Abstract
AIM: To create a rabbit model of pediatric nonalcoholic steatohepatitis (NASH) and to evaluate the role of adiponectin in the process.
METHODS: Thirty-two specific pathogen-free male New Zealand rabbits were divided randomly into three groups: (1) the normal control group (n = 10) was fed with standard diet for 12 wk; (2) the model group A (n = 11); and (3) model group B (n = 11) were fed with a high-fat diet (standard diet + 10% lard + 2% cholesterol) for 8 and 12 wk, respectively. Hepatic histological changes were observed and biochemical parameters as well as serum levels of adiponectin, interleukin (IL)-6, IL-10 and tumor necrosis factor (TNF)-α were measured.
RESULTS: Typical histological hepatic lesions of NASH were observed in both model groups described as liver steatosis, liver inflammatory infiltration, cytologic ballooning, perisinusoidal fibrosis and overall fibrosis. Compared with the normal control group, there were significant increases in model groups A and B in weight gain (1097.2 ± 72.3, 1360.5 ± 107.6 vs 928.0 ± 58.1, P < 0.05, P < 0.01), liver weight (93.81 ± 6.64, 104.6 ± 4.42 vs 54.4 ± 1.71, P < 0.01), Lg (ALT) (1.9 ± 0.29, 1.84 ± 0.28 vs 1.60 ± 0.17, P < 0.01), and Lg (TG) (1.03 ± 0.24, 1.16 ± 0.33 vs 0.00 ± 0.16, P < 0.01). Weight gain was much more in model group B than in model group A (1360.5 ± 107.6 vs 1097.2 ± 72.3, P < 0.05). But, there was no significant difference between the two groups concerning the other indexes. Pro-inflammatory cytokines (IL-6 and TNF-α) increased in model group B compared with that of control and model group A (IL-6: 1.86 ± 0.21 vs 1.41 ± 0.33, 1.38 ± 0.42, P < 0.01; TNF-α: 1.18 ± 0.07 vs 0.66 ± 0.08, 0.86 ± 0.43, P < 0.01, P < 0.05), whereas serum adiponectin and IL-10 decreased in model groups compared with that in the control (adiponectin: A: 21.87 ± 4.84 and B: 21.48 ± 4.60 vs 27.36 ± 7.29, P < 0.05. IL-10: A: 1.72 ± 0.38 and B: 1.83 ± 0.39 vs 2.26 ± 0.24, P < 0.01). Lg (TC) and the degree of liver fatty infiltration was an independent determinant of serum adiponectin level analyzed by stepwise multiple regressions, resulting in 29.4% of variances.
CONCLUSION: This rabbit model produces the key features of pediatric NASH and may provide a realistic model for future studies. Adiponectin level partially reflects the severity of liver steatosis, but not the degree of liver inflammation.
Keywords: Nonalcoholic steatohepatitis, Pediatric animal model, Adiponectin, Interleukin 6, Tumor necrosis factor
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is recognized as the most common cause of liver disease in pediatrics, paralleling the rapid rise in prevalence of obesity in children globally. Cases have been reported in obese children with liver steatosis and fibrosis by liver biopsy[1,2]. The study of the pathogenic or therapeutic factors involved in childhood nonalcoholic steatohepatitis (NASH) has been hampered by the absence of a suitable experimental model. The existing models were either using rats with a genetic defect[3], or lack of pathogenic factors such as cytochrome P4502E1 (CYP2E1) in which rats were required to be treated for a long time (up to 1 year)[4], and were fed with a diet lacking choline and methionine[5], creating a nutritional deficiency that is not common in patients with NASH. High fat diet-induced NASH may be a good model close to human conditions[6]. Rats have very short pre-pubertal stage and soon develop into adulthood in one month, therefore, it is not an ideal animal model to reflect the physiological and pathological state of children. However, the current theories about the pediatric NASH were all from the adult rats or from the clinical studies. Rabbits have at least 8-mo pre-pubertal stage and are supposed to be more ideal for mimicking pediatric NASH. Rabbit NASH animal model was only seen in Otogawas’ research[7], but it did not investigate the pediatric NASH as its primary goal.
Adiponectin, one kind of adipocytokine, is known to modulate insulin effects. In the liver, it increases the sensitivity of insulin to inhibit gluconeogenesis and regulates hepatic nonesterified fatty acid (NEFA) metabolism via activation of NEFA oxidation and suppression of lipogenesis[8–10]. In our previous study and other clinical studies, serum adiponectin levels were inversely associated with body mass index (BMI) and NAFLD, and were positively associated with high-density lipoprotein (HDL)-cholesterol levels, which suggested that adiponectin might be a protective factor in NAFLD in obese children[11,12]. As in nonalcoholic steatohepatitis, endotoxins and certain endotoxin-inducible cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-10, are also thought to play a role in the process of NASH[13]. In order to further understand the role of adiponectin in the process of childhood NASH and its relationship with the severity of the disease, we used a high-fat diet induced animal model to evaluate the role of serum adiponectin in the process of NASH, and to evaluate cytokines such as IL-6, IL-10 and TNF-α in the pathologic changes in NASH.
MATERIALS AND METHODS
Animal experiment
Thirty-two specific pathogen-free male New Zealand rabbits with a weight of 827 ± 36 g (mean ± SE) and 4-6 wk old, were obtained from the Experimental Animal Department of Zhejiang University School of Medicine (Hangzhou, China). The animals were divided randomly into three groups, each group matched in body weight and age: (1) the normal group (n = 10) was fed with standard diet for 12 wk; (2) the model group A (n = 11) and (3) model group B (n = 11) were fed a high-fat diet (standard diet + 10% lard + 2% cholesterol) for 8 and 12 wk, respectively. All rabbits were fed freely, given tap water, and kept in a room with controlled temperature (22 ± 1°C) and humidity (65%-70%). All animals received humane care and study protocols comply with the institutional guidelines. At the end of 8 and 12 wk, after one night fasting, rabbits were weighed and blood samples were collected through external jugular vein puncture after general anesthesia by injection of 1 mL/kg 3% sodium pentobarbital into the vein of ears. Rabbits were then sacrificed sequentially by an overdose of sodium pentobarbital injection. Intact livers were taken out of the abdominal cavity and washed with ice-cold 0.9% saline. The weight of livers was measured. Hepatic index was calculated (wet weight of liver/body weight). The right lobes of the livers were dissected out and pieces soaked in 10% neutral buffered formalin for histological observation by HE, red-oil and Masson trichrome staining. The diagnosis of NASH and classification of severity were made according to the criteria of Chinese Medical Association Committee of Nonalcoholic Fatty Liver Disease in the year of 2006[14].
Serum biochemical analysis
Serum transaminase (ALT and AST) activities, total cholesterol (TC), and triglyceride (TG) were determined on the Backman CX4 Automatic Biochemistry Analyzers using kits from Sigma. Serum adiponectin, IL-10, IL-6 and TNF-α were detected by commercially available ELISA kit (DiaClone, France).
Determinations of oxidative stress levels
The liver portions were homogenized in ice-cold 0.15 mol/L KCl. The degree of lipid peroxidation in the liver was assessed by measuring malondialdehyde (MDA) levels using the thiobarbituric acid (TBA) method, and superoxide dismutase (SOD) activities using the xanthine oxidase method according to the manufacturers’ instructions. The assay kits for determining MDA and SOD were from Jiancheng Bioengineering Institute (Nanjing, China). Protein levels were determined by Coomassie brilliant blue, using bovine serum albumin as a standard.
Classification of the degree of liver inflammation and steatosis
Formalin-fixed and paraffin-embedded livers were processed routinely for hematoxylin and eosin, red-oil and Masson trichrome staining. Lobular inflammatory activity and severity of liver steatosis were determined according to the criteria of Chinese Medical Association Committee of Fatty Liver Disease in 2006 and Nouchi et al[15,16]. The inflammatory scores were leveled as Grade 1: focal collections of mononuclear inflammatory cells; Grade 2: diffuse infiltrates of mononuclear inflammatory cells; Grade 3: focal collections of polymorphonuclear cells in addition to mononuclear cell infiltrates; and Grade 4: diffuse infiltrates of polymorphonuclear cells in the parenchymal area or lobular area. Severity of liver steatosis: Grade 1: < 30%; Grade 2: 30%-50%; Grade 3: 51%-75%; and Grade 4: > 75% under the low power microscope detection. Hepatocellular ballooning, Grade 0: absent; Grade 1: mild; Grade 2: marked. Perisinusoidal fibrosis, Grade 0: absent; Grade 1: up to 33%; Grade 2: 34%-66%; and Grade 3: > 66%. Overall fibrosis, Stage 1: none; Stage 2: either pericentral/pericellular or focal portal fibrosis; Stage 3: bridging fibrosis (central to central/central to portal); and stage 4: cirrhosis.
Statistical analysis
Statistical analyses were conducted using SPSS software (version 10.0). Quantitative data were presented as mean ± SD. Skewed distribution data were first logarithm transformed and tested for normality of distribution by examination of skewness and kurtosis. The statistical significance between means was estimated by one-way ANOVA followed by LSD multiple comparisons or an unpaired Student’s t test where appropriate. Stepwise multiple linear regression models were used to examine the determinant of serum adiponectin. In the analysis of histological grading, nonparametric tests (Kruskal-Wallis H test) were used. A two-tailed P < 0.05 was considered statistically significant.
RESULTS
Twenty-two male New Zealand rabbits were fed with a high-fat diet (standard diet + 10% lard + 2% cholesterol). All animals developed NASH at the end of 8 and 12 wk, according to the criteria of NASH diagnosis. Liver histology showed different grades of inflammation marked with different degree of mononuclear and polymorphonuclear cell infiltration, hepatocellular ballooning, and some showed portal and (or) perisinusoidal fibrosis.
Effects of fat diet on body weight, liver weight, weight gain and liver index
All rabbits completed the 8 wk or 12 wk experiment. There were no significant differences in the initial body weight among the three groups (P > 0.05). At the end of 8 wk or 12 wk, the liver weight and liver index (liver weight/body weight) were all significantly higher than that in control, but no difference was found between the two fat diet groups. Body weight gain (weight gain = body weight before death-initial body weight) increased stepwise in the control, group A (8 wk) and group B (12 wk) (P < 0.05) (Table 1).
Table 1.
Comparison of indexes among three groups
| Control (n = 10) | Group A (n = 11) | Group B (n = 11) | |
| BW (g) | 809.0 ± 58.0 (625-1067) | 973.0 ± 86.39 (545-1350) | 816.1 ± 67.5 (545-1200) |
| WG (g) | 928.0 ± 58.1 (570-1268) | 1097.2 ± 72.3a (779.0-1485) | 1360.5 ± 107.6c (537-1795) |
| LW (g) | 54.4 ± 1.71 (47-67.5) | 93.81 ± 6.64b (62.2-121.9) | 104.6 ± 4.42b (74.4-123.5) |
| Liver index | 0.032 ± 0.001 (0.02-0.04) | 0.045 ± 0.002a (0.03-0.06) | 0.051 ± 0.005a (0.04-0.1) |
| Adiponectin (μg/L) | 27.36 ± 7.29 (18.14-39.55) | 21.87 ± 4.84a (12.12-28.47) | 21.48 ± 4.60a (16.71-32.16) |
| ALT/AST | 1.00 ± 0.62 | 0.73 ± 0.38 | 0.70 ± 0.1 |
| Lg (ALT) | 1.60 ± 0.17 | 1.9 ± 0.29b | 1.84 ± 0.28b |
| Lg (AST) | 1.67 ± 0.18 | 2.08 ± 0.31b | 2.15 ± 0.30b |
| Lg (TC) | 0.24 ± 0.16 | 1.56 ± 0.46b | 1.56 ± 0.96b |
| Lg (TG) | 0.00 ± 0.16 | 1.03 ± 0.24b | 1.16 ± 0.33b |
| Lg (IL-10) | 2.26 ± 0.24 | 1.72 ± 0.38b | 1.83 ± 0.39b |
| Lg (IL-6) | 1.41 ± 0.33 | 1.38 ± 0.42 | 1.86 ± 0.21bd |
| Lg (INF-α) | 0.66 ± 0.08 | 0.86 ± 0.43 | 1.18 ± 0.07bc |
Group A: 8 wk model; Group B: 12 wk model. Compared with control,
P < 0.05,
P < 0.01. Compared with group A,
P < 0.05,
P < 0.01. BW: Body weight; WG: Weight gain; LW: Liver weight.
Serum biochemical parameters
Serum transaminase (ALT, AST) and lipids (TG, TC) elevated remarkably, while serum adiponectin and IL-10 decreased significantly in two model groups, compared with the control. No difference was found between the two model groups. Pro-cytokines (IL-6 and TNFα) increased significantly in group B, compared either with group A or the control group (P < 0.01), but no difference was found between group A and control group. There was no difference in ALT/AST among all three groups (Table 1).
Determinations of oxidative stress levels and histological examinations
MDA levels and SOD activities were significantly increased in groups A and B, compared to the control group (Table 2). Histological examinations showed normal liver or mild microvesicular steatosis without inflammatory infiltration or fibrosis in the normal group. Mild to severe macro- and micro-vesicular steatosis, infiltration of mild lobular mixed neutrophilic and mononuclear cells, even focal necrosis, perisinusoidal and portal fibrosis, severe hepatocellular ballooning were observed in the 8 wk or 12 wk model groups (P < 0.01). The liver index, hepatic cell steatosis, inflammatory activity score and overall fibrosis stage were all significantly higher in the two model groups than in the control group. Liver steatosis and inflammatory infiltration were more severe in model group B than in model group A (P < 0.05), but no difference was found in other indexes between the two model groups (P > 0.05) (Table 3, Figure 1 and Figure 2).
Table 2.
Hepatic malondialdehyde (MDA) levels and hepatic superoxide dismutase (SOD) activities among three groups
| Group | MDA (nmol/mg protein) | SOD (U/mg protein) |
| Control | 2.73 ± 0.45 | 36.39 ± 5.2 |
| Group A | 4.84 ± 0.72b | 39.08 ± 3.5b |
| Group B | 5.51 ± 0.91b | 41.28 ± 3.2b |
P < 0.01 compared with control. There is no difference between group A and group B.
Table 3.
Comparison of histopathology among three groups
| Group | 0 | 1 | 2 | 3 | 4 | χ2 | |
| Steatosis | Control | 10 | 0 | 0 | 0 | ||
| Model A | 0 | 1 | 3 | 7 | 17.6b | ||
| Model B | 0 | 1 | 1 | 9 | 18.4b 7.51d | ||
| Inflammatory score | Control | 10 | 0 | 0 | 0 | ||
| Model A | 3 | 3 | 1 | 4 | 17.3b | ||
| Model B | 2 | 4 | 0 | 5 | 17.2b 4.9c | ||
| Cytologic ballooning | Control | 9 | 1 | 0 | 0 | 0 | |
| Model A | 0 | 5 | 6 | 0 | 0 | 10.8b | |
| Model B | 0 | 3 | 8 | 0 | 0 | 11.1b | |
| Perisinusoidal fibrosis | Control | 10 | 0 | 0 | 0 | 0 | |
| Model A | 0 | 8 | 3 | 0 | 0 | 17.9b | |
| Model B | 0 | 7 | 4 | 0 | 0 | 17.6b | |
| Overall fibrosis | Control | 10 | 0 | 0 | 0 | ||
| Model A | 5 | 6 | 0 | 0 | 17.5b | ||
| Model B | 4 | 7 | 0 | 0 | 17.6b |
Compared with control, aP < 0.05,
P < 0.01. Compared with model A,
P < 0.05,
P < 0.01.
Figure 1.
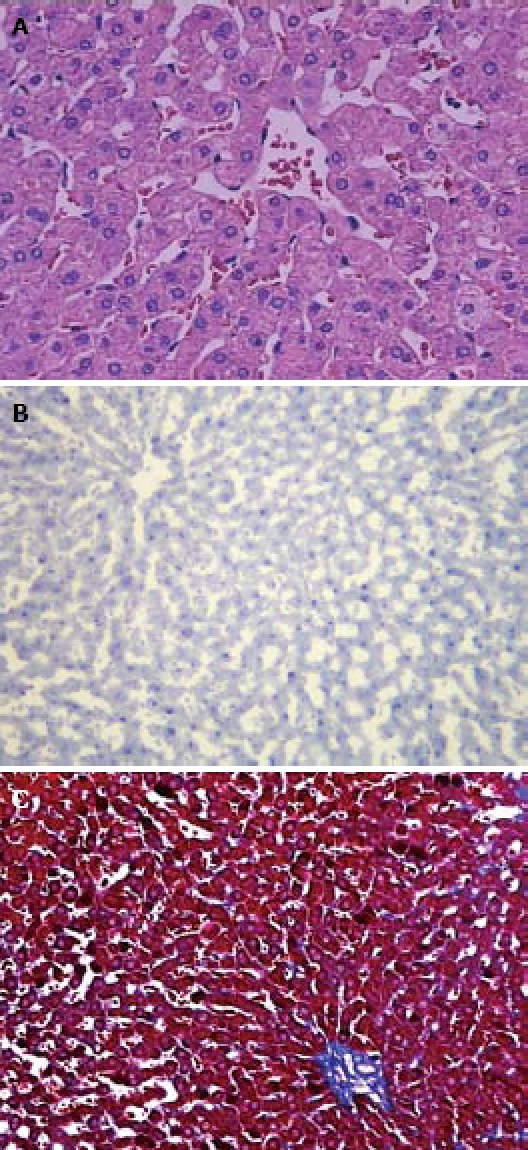
The rabbits fed with the standard diet. A: Normal liver cells without vacuolar degeneration and no distinct inflammatory cell infiltration by HE staining (× 400); B: No positive fat deposition by red-oil staining (× 100); C: Normal liver cells without apparent collagen fibrosis by Masson trichrome staining (× 100).
Figure 2.
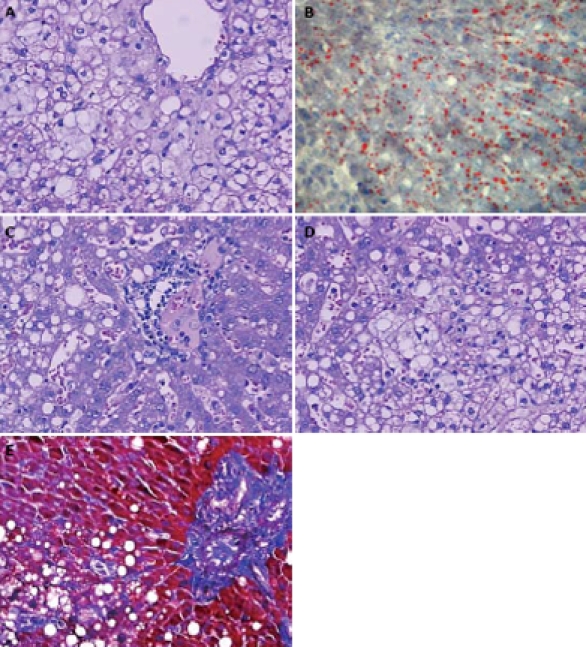
The rabbits fed with the high-fat diet. A: Pronounced hepatic steatosis. Hepatocellular ballooning with clear vacuolar degeneration was apparent by HE staining (× 400); B: Pronounced hepatic steatosis with positive fat infiltration by red-oil staining (× 200); C: Abundant mononuclear and polymorphonuclear inflammatory cells infiltrates by HE staining (× 400); D: Pieces necrosis by HE staining (× 400); E: Significant collagen and reticulin fibrosis among hepatic cells in blue color by Masson trichrome staining (× 200).
Association between serum adiponectin and other factors
Serum adiponectin levels were inversely associated with liver steatosis (r = -0.303, P = 0.046), Lg (TC) (r = -0.424, P = 0.008), Lg (TG) (r = -0.33, P = 0.03), Ig (TNF-α) (r = -0.412, P = 0.01) and liver index (r = -0.313, P = 0.04) (Table 3), but not related to the degree of liver inflammatory infiltration, wet liver weight, Lg (ALT), Lg (AST), Lg (IL-10) and Lg (IL-6). The degree of liver cell steatosis and Lg (TC) were independent determinants of serum adiponectin levels analyzed by stepwise multiple regression analysis, which resulted in a 29.4% variance (Tables 4 and 5).
Table 4.
Pearson correlation of adiponectin with other variants
| Liver steatosis | Lg (TC) | Lg (Tg) | Lg (TNF-α) | Liver index | |
| Adiponectin | -0.303 | -0.424 | -0.33 | -0.412 | -0.313 |
| P value | 0.046 | 0.008 | 0.03 | 0.01 | 0.04 |
Table 5.
Degree of liver cell steatosis and Lg (TC) by stepwise multiple regression analysis
| B | t | P | R2 | |
| Constant | 27.890 | 14.322 | 0.000 | |
| Lg (TC) | -1.333 | 2.978 | 0.006 | |
| Liver steatosis | -0.97 | 2.167 | 0.038 | 0.294 |
DISCUSSION
By feeding rabbits a high-fat diet, we produced the typical hepatic lesions of NASH. The distinctive morphological histological features of NASH included steatosis and lobular inflammation, which contained polymorphonuclear leukocytes and perisinusoidal fibrosis in zone 3 of the acinus. Other common features were also found such as hepatocellular ballooning and poorly formed Mallory’s hyaline. Moreover, the MDA levels and SOD activities were significantly increased in the model groups demonstrating oxidative stress in the pathogenesis of NASH. Together with elevation of serum transaminases and lipids, we successfully established an animal model of pediatric NASH. Model A is not significantly different from model B in serum biochemical analysis and main histological changes.
Adiponectin is much implicated in the pathogenesis of NAFLD/NASH. We found that serum adiponectin was much lower in the model groups as the pathologic changes showed much deteriorated stetosis, inflammatory infiltration and overall fibrosis in the latter. It was in agreement with our previous study and other studies that serum adiponectin values were lower in NASH patients than in non-NASH persons, and hepatic expression of adiponectin and its type II receptor were also less in NASH than those in fatty liver[17–19]. Furthermore, reduced adiponectin levels were associated with increased fat content and extensive necroinflammation in NAFLD patients[20]. In our models, adiponectin was inversely associated with liver steatosis, Lg (TC), Lg (TG) and liver index, but not related to the degree of liver inflammatory infiltration. Stepwise multiple regression analysis showed that the degree of liver cell steatosis and Lg (TC) was independent determinants of serum adiponectin levels, which resulted in a 29.4% variance. These coincide with other studies. Available evidence from cross-sectional studies suggests that adiponectin levels in human are strictly associated with the amount of centrally located fat[21,22]. And clinical imaging studies also support a physiological link between adiponectin and liver fat accumulation[23,24]. Hypoadiponemia may predict a high percentage of hepatic fat content, but not the score of necroinflammation or fibrosis. The actions of adiponectin on the liver are supposed to oppose fatty acid synthesis and promote mitochondrial β-oxidation, which are thought to exert through activation of the cyclic-AMP dependent protein kinase (AMPK)[25]. Whether a single fasting adiponectin value can reliably distinguish an individual patient with NASH from someone with only steatosis or early fibrosis needs to be evaluated in the future studies.
Adiponectin has anti-inflammatory properties in the liver, and its deficiency might account for high aminotransferase and liver disease progression. However, we could not confirm a direct association between adiponectin and ALT. Our study does not support the findings of a recent pediatric study which demonstrated a correlation between ALT and adiponectin in obese subjects with or without abnormal ALT[26]. This suggests that although adiponectin has an anti-inflammatory effect, it cannot predict the inflammatory severity and deterioration of aminotransferase in NASH. In NASH, oxidative stress causes various types of functional and structural damage and frequently increases proinflammatory cytokine production. Serum levels of TNF-α and IL-6 increased stepwise in the control and model groups, while adiponectin and IL-10 markedly decreased in the two model groups. This is in agreement with the studies that cytokines producing cells in ob/ob livers are Th1 polarized, while Th2 anti-inflammatory cytokines were down-regulated[27]. This may be also due to the loss of the protective effect of adiponectin since it was found to have anti-inflammatory effects by opposing the synthesis and release of TNF-α from macrophages within adipose tissue in obesity[28]. Adiponectin may also have the effect of inducing the regulatory T cells in its tolerate state by mainly producing the Th2 cytokine of IL-10. Thus the imbalance of protective and proinflammatory cytokines probably contributes to the histological inflammation and steatosis in NASH. Moreover, some studies have shown that administration of exogenous adiponectin reverses experimental forms of NAFLD and steatohepatitis[29]. However, the cross talk among the immune cells (especially T cells), cytokines and endocrine cells need to be clarified in the future studies.
In conclusion, the pediatric rabbit NASH model has been successfully produced in this study, which may provide a realistic model for future studies. Adiponectin level partially reflects the severity of liver steatosis, but not the degree of liver inflammation. The imbalance of anti-inflammatory cytokines (adiponectin, IL-10) and proinflammatory cytokines (TNF-α, IL-6) may contribute to the histological process in NASH.
COMMENTS
Background
The study of the pathogenic or therapeutic factors involved in childhood nonalcoholic steatohepatitis (NASH) has been hampered by the absence of a suitable experimental model. The existing models were either using rats with a genetic defect, or lack of pathogenic factors such as cytochrome P4502E1 (CYP2E1), or involved feeding rats a diet lacking choline and methionine, which all were not the natural course of developing NASH in patients. High fat diet-induced NASH may be a good model close to human conditions. Rabbits have at least an 8-mo pre-pubertal stage and are supposed to be more ideal for mimicking peadiatric NASH. Adiponectin, one kind of adipocytokine, was known to modulate insulin effects. But its role in the process of peadiatric NASH and its relationship with the severity of the disease is not clear.
Research frontiers
Nonalcoholic fatty liver disease is now recognized as the most common cause of liver disease in pediatrics, paralleling the rapid rise in prevalence of obesity in children globally. Most studies focus on the mechanisms of NASH such as “second hit theories”, the pathogenic changes in NASH, or efforts on the therapeutic areas. However it is still a “bottle neck” to establish a suitable animal model.
Innovations and breakthroughs
For the first time, an easier and less expensive high-fat diet induced pediatric NASH model has been successfully produced in this study. Moreover, the study of the cytokine adiponectin in the process of the disease showed that it partially reflects the severity of liver steatosis but not the degree of liver inflammation. The imbalance of anti-inflammatory cytokines (adiponectin, IL-10) and proinflammatory cytokines (TNF-α, IL-6) may contribute to the histological process in NASH.
Applications
This high-fat diet induced rabbit NASH model may provide a realistic pediatric NASH model for future studies.
Peer review
Development of a simple and inexpensive rodent model of NASH is an important issue in the study of the etiology of this disease. As a model of NASH, the rabbit model looks promising and this manuscript may represent an important contribution to this field. Another major contribution is the development of fibrosis in a simple model with a high fat diet for 8 wk.
Supported by The funds for programs of Zhejiang Provincial Natural Science, No.Y2080047; Major Programs of Zhejiang Provincial Medical and Health Science and Technology and Chinese Ministry of Health, No.WKJ2008-2-026; Special Major Programs of Zhejiang Provincial Science and Technology, No. 2008c03002-1
Peer reviewer: Carlos J Pirola, PhD, FAHA, Instituto de Investigaciones Medicas A Lanari, Combatientes de Malvinas 3150, Buenos Aires-1427, Argentina
S- Editor Cheng JX L- Editor Ma JY E- Editor Zheng XM
References
- 1.Rashid M, Roberts EA. Nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr. 2000;30:48–53. doi: 10.1097/00005176-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Lavine JE, Schwimmer JB. Nonalcoholic fatty liver disease in the pediatric population. Clin Liver Dis. 2004;8:549–558, viii-ix. doi: 10.1016/j.cld.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Irizar A, Barnett CR, Flatt PR, Ioannides C. Defective expression of cytochrome P450 proteins in the liver of the genetically obese Zucker rat. Eur J Pharmacol. 1995;293:385–393. doi: 10.1016/0926-6917(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 4.Enriquez A, Leclercq I, Farrell GC, Robertson G. Altered expression of hepatic CYP2E1 and CYP4A in obese, diabetic ob/ob mice, and fa/fa Zucker rats. Biochem Biophys Res Commun. 1999;255:300–306. doi: 10.1006/bbrc.1999.0202. [DOI] [PubMed] [Google Scholar]
- 5.Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645–1653. doi: 10.1016/s0016-5085(96)70028-8. [DOI] [PubMed] [Google Scholar]
- 6.Lieber CS, Leo MA, Mak KM, Xu Y, Cao Q, Ren C, Ponomarenko A, DeCarli LM. Model of nonalcoholic steatohepatitis. Am J Clin Nutr. 2004;79:502–509. doi: 10.1093/ajcn/79.3.502. [DOI] [PubMed] [Google Scholar]
- 7.Otogawa K, Kawada N. [Rabbit model for the study of human NASH] Nippon Rinsho. 2006;64:1043–1047. [PubMed] [Google Scholar]
- 8.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 9.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 11.Zou CC, Liang L, Hong F, Fu JF, Zhao ZY. Serum adiponectin, resistin levels and non-alcoholic fatty liver disease in obese children. Endocr J. 2005;52:519–524. doi: 10.1507/endocrj.52.519. [DOI] [PubMed] [Google Scholar]
- 12.Asayama K, Hayashibe H, Dobashi K, Uchida N, Nakane T, Kodera K, Shirahata A, Taniyama M. Decrease in serum adiponectin level due to obesity and visceral fat accumulation in children. Obes Res. 2003;11:1072–1079. doi: 10.1038/oby.2003.147. [DOI] [PubMed] [Google Scholar]
- 13.Larter CZ, Farrell GC. Insulin resistance, adiponectin, cytokines in NASH: Which is the best target to treat? J Hepatol. 2006;44:253–261. doi: 10.1016/j.jhep.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Guidelines for diagnosis and treatment of nonalcoholic fatty liver diseases. Zhonghua Ganzangbing Zazhi. 2006;14:161–163. [PubMed] [Google Scholar]
- 15.Nouchi T, Worner TM, Sato S, Lieber CS. Serum procollagen type III N-terminal peptides and laminin P1 peptide in alcoholic liver disease. Alcohol Clin Exp Res. 1987;11:287–291. doi: 10.1111/j.1530-0277.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 16.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 17.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 18.Targher G, Bertolini L, Zenari L. Hypoadiponectinemia is closely associated with nonalcoholic hepatic steatosis in obese subjects. Diabetes Care. 2004;27:2085–2086. doi: 10.2337/diacare.27.8.2085. [DOI] [PubMed] [Google Scholar]
- 19.Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, Ebenbichler CF, Patsch JR, Tilg H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005;54:117–121. doi: 10.1136/gut.2003.037010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bugianesi E, Pagotto U, Manini R, Vanni E, Gastaldelli A, de Iasio R, Gentilcore E, Natale S, Cassader M, Rizzetto M, et al. Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab. 2005;90:3498–3504. doi: 10.1210/jc.2004-2240. [DOI] [PubMed] [Google Scholar]
- 21.Fishbein MH, Mogren C, Gleason T, Stevens WR. Relationship of hepatic steatosis to adipose tissue distribution in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2006;42:83–88. [PubMed] [Google Scholar]
- 22.Busetto L, Tregnaghi A, De Marchi F, Segato G, Foletto M, Sergi G, Favretti F, Lise M, Enzi G. Liver volume and visceral obesity in women with hepatic steatosis undergoing gastric banding. Obes Res. 2002;10:408–411. doi: 10.1038/oby.2002.56. [DOI] [PubMed] [Google Scholar]
- 23.Burgert TS, Taksali SE, Dziura J, Goodman TR, Yeckel CW, Papademetris X, Constable RT, Weiss R, Tamborlane WV, Savoye M, et al. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab. 2006;91:4287–4294. doi: 10.1210/jc.2006-1010. [DOI] [PubMed] [Google Scholar]
- 24.Sabir N, Sermez Y, Kazil S, Zencir M. Correlation of abdominal fat accumulation and liver steatosis: importance of ultrasonographic and anthropometric measurements. Eur J Ultrasound. 2001;14:121–128. doi: 10.1016/s0929-8266(01)00153-7. [DOI] [PubMed] [Google Scholar]
- 25.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 26.Louthan MV, Barve S, McClain CJ, Joshi-Barve S. Decreased serum adiponectin: an early event in pediatric nonalcoholic fatty liver disease. J Pediatr. 2005;147:835–838. doi: 10.1016/j.jpeds.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 27.Copaci I, Micu L, Voiculescu M. The role of cytokines in non-alcoholic steatohepatitis. A review. J Gastrointestin Liver Dis. 2006;15:363–373. [Google Scholar]
- 28.Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, Yoshimatsu H. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40:177–184. doi: 10.1002/hep.20282. [DOI] [PubMed] [Google Scholar]
- 29.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]


