INR (SI) | 2.09 ± 0.90 (1.00-4.06) | 1.80 ± 0.62 (1.26-3.02) | 1.95 ± 0.77 (1.00-4.06) |
Patient outcomes
Thirteen patients ultimately recovered from their illness, based on both clinical and biochemical assessments (average recovery time 28 d, SD 22 d); two patients died (1 autoimmune hepatitis, 1 neoplastic disease); and none of the patients were transplanted. The first patient that showed an unfavorable course of illness died of sepsis before a transplant could be provided. The second patient died from metastatic cancer.
Correlation of MBT with clinical course
Figure 1 illustrates the method of comparing results from the breath test versus the blood tests along the clinical course of recovery. In all patients that eventually recovered, clinical improvement and normalization of biochemical parameters followed the progressive improvement of BreathID® 13C-MBT scores (Figure 2A). In the two patients that died, breath test parameters deteriorated or failed to improve (Figure 2B). Overall, breath test parameters showed better correlation with clinical improvement than all other tests. Correlations between the breath test parameters and the blood test values varied between 0.2 and 0.7 (Table 4). The comparisons showed a trend for correlation; however, statistical significance was not reached due to the small number of patients and tests.
Figure 1.
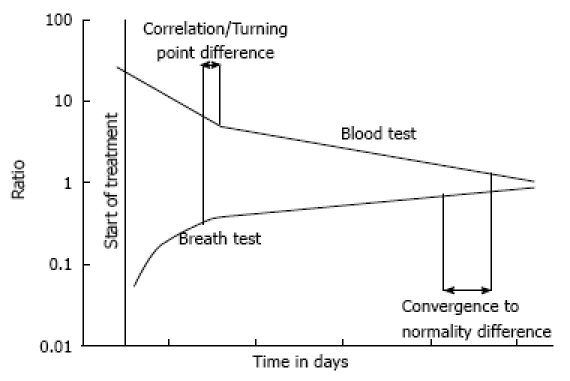
This is an illustration of typical breath test and blood test results plotted over the clinical time course. The wide green line indicates the range of normal values. As the patient recovered, test results converged toward normality.
Figure 2.
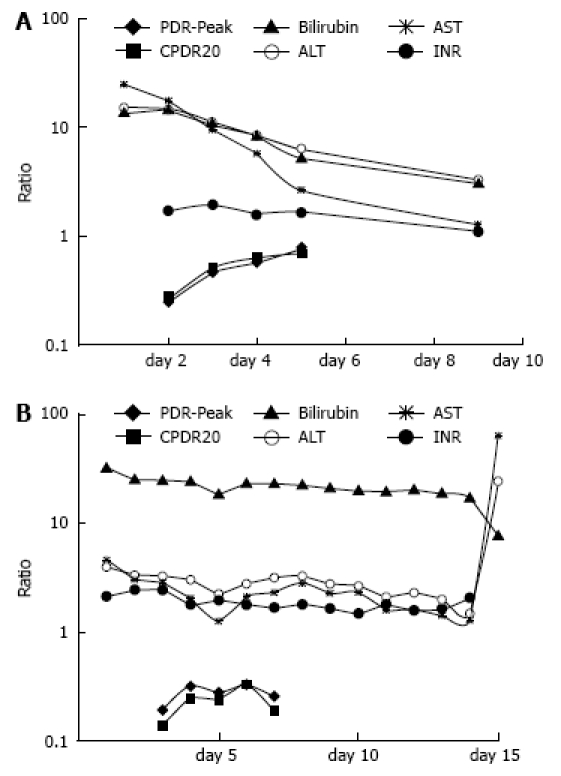
The clinical course of a patient with fulminant auto-immune hepatitis. A: Upon admission, aminotransferase levels were well above 10 × ULN, INR was increased, and breath test values were low. The patient received treatment with steroids and recovered rapidly. The breath test values normalized within 3 d, but blood tests began normalizing 2 d later; B: Despite therapy with steroids, this patient failed to improve, developed gram negative sepsis, and died. Note that despite stable blood tests, there was a lack of improvement in breath test values. The low breath test values may have been a negative prognostic factor in this patient and may have provided a rationale for early transplantation.
Table 4.
Correlation coefficients for blood test versus breath test parameters
| PDR peak | Peak time | PDR10 | PDR15 | PDR20 | PDR30 | PDR60 | CPDR10 | CPDR15 | CPDR20 | CPDR30 | CPDR60 | |
| BIL | -0.556 | 0.394 | -0.519 | -0.544 | -0.575 | -0.588 | -0.519 | -0.456 | -0.513 | -0.540 | -0.580 | -0.595 |
| ALT | -0.276 | 0.235 | -0.258 | -0.279 | -0.293 | -0.318 | -0.403 | -0.248 | -0.266 | -0.276 | -0.306 | -0.326 |
| AST | -0.338 | 0.226 | -0.306 | -0.337 | -0.358 | -0.384 | -0.451 | -0.279 | -0.311 | -0.329 | -0.362 | -0.405 |
| AP | -0.239 | 0.219 | -0.308 | -0.280 | -0.268 | -0.172 | -0.110 | -0.283 | -0.301 | -0.296 | -0.282 | -0.198 |
| GGT | 0.504 | -0.344 | 0.510 | 0.538 | 0.507 | 0.418 | 0.203 | 0.340 | 0.449 | 0.483 | 0.497 | 0.438 |
| LDH | -0.313 | 0.221 | -0.313 | -0.329 | -0.329 | -0.325 | -0.314 | -0.264 | -0.302 | -0.317 | -0.333 | -0.332 |
| INR | -0.436 | 0.441 | -0.412 | -0.435 | -0.452 | -0.431 | -0.386 | -0.390 | -0.420 | -0.437 | -0.458 | -0.562 |
| PTT | -0.565 | 0.239 | -0.413 | -0.509 | -0.579 | -0.632 | -0.471 | -0.360 | -0.420 | -0.471 | -0.543 | -0.598 |
| PT% | 0.628 | -0.364 | 0.475 | 0.573 | 0.635 | 0.673 | 0.513 | 0.440 | 0.489 | 0.540 | 0.609 | 0.660 |
| F-VII% | 0.648 | -0.828 | 0.720 | 0.737 | 0.723 | 0.613 | 0.581 | 0.661 | 0.694 | 0.715 | 0.725 | 0.688 |
| F-V% | 0.576 | -0.495 | 0.598 | 0.521 | 0.546 | 0.579 | 0.503 | 0.527 | 0.548 | 0.554 | 0.582 | 0.591 |
Results obtained from the device were expressed as percentage of administered dose of 13C recovery (PDR) and the cumulative percentage of 13C recovery over time (CPDR) at 10, 15, 20, 30 and 60 min after ingestion of methacetin, as well as the PDR peak, and peak time. PDR refers to the rate at which the 13C substrate is metabolized and exhaled expressed as percent per hour. PDR is based on the change in 13C/12C ratio for each patient. CPDR is the numeric integral of PDR, and describes the total percent of substrate metabolized at any given accumulated time. Data are expressed in units of percent. ALT: Alanine aminotransferase; AST: Aspartate amino transferase; GGT: Gamma glutamyl transpepetidase; AP: Alkaline phosphatase; F-V, F-VII: Factor 5 and 7; PDR: Percent dose recovery at different time points in minutes; CPDR: Cumulative percent dose recovery at different time points.
MBT results detect improvement in liver function prior to standard tests
The MBT indicated improvement in hepatic function prior to lab results. For this analysis, we defined 2 clinically relevant points. The first was the “point of stable improvement”, which was defined as the point in time after which a continuous improvement was observed in patient MBT scores or blood tests results. The second was “convergence to normality,” which was the point in time at which normal values were achieved (normal values are defined in the Methods). As shown in Table 5, the average point of stable improvement for MBT parameters occurred at 3 d, while for blood tests it occurred between 7 and 9 d after treatment. Later, the average convergence to normality for MBT parameters occurred at 9 d, while for blood tests it occurred within 13 to 28 d after treatment.
Table 5.
Blood tests versus breath test parameters (mean, SD)
| Point of stable improvement (days after treatment) | Convergence to normality (days after treatment) | |
| Breath test | 2.85, 2.23 | 8.89, 11.5 |
| ALT | 7.33, 11.06 | 28.24, 21.69 |
| AST | 7.62, 11.44 | 12.96, 6.96 |
| INR | 8.62, 12.1 | 16.02, 24.71 |
| BIL | 7, 10.61 | 23.63, 22.61 |
MBT parameters detect deterioration in liver function prior to other tests
In both patients that died, MBT parameters were extremely low at presentation and remained low throughout the course of the illnesses (despite medical therapy in the case of autoimmune hepatitis). In contrast, patient blood test parameters fluctuated during the course of the illnesses and finally deteriorated shortly before death (< 24 h).
DISCUSSION
The BreathID® 13C-MBT is a rapid, non-invasive tool for the assessment of liver function in acute liver disease. This study showed that using the MBT to evaluate patients with acute severe liver disease enabled detection of improvement 4-6 d earlier than the currently used laboratory tests, irrespective of disease etiology. Furthermore, breath test results indicated normalization of liver function 4-19 d earlier than blood test values in patients that recovered from their illnesses. The data of the present study suggests that the MBT may serve as a more sensitive decision-making tool than standard test parameters in the setting of severe acute liver disease.
In fulminant hepatic failure (FHF) an early prognosis is essential in determining the need and appropriate timing of orthotopic liver transplantation (OLT). Several reports have described laboratory parameters that served as predictive criteria. Since its publication by O’Grady and colleagues in 1989[38], the King’s College Criteria have been widely used to define patients with poor prognoses using pH, thrombin time, serum creatinine, and bilirubin levels. Recently, the Acute Physiology and Chronic Health Evaluation (APACHE II) score was described for determining prognosis in the setting of FHF. The APACHE II used a combination of both clinical and laboratory parameters and its sensitivity was comparable to that of the King’s College Criteria[39]. A recent study in 120 consecutive patients with FHF investigated the prognostic efficacies of King’s College criteria, Clichy’s criteria, the Model for End-Stage Liver Disease (MELD), and the Pediatric End-Stage Liver Disease (PELD). MELD scores were significantly higher in patients that died compared to those that survived without OLT. Logistic regression analysis yielded concordance statistics that were significantly higher for MELD (0.95) and PELD (0.99) compared to King’s College (0.74) and Clichy’s criteria (0.68). In a Cox model analysis, the data included patients that received transplants and censored the time from admission; this analysis showed the concordance statistics for MELD (0.77) and PELD (0.79) remained significantly higher than that of King’s College criteria, but not higher than that of Clichy’s criteria[40]. However, the MELD and PELD were not effective for follow-up on a daily basis, thus they were not useful for decision-making in the acute setting. The lack of an effective measure of liver function in the acute setting makes it difficult to reach an appropriate clinical decision in hepatology[41,42].
Noninvasive tests were used previously to assess liver function in acute liver disease. In 1993, Luketic et al[43] demonstrated that hepatic lidocaine metabolism was useful in the selection of patients for liver transplantation[44]. The study evaluated hepatic conversion of lidocaine to its primary metabolite, monoethylglycinexylodide, and compared the results to liver histological findings in 225 patients with chronic hepatitis. A decline in monoethylglycinexylodide production was correlated with worsening liver histological findings. A further stepwise decline in monoethylglycinexylodide production was correlated with a worsening Child class score. In contrast, no relationship was observed between liver histological status and serum transaminases (AST or ALT), bilirubin, albumin, or prothrombin time. Thirty-five patients underwent a follow-up histological evaluation and monoethylglycinexylodide testing after receiving at least 6 mo treatment for chronic hepatitis (interferon for hepatitis B and C and corticosteroids for autoimmune hepatitis). The change in monoethylglycinexylodide production was linearly related to the change in the Knodell histological index.
MBT has several advantages compared with the above tests. It is a noninvasive, easily preformed, bed-side test and is not associated with patient discomfort. It provides real time results and does not require any expertise for data analysis. It has no known side effects and is not limited by patient-specific parameters. Finally, the test is sensitive to minor changes in liver metabolism, thus enabling daily follow-ups in the acute setting.
The cause of acute liver failure (ALF)[6] is a major determinant of its outcome. Thus, the prognosis for ALF due to acetaminophen (paracetamol) overdose is relatively good, but the mortality rate for ALF from other causes may be significantly higher. In the current study, only 2 patients (13%) had acute liver damage due to paracetamol, but a far greater percent presented with acute autoimmune hepatitis. This may be explained by the observation that paracetamol is the etiological agent for a large percentage of ALF cases in England and the US, but it accounts for far fewer incidents in Europe and Israel (unpublished results). In a study performed in Spain, 267 cases of ALF were analyzed retrospectively. Acetaminophen overdose accounted for only 2.2% of the patients and overall survival was 58%. Liver transplants were performed in 150 patients, with a survival of 69%. Patients that fulfilled the criteria, but were not provided transplants due to contraindications, had a survival rate of only 7.8%. Those that did not fulfill the transplant criteria had a 85.5% survival rate[45]. The high survival rate in our group of patients might be explained by the fact that only 2 patients (13%) fulfilled the transplant criteria. One (autoimmune hepatitis) patient died from sepsis before transplantation was possible, and the other patient began to recover spontaneously a few hours before the scheduled transplantation. In this patient, the breath test made it possible to follow liver function even after correction of coagulopathy with fresh frozen plasma in preparation for liver transplantation.
Orthotopic liver transplantation is the most definitive solution to the problem of sudden hepatocyte loss. However, the selection of a lifetime surgical solution for an acute, potentially self-limiting problem is a stressful decision for physicians. Several obstacles to successful transplantation may arise, including transporting a patient with cerebral edema safely to the transplantation center, securing funding in a timely fashion, and obtaining a suitable organ rapidly. The overall one year survival rate of patients with acute liver failure that undergo liver transplantation exceeds 60 percent. However, due to the logistic hurdles that must be overcome, it is estimated that currently only 10 percent of patients with acute liver failure receive a liver graft. Subsequently, any test that may help in determining recovery at an earlier stage, thus deferring the need for transplantation, is of great value.
The current study had several limitations. The cohort only included 15 patients. The requirement for obtaining informed consent excluded patients with encephalopathy; thus, patients on that end of the spectrum were not assessed. Indeed, only two patients in this cohort displayed an indication for liver transplantation. In order to demonstrate an effect of MBT on decisions regarding liver transplantation, a larger group of patients with fulminant liver failure must be studied. However, even in the limited number of cases studied here, we found the MBT test offered an advantage as a decision-making tool because improvement in MBT parameters provided a rationale for avoiding unnecessary liver transplantation in one of these patients.
In summary, the results of the present preliminary study suggest that a point of care, on-line breath test system may provide an important tool for decision-making in clinical hepatology in the setting of acute severe liver disease. Further large scale studies are required for assessment of the general utility of this test.
Peer reviewers: Wing-Kin Syn, MD, Division of Gastroenterology, GSRB-1, Suite 1073, DUMC 3256, 595 LaSalle Street, Durham, NC 27710, United States; Yogesh K Chawla, Dr, Professor, Department of Hepatology, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India
S- Editor Cheng JX L- Editor Rippe RA E- Editor Zheng XM
References
- 1.Elias E. Liver failure and liver disease. Hepatology. 2006;43:S239–S242. doi: 10.1002/hep.21041. [DOI] [PubMed] [Google Scholar]
- 2.Davis CL, Gonwa TA, Wilkinson AH. Identification of patients best suited for combined liver-kidney transplantation: part II. Liver Transpl. 2002;8:193–211. doi: 10.1053/jlts.2002.32504. [DOI] [PubMed] [Google Scholar]
- 3.Han MK, Hyzy R. Advances in critical care management of hepatic failure and insufficiency. Crit Care Med. 2006;34:S225–S231. doi: 10.1097/01.CCM.0000231882.85350.71. [DOI] [PubMed] [Google Scholar]
- 4.Blei AT. Selection for acute liver failure: have we got it right? Liver Transpl. 2005;11:S30–S34. doi: 10.1002/lt.20595. [DOI] [PubMed] [Google Scholar]
- 5.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 6.Mullin EJ, Metcalfe MS, Maddern GJ. How much liver resection is too much? Am J Surg. 2005;190:87–97. doi: 10.1016/j.amjsurg.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 7.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 8.Stadlbauer V, Jalan R. Acute liver failure: liver support therapies. Curr Opin Crit Care. 2007;13:215–221. doi: 10.1097/MCC.0b013e328052c4cc. [DOI] [PubMed] [Google Scholar]
- 9.Singhal A, Neuberger J. Acute liver failure: bridging to transplant or recovery--are we there yet? J Hepatol. 2007;46:557–564. doi: 10.1016/j.jhep.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Sass DA, Shakil AO. Fulminant hepatic failure. Liver Transpl. 2005;11:594–605. doi: 10.1002/lt.20435. [DOI] [PubMed] [Google Scholar]
- 11.Sass DA, Shakil AO. Fulminant hepatic failure. Gastroenterol Clin North Am. 2003;32:1195–1211. doi: 10.1016/s0889-8553(03)00088-8. [DOI] [PubMed] [Google Scholar]
- 12.Neuberger J. Prediction of survival for patients with fulminant hepatic failure. Hepatology. 2005;41:19–22. doi: 10.1002/hep.20562. [DOI] [PubMed] [Google Scholar]
- 13.Schiødt FV, Lee WM. Fulminant liver disease. Clin Liver Dis. 2003;7:331–349, vi. doi: 10.1016/s1089-3261(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 14.Larsen FS. Optimal management of patients with fulminant hepatic failure: targeting the brain. Hepatology. 2004;39:299–301. doi: 10.1002/hep.20071. [DOI] [PubMed] [Google Scholar]
- 15.Vaquero J, Blei AT. Etiology and management of fulminant hepatic failure. Curr Gastroenterol Rep. 2003;5:39–47. doi: 10.1007/s11894-003-0008-8. [DOI] [PubMed] [Google Scholar]
- 16.Van Thiel DH, Brems J, Nadir A, Idilman R, Colantoni A, Holt D, Edelstein S. Liver transplantation for fulminant hepatic failure. J Gastroenterol. 2002;37 Suppl 13:78–81. doi: 10.1007/BF02990105. [DOI] [PubMed] [Google Scholar]
- 17.Farmer DG, Anselmo DM, Ghobrial RM, Yersiz H, McDiarmid SV, Cao C, Weaver M, Figueroa J, Khan K, Vargas J, et al. Liver transplantation for fulminant hepatic failure: experience with more than 200 patients over a 17-year period. Ann Surg. 2003;237:666–675; discussion 675-676. doi: 10.1097/01.SLA.0000064365.54197.9E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGuire BM. The critically ill liver patient: fulminant hepatic failure. Semin Gastrointest Dis. 2003;14:39–42. [PubMed] [Google Scholar]
- 19.Rinella ME, Sanyal A. Intensive management of hepatic failure. Semin Respir Crit Care Med. 2006;27:241–261. doi: 10.1055/s-2006-945528. [DOI] [PubMed] [Google Scholar]
- 20.Schneider A, Caspary WF, Saich R, Dietrich CF, Sarrazin C, Kuker W, Braden B. 13C-methacetin breath test shortened: 2-point-measurements after 15 minutes reliably indicate the presence of liver cirrhosis. J Clin Gastroenterol. 2007;41:33–37. doi: 10.1097/MCG.0b013e31802dd4b9. [DOI] [PubMed] [Google Scholar]
- 21.Hepner GW, Vesell ES. Assessment of aminopyrine metabolism in man by breath analysis after oral administration of 14C-aminopyrine. Effects of phenobarbital, disulfiram and portal cirrhosis. N Engl J Med. 1974;291:1384–1388. doi: 10.1056/NEJM197412262912605. [DOI] [PubMed] [Google Scholar]
- 22.Ilan Y. Review article: the assessment of liver function using breath tests. Aliment Pharmacol Ther. 2007;26:1293–1302. doi: 10.1111/j.1365-2036.2007.03519.x. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh CB, Yu CY, Tzao C, Chu HC, Chen TW, Hsieh HF, Liu YC, Yu JC. Prediction of the risk of hepatic failure in patients with portal vein invasion hepatoma after hepatic resection. Eur J Surg Oncol. 2006;32:72–76. doi: 10.1016/j.ejso.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Park GJ, Katelaris PH, Jones DB, Seow F, Lin BP, Le Couteur DG, Ngu MC. The C-caffeine breath test distinguishes significant fibrosis in chronic hepatitis B and reflects response to lamivudine therapy. Aliment Pharmacol Ther. 2005;22:395–403. doi: 10.1111/j.1365-2036.2005.02623.x. [DOI] [PubMed] [Google Scholar]
- 25.Utecht KN, Hiles JJ, Kolesar J. Effects of genetic polymorphisms on the pharmacokinetics of calcineurin inhibitors. Am J Health Syst Pharm. 2006;63:2340–2348. doi: 10.2146/ajhp060080. [DOI] [PubMed] [Google Scholar]
- 26.Liu L, Pang KS. An integrated approach to model hepatic drug clearance. Eur J Pharm Sci. 2006;29:215–230. doi: 10.1016/j.ejps.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Barstow L, Small RE. Liver function assessment by drug metabolism. Pharmacotherapy. 1990;10:280–288. [PubMed] [Google Scholar]
- 28.Klatt S, Taut C, Mayer D, Adler G, Beckh K. Evaluation of the 13C-methacetin breath test for quantitative liver function testing. Z Gastroenterol. 1997;35:609–614. [PubMed] [Google Scholar]
- 29.Adamek RJ, Goetze O, Boedeker C, Pfaffenbach B, Luypaerts A, Geypens B. 13C-methacetin breath test: isotope-selective nondispersive infrared spectrometry in comparison to isotope ratio mass spectrometry in volunteers and patients with liver cirrhosis. Z Gastroenterol. 1999;37:1139–1143. [PubMed] [Google Scholar]
- 30.Matsumoto K, Suehiro M, Iio M, Kawabe T, Shiratori Y, Okano K, Sugimoto T. [13C]methacetin breath test for evaluation of liver damage. Dig Dis Sci. 1987;32:344–348. doi: 10.1007/BF01296285. [DOI] [PubMed] [Google Scholar]
- 31.Braden B, Faust D, Sarrazin U, Zeuzem S, Dietrich CF, Caspary WF, Sarrazin C. 13C-methacetin breath test as liver function test in patients with chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2005;21:179–185. doi: 10.1111/j.1365-2036.2005.02317.x. [DOI] [PubMed] [Google Scholar]
- 32.Braden B, Lembcke B, Kuker W, Caspary WF. 13C-breath tests: current state of the art and future directions. Dig Liver Dis. 2007;39:795–805. doi: 10.1016/j.dld.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Israeli E, Ilan Y, Meir SB, Buenavida C, Goldin E. A novel 13C-urea breath test device for the diagnosis of Helicobacter pylori infection: continuous online measurements allow for faster test results with high accuracy. J Clin Gastroenterol. 2003;37:139–141. doi: 10.1097/00004836-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Lysy J, Israeli E, Strauss-Liviatan N, Goldin E. Relationships between hypoglycaemia and gastric emptying abnormalities in insulin-treated diabetic patients. Neurogastroenterol Motil. 2006;18:433–440. doi: 10.1111/j.1365-2982.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 35.Goetze O, Selzner N, Fruehauf H, Fried M, Gerlach T, Mullhaupt B. 13C-methacetin breath test as a quantitative liver function test in patients with chronic hepatitis C infection: continuous automatic molecular correlation spectroscopy compared to isotopic ratio mass spectrometry. Aliment Pharmacol Ther. 2007;26:305–311. doi: 10.1111/j.1365-2036.2007.03360.x. [DOI] [PubMed] [Google Scholar]
- 36.Nista EC, Fini L, Armuzzi A, Candelli M, Zocco MA, Cazzato IA, Merra G, Finizio R, Miele L, Grieco A, et al. 13C-breath tests in the study of microsomal liver function. Eur Rev Med Pharmacol Sci. 2004;8:33–46. [PubMed] [Google Scholar]
- 37.Schoeller DA, Baker AL, Monroe PS, Krager PS, Schneider JF. Comparison of different methods expressing results of the aminopyrine breath test. Hepatology. 1982;2:455–462. doi: 10.1002/hep.1840020411. [DOI] [PubMed] [Google Scholar]
- 38.O'Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 39.Bailey B, Amre DK, Gaudreault P. Fulminant hepatic failure secondary to acetaminophen poisoning: a systematic review and meta-analysis of prognostic criteria determining the need for liver transplantation. Crit Care Med. 2003;31:299–305. doi: 10.1097/00003246-200301000-00048. [DOI] [PubMed] [Google Scholar]
- 40.Yantorno SE, Kremers WK, Ruf AE, Trentadue JJ, Podestá LG, Villamil FG. MELD is superior to King's college and Clichy's criteria to assess prognosis in fulminant hepatic failure. Liver Transpl. 2007;13:822–828. doi: 10.1002/lt.21104. [DOI] [PubMed] [Google Scholar]
- 41.Gow PJ, Warrilow S, Lontos S, Lubel J, Wongseelashote S, MacQuillan GC, Jones RM, Bellomo R, Angus PW. Time to review the selection criteria for transplantation in paracetamol-induced fulminant hepatic failure? Liver Transpl. 2007;13:1762–1763. doi: 10.1002/lt.21301. [DOI] [PubMed] [Google Scholar]
- 42.O'Grady JG. Prognostication in acute liver failure: a tool or an anchor? Liver Transpl. 2007;13:786–787. doi: 10.1002/lt.21159. [DOI] [PubMed] [Google Scholar]
- 43.Luketic VA, Shiffman ML, Fisher RA, Sanyal AJ, Purdum PP 3rd, Posner MP. Hepatic lidocaine metabolism is useful in the selection of patients in need of liver transplantation. Transplant Proc. 1993;25:1072–1074. [PubMed] [Google Scholar]
- 44.Shiffman ML, Luketic VA, Sanyal AJ, Duckworth PF, Purdum PP 3rd, Contos MJ, Mills AS, Edinboro LE, Poklis A. Hepatic lidocaine metabolism and liver histology in patients with chronic hepatitis and cirrhosis. Hepatology. 1994;19:933–940. [PubMed] [Google Scholar]
- 45.Escorsell A, Mas A, de la Mata M. Acute liver failure in Spain: analysis of 267 cases. Liver Transpl. 2007;13:1389–1395. doi: 10.1002/lt.21119. [DOI] [PubMed] [Google Scholar]


