Abstract
Pollen from cedar and cypress trees is a major cause of seasonal hypersensitivity in humans in several regions of the Northern Hemisphere. We report the first crystal structure of a cedar allergen, Jun a 1, from the pollen of the mountain cedar Juniperus ashei (Cupressaceae). The core of the structure consists primarily of a parallel β-helix, which is nearly identical to that found in the pectin/pectate lyases from several plant pathogenic microorganisms. Four IgE epitopes mapped to the surface of the protein are accessible to the solvent. The conserved vWiDH sequence is covered by the first 30 residues of the N terminus. The potential reactive arginine, analogous to the pectin/pectate lyase reaction site, is accessible to the solvent, but the substrate binding groove is blocked by a histidine-aspartate salt bridge, a glutamine, and an α-helix, all of which are unique to Jun a 1. These observations suggest that steric hindrance in Jun a 1 precludes enzyme activity. The overall results suggest that it is the structure of Jun a 1 that makes it a potent allergen.
Common allergic reactions, including allergic rhinitis and asthma, are initiated when protein or glycoprotein allergens cross-link specific IgE antibody molecules on the surface of mast cells or basophils. However, our understanding of the structural basis of this process is incomplete. For instance, the structures of the receptors that anchor the IgE molecules to cells and the signaling process that ensues after cross-linking are known in some detail. On the other hand, our understanding of the structural requirements for proteins to function as allergens is very limited. It is well known that not all proteins are naturally allergenic, and those that are allergenic belong to a relatively small number of protein families. This suggests that certain structural features or biochemical activities are required for proteins to promote an allergenic response.
We have performed studies of the extremely allergenic glycoproteins from the pollen of the mountain cedar tree, Juniperus ashei, a major cause of seasonal hypersensitivity in the central United States (1). The propensity for cedar allergens to induce IgE antibody responses and mediate allergic reactions is indicated by the finding that approximately half of those who suffer from mountain cedar pollinosis do not have reactions to any other allergens. The pollen of related species are responsible for severe, seasonal allergic diseases in Japan (Cryptomeria japonica (2, 3) and Chamaecyparis obtusa (4)) and Europe (Cupressus arizonica (5) and Cupressus sempervirens (6)). Furthermore, the pollen from Juniperus ashei is cross-reactive with those from other cedars and cypresses (1), suggesting that homologues of the mountain cedar allergen participate in the vigorous allergic responses in diverse geographic regions and human populations.
One of the unresolved questions in immunology is whether the propensity of allergens to induce pathological responses is due to their unique structural features or biochemical activities. Resolving the three-dimensional structures of the cedar allergens and defining their relationship with their biochemical activity and binding to IgE antibodies to form a pathogenic complex on the surface of cells should advance our understanding of the allergic process. In 1999, we isolated and characterized a major mountain cedar allergen, Jun a 11 (7), and found it to be a glycoprotein of 346 amino acid residues with a >80% amino acid sequence identity to the group 1 allergens isolated from the Japanese cedar (2, 3), the Japanese cypress (4), the Arizona cypress (5), the Mediterranean Italian cypress (6), and the North American eastern red cedar (Juniperus virginiana) (8). This high percentage of sequence identity implies that the group 1 cedar allergens have similar tertiary structures that may be responsible for their allergenicity and the extensive cross-reactivities between the cedar pollen allergens.
Jun a 1 has less extensive sequence identity (20–50%) with the Pel and Pnl of microorganisms. The Pels and Pnls depolymerize the cell walls of plants in the presence of Ca2+ ions in a process classically called “maceration” (9, 10). These enzymes injure or destroy fruits by cleaving the α-1,4 glycosidic bond of pectate and pectin, the major components of plant cell walls (11). However, Pnl and Pel are also produced by higher plants where they are thought to promote germination by pollen grains and the ripening (softening) of fruits (12, 13). Despite the relatively low degree of overall sequence identity between the microbial and plant Pel, the residue sequences vWiDH and RXPXXR (uppercase letters indicate identity residues between enzymes, the v and i indicate conserved residues, and X represents any residue) are highly conserved (14). The crystal structures of several Pnl and Pel from the plant microbial pathogens Aspergillus niger, Erwinia chrysanthemi, and Bacillus subtilis have been reported (15–19). None of the crystal structures of the Pnl and Pel have unequivocally identified the vWiDH and RXPXXR sequences as being the active sites of the lyases. Scavetta et al. (20) calculated the pKa values for all of the Pel C arginine groups. All except the first arginine in the RXPXXR sequence (Arg218) had normal values. The crystal structure of the Pel C indicated that the Arg218 was oriented in such a manner to suggest a catalytic role. Scavetta et al. (20) modified the Pel C by an R218K mutation that inactivated the pectolytic activity of the enzyme without affecting the tertiary structure of the enzyme. When this mutant Pel C was complexed with a galacturonopentaose oligosaccharide substrate, the crystal structure showed that the substrate bound in a cleft encompassing the RXPXXR sequence as well as the postulated Ca2+ binding sites (21).
The relationship between the three-dimensional structures of the group 1 cedar pollen allergens and the microbial Pnl and Pel has not been established, because none of the tertiary structures of the cedar pollen allergens had been determined. Our homology modeling studies have suggested that their tertiary structures may be similar (22). However, our pectolytic assays of Jun a 1 and the Jun a 1 homologue from the Japanese cedar pollen, Cry j 1, indicate that there are functional differences. We have not been able to demonstrate pectolytic activity in Jun a 1, and the pectolytic activity of Cry j 1 was very low relative to the Pels from microbial sources.2 As part of our efforts to explain these observations, we initiated a structural investigation.
We recently reported the crystallization of the Jun a 1 allergen (23). We report here the three-dimensional crystal structure of Jun a 1, the first for a cedar pollen allergen and the first putative plant Pel to be determined. Analysis of the crystal structure revealed that the predominate conformation of Jun a 1 is fundamentally identical to that of the microbial Pnl and Pel. The lack of Pel or Pnl activity of purified Jun a 1 provides an opportunity to investigate the structural requirements for the activity of these enzymes. Furthermore, this is the first report of the structure of a protein isolated from a non-microbial source that contains the parallel β-helical motif.
MATERIALS AND METHODS
Crystallization and Native Data Collection
The Jun a 1 allergen was isolated from mountain cedar pollen (J. ashei) collected in northwestern Bexar County, Texas. The allergen was purified using concanavalin A-Sepharose (Amersham Biosciences) chromatography as described previously (7). A preliminary description of the crystallization and preliminary x-ray diffraction data has been published elsewhere (23). In summary, crystals were obtained after 6–7 weeks at 277 K from solutions containing sodium acetate, ammonium acetate, and 23% polyethylene glycol 4000, pH 5.5. The crystals are monoclinic (space group P21) with four molecules in the unit cell (Table I). All native and heavy atom derivative data were collected from crystals cryoprotected with glycerol and rapidly cooled to 100 K in liquid nitrogen for storage and data collection. A 2.5-Å resolution native data set and heavy atom derivative data sets were collected on a MAC-Science DIP 2030 Image Plate or a Bruker SMART2K charge-coupled device using copper Kα x-rays produced by MacScience M06HF rotating anode generators equipped with Bruker Göbel optics. Image processing and data reduction were performed using DENZO and SCALEPACK from the HKL package (24). A 1.7-Å resolution native data set was collected at the Advanced Photon Source 14-BM-C beamline, λ = 0.9 Å. Data collection statistics of all data sets are summarized in Table I.
TABLE I.
Crystallographic data collection and refinement statistics
| Native | Native | K2PtCl4 | UO2(OAc)2 | |
|---|---|---|---|---|
| X-ray source | APS 14-BM-C | MacScience DIP2030H | MacScience DIP2030H | Bruker CCD |
| Wavelength (Å) | 0.9 | 1.5418 | 1.5418 | 1.5418 |
| Resolution (Å) | 1.7 | 2.5 | 2.5 | 2.5 |
| Space Group | P21 | P21 | P21 | P21 |
| a (Å) | 53.6 | 53.4 | 53.5 | 53.6 |
| b (Å) | 115.0 | 113.5 | 112.9 | 113.7 |
| c (Å) | 73.5 | 72.4 | 72.5 | 72.6 |
| β (°) | 95.8 | 96.4 | 96.3 | 96.4 |
| Molecules per a.u.a | 2 | 2 | 2 | 2 |
| Measured reflections | 286,470 | 108,027 | 114,672 | 76,152 |
| Unique reflections | 89,001 | 29,648 | 29,615 | 29,989 |
| Redundancyb | 3.2 (2.0) | 3.7 (3.6) | 3.9 (3.8) | 3.2 (2.4) |
| Completenessb (%) | 91.3 (47.3) | 99.6 (99.9) | 99.9 (100.0) | 80.1 (29.4) |
| I/σb | 14.5 (4.1) | 14.7 (7.5) | 11.6 (5.6) | 12.4 (4.1) |
| Rsymb,c (%) | 5.0 (22.5) | 6.6 (19.2) | 8.5 (27.4) | 6.2 (18.4) |
| Refinement statistics | ||||
| Reflections used | 84,532 | |||
| Rcrystd | 0.193 | |||
| Rfreee | 0.241 | |||
| Dimer | Molecule A | Molecule B | Water | |
| Number of atoms | 5290 | 2645 | 2645 | 701 |
| Average B-factor (Å2) | 19.59 | 18.27 | 20.90 | 33.72 |
| Backbone | 16.05 | 14.88 | 17.22 | |
| Side chain | 23.74 | 21.99 | 24.95 | |
| R.M.S.f deviation (Å) | ||||
| Bond lengths | 0.017 | |||
| Angle distances | 0.025 | |||
| Ramachandran analysis (%) | ||||
| Most favored | 519 (87.7) | 257 (86.8) | 262 (88.5) | |
| Allowed | 67 (11.3) | 37 (12.5) | 30 (10.1) | |
| Generously allowed | 4 (0.7) | 1 (0.3) | 3 (1.0) | |
| Disallowed | 2 (0.3) | 1 (0.3) | 1 (0.3) | |
Asymmetric unit.
Numbers in parentheses are for the highest resolution shell.
Rsym = ∑i∑hkl|Ii(hkl) – 〈I(hkl)〉|/∑hkl〈I(hkl)〉, where Ii(hkl) is the Ith measured diffraction intensity and 〈I(hkl)〉 is the mean of the intensity for the Miller index (hkl).
Rcryst = ∑hkl‖Fo(hkl)| – |Fc(hkl)‖/∑hkl|Fo(hkl)|.
Rfree = Rcryst for a test set of reflections (5%).
Root mean square.
Isomorphous Replacement
Heavy atom derivatives were prepared by soaking the native crystals in mother liquor containing the heavy atom reagent K2PtCl4 or UO2 (NO2)2. Data to 2.5-Å resolution from the PtCl42− and UO2(NO2)2 derivatives were collected as described above for the native 2.5-Å resolution data. The three 2.5-Å resolution data sets were used to solve the structure using the multiple isomorphous replacement procedure in the program SOLVE (25). Four PtCl42− sites and four UO22+ sites were found with a figure of merit of 0.45. Solvent flattening and non-crystallographic symmetry averaging in RESOLVE (25) increased the figure of merit to 0.66.
Phase Improvement and Refinement
The initial Fourier map showed clearly the two molecules in the asymmetric unit. Many of the side chains were readily identifiable. The preponderance of β-structure was clearly visible, thereby establishing the directionality of the coils. However, the connectivity was not evident, and only about one-third of the residues of the two molecules could be built into the model. The high resolution synchrotron data was not isomorphous with the low resolution data. The phases obtained from the 2.5-Å data were extended to 1.7 Å using the SOLVE/RESOLVE programs after rigid body, positional, and B-factor refinements of the 2.5-Å model were made using the program CNS (26). This was followed by several cycles of REFMAC (27)/RESOLVE refinement and automated model building with the figure of merit at each stage being 0.33, 0.59, and 0.74, respectively. A nearly complete model of the Jun a 1 structure was built using XTALVIEW (28). Several cycles of molecular dynamics refinement with simulated annealing using the program CNS were performed and interspersed with model building using XTALVIEW. Further refinement was performed using SHELXL (29) interspersed with model building. The refinement converged at a final R of 0.193 and an Rfree of 0.242 for all data. The final structure consists of 692 amino acid residues in two molecules with 701 water molecules. The refinement statistics are summarized in Table I.
Model Quality
The amino acid geometries, as determined using PROCHECK (30), are normal in each molecule in the asymmetric unit with three exceptions. In each molecule there are two cis-proline residues (224 and 231). One tyrosine residue (235) has φψ angles (~67,−48) that place the residue in the disallowed region of the Ramachandran plot (30). The electron density corresponding to the Pro224, Pro231, and Tyr235 residues is unequivocal in each molecule in the asymmetric unit.
All figures were drawn using PyMOL.3 Solvent accessibility calculations were performed using WhatIF (31).4 Secondary structure alignments were calculated by the method of Krissinel and Henrick (32, 33).5
RESULTS
Overall Structure
Jun a 1 crystallized in the P21 space group with two molecules of Jun a 1 in the asymmetric unit. The structures of both molecules were refined independently and are virtually identical. The root mean square deviation between 311 (90%) of the Cα atoms of the two molecules is 0.2 Å, whereas 35 residues grouped in the various loops have a root mean square deviation of >1.6 Å. The results and discussion which follow apply equally to both molecules, unless stated otherwise.
Jun a 1 is a single chain polypeptide. The right-handed parallel β-helix is the predominant structural motif (Fig. 1). The parallel β-helix conformation of the Jun a 1 is very similar to those of the Pel and Pnl, for which crystal structures have been published and whose coordinates are available from the Protein Data Bank (16, 17, 19, 21, 34, 35) (Fig. 2). The three parallel β-sheets, PB1 (Fig. 2, green), PB2 (red), and PB3 (yellow), are separated by turns, i.e. sequences of residues in random coil conformations of variable lengths. Secondary structural elements are identified as described by Yoder et al. (15). Turn T1, usually two residues long, connects PB1 with PB2. T2 (connects PB2 to PB3) and T3 (connects PB3 to PB1 of the next coil) are variable in length. The coils are numbered from the N-terminal end of the parallel β-helix (Fig. 1).
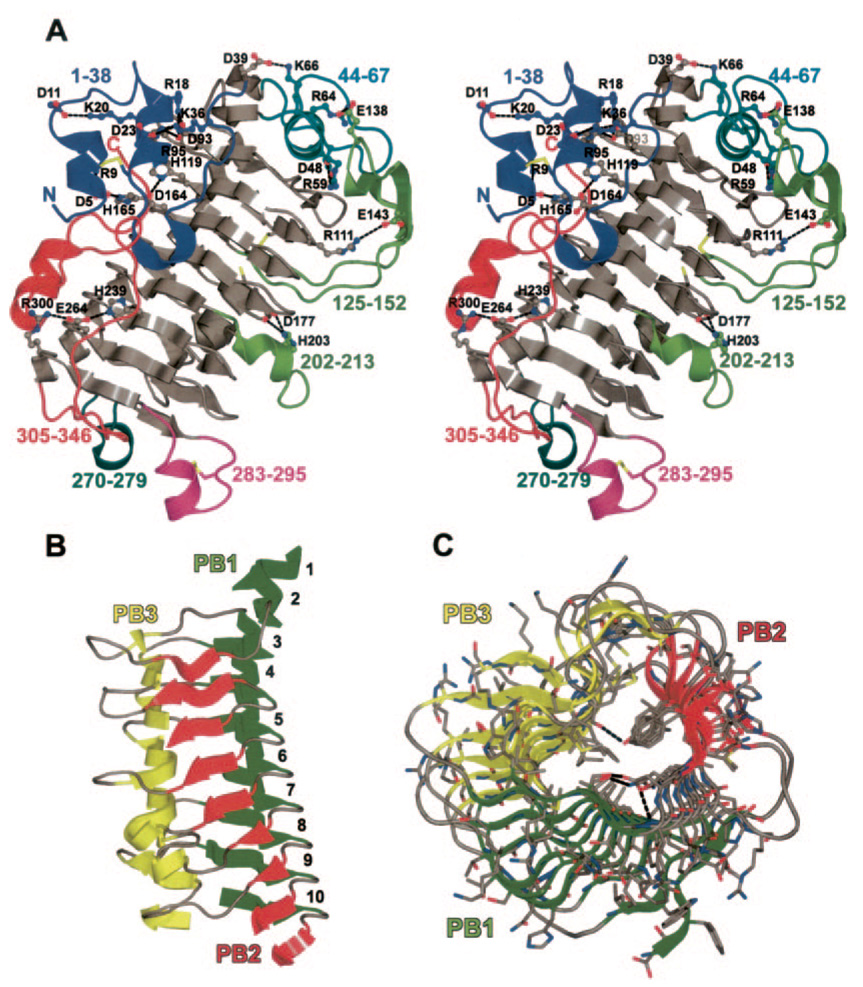
FIG. 1. Structure of Jun a 1.
A, stereo view showing the loops and the salt bridges (ball and sticks) we identified. The color of the numbers and the carbon atoms of the salt bridges refer to the loop of the same color. The three disulfides and free sulfhydryl are shown as sticks with black colored carbons. Single letter amino acid abbreviations are used with position numbers. B, parallel β-helical structure of Jun a 1 with loops removed for clarity. Secondary structures are colored as follows: green, parallel β-helical sheet PB1; red, PB2; yellow, PB3. Numbers refer to the PB sheet strand from the N (top) to C (bottom) termini. C, previous figure rotated 90° about the horizontal axis. The view is from the C terminus toward the N terminus of the β-helical core.
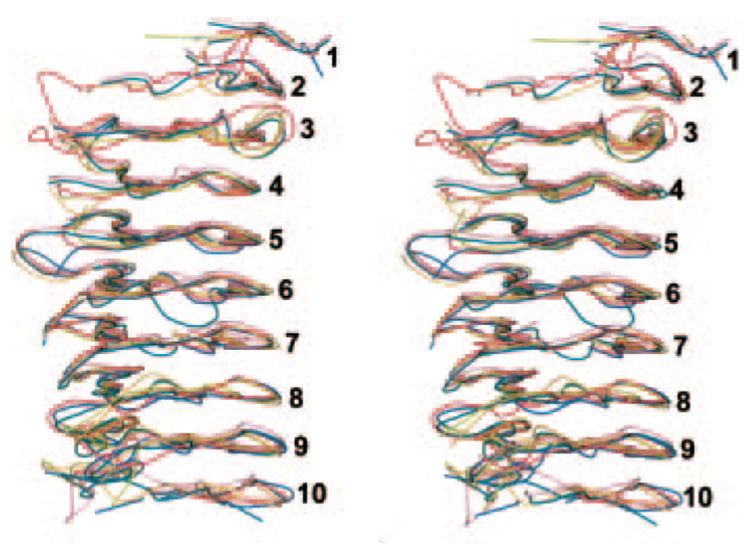
FIG. 2. Stereo view showing similarity of the β-helical cores of Jun a 1 (red) and Pnl A (turquoise), Pnl B (magenta), Pel A (orange), Pel C (marine), and B. subtilis Pel (olive).
The Pels and Pnls are superimposed on the Jun a 1 by their secondary structural elements. Numbers refer to the PB sheet strand from the N (top) to C (bottom) termini. Orientation is similar to that of Fig. 1B.
The Jun a 1 β-helix is shaped much like the two sheet β-helix but with a short β-strand instead of random coils connecting the two β-sheets (36), giving the cross-sectional shape of the β-helical core the appearance of an isosceles triangle with PB1 anti-parallel to PB3. PB1, T1, and PB2 are regular in length. The average φψ angles of the first, (54° and 33°) and second (−91° and 156°) residues in T1 are similar to that of the Pel and Pnl. The result is that the PB2 sheet is pointed in a direction nearly perpendicular to the PB1 sheet. T2 and T3 are less regular in length and planarity and contain all but one of the intra-coil loops. Furthermore, the φψ angles in PB3 vary widely from coil to coil.
Loops
There are five loops of various types (ζ and Ω) (37), motifs, and lengths extending from the β-helical core. Except for the N- and C-terminal loops, the loops invariably begin and end within T2 (Fig. 1). The Thr44-Ala67 loop is an exception, as it begins on coil 1 and ends on coil 2. The closeness of the Cα to Cα distances indicates that the loops do not alter the spatial continuity of the β-helical core.
One loop (Thr44-Ala67) traverses the N-terminal, and one (Ala270-Val279) covers the C-terminal end of the β-helix. These loops preclude the end-to-end binding of other Jun a 1 molecules to the β-helix in the manner described by Richardson and Richardson (38).
Salt Bridges
There are 10 salt bridges in each of the two molecules of Jun a 1 (Fig. 1). Most of these salt bridges are between residues in the parallel β-helix coils and residues in the various loops. Two of the salt bridges are unique to Jun a 1. The salt bridge between Asp5 in the N-terminal helix-turn-helix loop and His165 on T1.5 of the β-helix aids in holding the N-terminal helix-turn-helix loop in place and shields the vWiDH sequence, which is always present in the Pnl and Pel and proposed to be an enzymatically active site (20), from the solvent (Table II). In addition, the salt bridge between Asp177 and His203 traverses the presumed substrate binding groove in the vicinity of the putative active site Arg229.
TABLE II.
Solvent accessibilities (Å2) of the vWiDH sequence side chains of Jun a 1 and the pectate lyases
| PDB identifier | Type | Ala, Ile, or Val | Trp | Ile or Val | Asp | His |
|---|---|---|---|---|---|---|
| 1PXZ | Jun a 1a | 0.17 | 0.00 | 0.52 | 0 | 0.17 |
| 1BN8 | BsPelb | 0 | 5.59 | 0 | 0.42 | 5.27 |
| 1IDK | Pnl Ac | 0 | 2.78 | 0 | 0.67 | 4.33 |
| 1JTA | Pel Ad | 0 | 3.49 | 0.87 | 0.17 | 3.29 |
| 1QCX | Pnl Bc | 0 | 3.48 | 0.17 | 0.98 | 12.53 |
| 1AIR | Pel Cd | 0 | 14.85 | 0 | 0.63 | 0.87 |
| 1PCL | Pel Ed,e | 13.98 | 13.80 | 14.68 | 11.71 | 8.04 |
Source organism, Juniperus ashei.
Source organism, Bacillus subtilis.
Source organism, Aspergillus niger.
Source organism, Erwinia chrysanthemi.
1PCL Pel E structure is Cα only.
Stacks
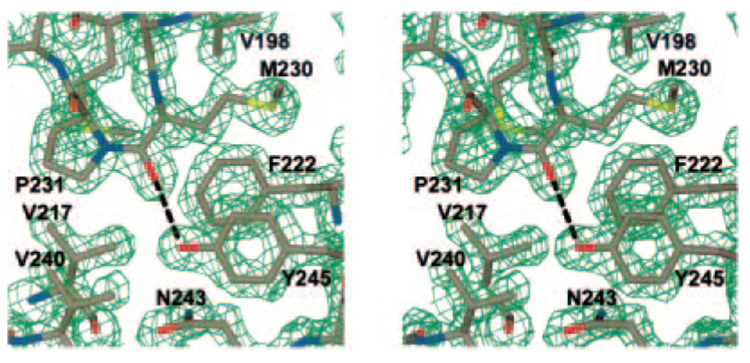
The Pnl and Pel structures are also characterized by their unique stacks of asparagine, aliphatic, and aromatic residues in the interior of the β-helical core. Similar stacks are found in Jun a 1 in the same relative locations in the interior of the β-helical core (Fig. 1). The aromatic stack of Jun a 1 has a tyrosine in the middle; however, the non-polar character of the interior is maintained by a hydrogen bond between the Oη of Tyr245 and the oxygen of Met230 (Fig. 3). The significance of this unique interaction for any potential Pel and Pnl activity of Jun a 1 is discussed below.
FIG. 3. Stereo view of the 2Fo – Fc map of the β-helical strand (PB3.7-PB2.8) showing the cis-Pro231 configuration and the internal hydrogen bond (black dashed line) between Tyr245 and Met230 positioned by the cis-Pro231.
Also shown are residues of the aliphatic stack (Val217 and Val240), the asparagine stack (Asn243), and the aromatic stack (Phe222 and Tyr245). Electron density is contoured at the 2 σ level. Single letter amino acid abbreviations are used with position numbers.
The asparagine and aliphatic stacks are very similar to those found in the Pel and Pnl. However, there are two serines (Ser263 and Ser298) on the C-terminal end of the aliphatic stack with their side chains inside the β-helical core. These two serines interact with the asparagine stack, which is formed by the asparagine usually present as the second residue of T1.4 through T1.10. The second residue in T1 is invariably an Asn, which forms a stack in the interior of the β-helix. The one exception is Asp301 of T1.10, which forms a hydrogen bond with the Oγ of Ser298. This arrangement, along with the hydrogen bond between Asn266 and the Ser263 Oγ, maintains the hydrophobic character of the interior of the β-helical core.
Cysteine
The Jun a 1 structure differs from those of the Pnl and Pel structures in the number and placement of the cysteine residues. Jun a 1 has three disulfide bonds and a free sulfhydryl group, whereas the microbial Pels and Pnls have only one or two disulfide bonds in locations different from those in Jun a 1. Furthermore, the Pels and Pnls do not have a free sulfhydryl.
cis-Proline
There are two cis-prolines in the Jun a 1 structure, Pro224 and Pro231. The cis-Pro224 residue is in the same locale as the cis-Pro231 but does not seem to have any unusual interactions as a result of the cis configuration. On the other hand, cis-Pro231 is invariant in the Pnl and Pel. This residue positions the carbonyl oxygen of Met230 to form a hydrogen bond with the OH moiety of Tyr245. The electron density map quite clearly shows that the cis configuration of Pro231 is correct (Fig. 3). There are further ramifications of this configuration, which will be discussed below.
Dihedral Angles
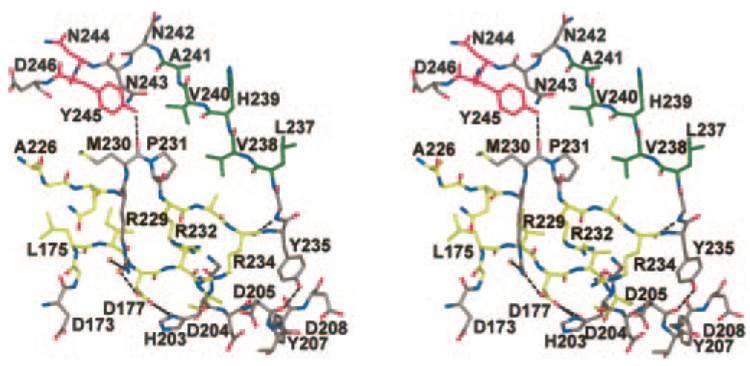
In all of the Pnl and Pel structures published to date, all of the φψ angles in the polypeptide chains are within the normal limits (30). However, in the Jun a 1 structure the φψ angles of Tyr235 (64° and −48°, respectively) place the residue in the disallowed region of the Ramachandran plot below the left-handed helix region. These φψ angles create a βEγ- (or a γβE)-type turn (39) between PB3.7 and PB1.8. The only hydrogen bond between the polypeptide backbone nitrogen and oxygen wholly within this β-coil is formed between the Arg234 oxygen and the Gly236 nitrogen (Fig. 4). As a result, the OH group of Tyr235 is positioned to form a hydrogen bond with the 202–213 loop (T3.6).
FIG. 4. Stereo view of residues around the putative pectolytic site (Asp177, His203, Arg229), showing its relationship with the Tyr245-cis-Pro231 interaction.
Carbon atoms are colored to correspond to the β-pleated sheets (PB3.5, T3.6, PB3.7-PB2.8) depicted in Fig. 1. Single letter amino acid abbreviations are used with position numbers.
Proposed Pectolytic Site
The Pnl and Pel contain two highly conserved sequences, vWiDH and RXPXXR. The biological function of the vWiDH sequence has not yet been determined with certainty. Non-conserved mutations in this sequence in Pel C produced proteins with pectolytic activity, but these proteins were not well exported and remained associated with the bacterial membrane fraction (40). In Jun a 1, Ala is substituted for Val in position 161 of the conserved vWiDH sequence (161–165). Unlike the Pnl and Pel, the aWiDH sequence of Jun a 1 is covered by a complex loop that extends to the N-terminal end of the β-helical core (Fig. 5).
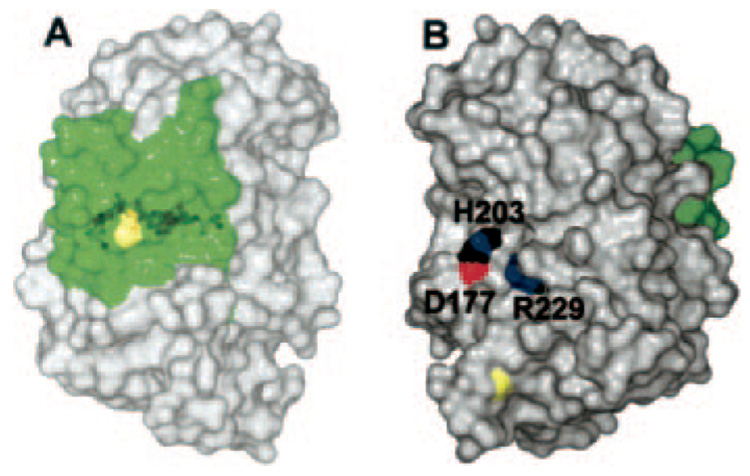
FIG. 5. The surface of Jun a 1 showing the locations of the RXPXXR and aWiDH sites.
A, the aWiDH residues covered by residues 1–30 (green). Yellow depicts the disulfide bond between Cys7 and Cys27. B, view of panel A rotated 90° about the vertical axis showing the potential substrate binding groove around the active site. His203 (H203) and Arg229 (R229) are shown in Corey-Pauling-Koltun colors. Yellow depicts the disulfide bond between Cys285 and Cys291. The aWiDH site is the green area on the right. D177, Asp177.
The RXPXXR sequence has been shown to be required for Pnl or Pel pectolytic activity (40–43). The crystal structure of a pectolytic inactive mutant of Pel C (R218K), with a plant cell wall fragment complexed to the RXPXXR sequence of the protein, confirmed this sequence as the pectolytic site (20). Although Jun a 1 does not exhibit pectolytic activity, it does possess the RXPXXR sequence (229–234), which is located on the side opposite the aWiDH site (Fig. 5). The cis-Pro231 is strictly conserved, as is Arg229. Residues Arg229, cis-Pro231, and Arg234 are part of the intricate interactions involving Tyr245 in the aromatic stack and Tyr235, which has unfavorable φψ angles (Fig. 4). The Tyr245-Met230-Tyr235 complex appears to fashion the proper environment for Arg229 and His203. The cis-Pro231 is strictly conserved, as is Arg229. Arginine 234 is conserved in the Pel, but in the Pnl the equivalent residue is a glutamine. Although Jun a 1 is not enzymatically active, Arg229 is located in the putative enzymatic active site (20, 43) and is also accessible to the solvent (Fig. 6). In addition, the side chain of this putative reactive arginine is extended in the same direction as all of the equivalent arginines in the other Pnl (16, 35) and Pel (17, 19, 21, 34).
FIG. 6. The surfaces of Jun a 1 (wheat) aligned with the R218K mutant of Pel C (violet) according to the secondary structural elements.
A, surface view of the pectolytic site of the Pel C mutant with the tetragalactouronate (yellow sticks, carbons) and Ca2+ ions (light gray spheres). Blue shows the nitrogen surface of the Lys218 Nζ. The surfaces of residues in this area unique to Jun a 1 are not shown. B, same view as in panel A with the surfaces of the residues unique to Jun a 1 included. The nitrogen surfaces of Arg229 of Jun a 1 are shown in blue. Lys197 is located to the left of Arg229 but is not visible in this view. Note that the nonreducing end of the substrate would have to be positioned within the ring of His203 if it were to bind to Jun a 1. Single letter amino acid abbreviations are used with position numbers.
Ca2+
Calcium ions are required for pectinolytic activity by the Pels and Pnls. Some of the residues identified as binding to Ca2+ and the galacturonopentaose oligosaccharide substrate in the Pel C mutant structure are also in the same spatial arrangement in Jun a 1 (32, 33). However, the two Ca2+ binding aspartates (Asp160 and Asp162) present in the Pel C are absent in Jun a 1. These two aspartates are part of a loop (152–164) that begins after the vWiDH site and extends into the edge of the active site (Fig. 6, lower). This loop is not present in Jun a 1, which reduces the potential for Ca2+ binding. Curiously, in Jun a 1 the free sulfhydryl of Cys171 is located near to the region where the Pel C loop would begin if it were present. Ser196, Arg245, and Ser308 bind to the substrate in Pel C, but in Jun a 1 these residues are His203 and Ser256, respectively, and a Ser308 equivalent residue is not present. Thus, it is not likely that Jun a 1 would bind Ca2+. In fact the electron density map does not show evidence of Ca2+ being present. Furthermore, crystals of Jun a 1 soaked in mother liquor solutions containing high concentrations of Ca2+ do not show the presence of Ca2+ in the difference electron density maps.
Water
There are 701 water molecules in the crystal structures of the two Jun a 1 molecules. In both of the Jun a 1 molecules in the asymmetric unit there are three waters in the interior of the hydrophobic β-helical core and four waters buried between the helix-loop-helix peptide (residues 1–30) and the aWiDH region (residues 161–165) exterior to the β-helical core. This arrangement adds stability to the association of the N terminus helix-loop-helix moiety with the aWiDH residues, thereby limiting the access of solvent or potential substrates to the aWiDH residues.
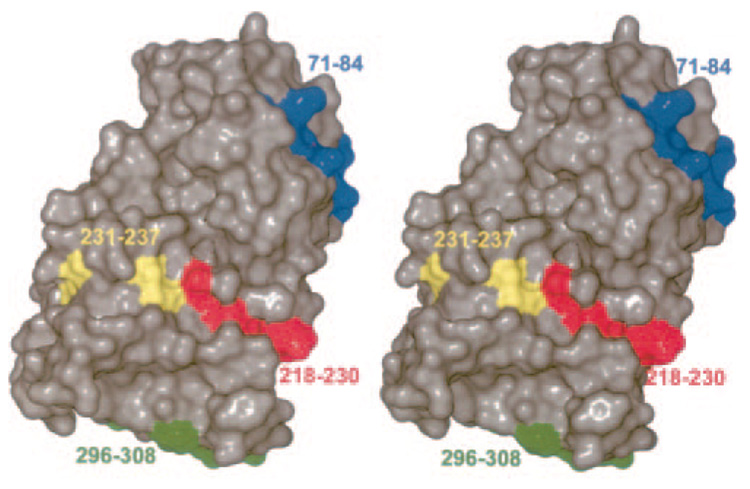
IgE Epitopes
The four sites on the Jun a 1 that have been defined as regions to which IgE antibodies bind are on the surface of the molecule (22) (Fig. 7). The backbone portions of these epitopes are part of the β-helix. Epitopes 1–3 are extended on the surface of the β-helix as a part of single rungs and present a linear appearance. Although epitope 4 is composed of the final rung of the β-helix, its C-terminal moiety is part of the random coil structure that extends across the C-terminal end of the β-helix. The result is that epitope 4 presents a more compact surface. The significance of this observation is not clear.
FIG. 7. Stereo view showing the location of the epitopes.
The view is the same as in Fig. 1A.
DISCUSSION
The predominant motif of Jun a 1 is a parallel β-helix. This is the first description of this type of structure in a higher plant or animal. However, many aspects of the Jun a 1 structure are very similar to those of the microbial Pel and Pnl. The similarity in the structures of Jun a 1 and Pel (17, 19, 21, 34) and the Pnl (16, 35) is particularly striking, given the limited amino acid sequence homology. Secondary structure alignments show that the structural similarity between Jun a 1 and the microbial Pnl A and B and the Pel A, C, and B. subtilis Pel is concentrated in the β-helical cores, where the differences in the distances between the Cα atoms are <0.7 Å (Fig. 2). Furthermore, the asparagine, aliphatic, and aromatic stacks in the interior of the β-helical core of Jun a 1 are in the same relative locations as those of the Pnl and Pel structures (Fig. 1). In contrast, differences in the distances between the Cα atoms that are not in the β-helical core are >1.8 Å, largely because the positions and conformations of the loops of Jun a 1 are quite different from those of the Pnl and Pel loops. This could explain the differences in biological activities between Jun a 1 and the microbial Pnl and Pel enzymes. Such differences could also explain the differences in allergenicity between Jun a 1 and its homologues from other plants (1).
Secondary structure alignments of the microbial Pel and Pnl indicate that the invariant vWiDH sequence is located in a similar region of Jun a 1. However, the aWiDH sequence is covered by a helix-turn-helix moiety not found in the microbial Pel and Pnl. Furthermore, solvent accessibility calculations indicate that unlike their counterparts in the microbial Pnl and Pel, the aWiDH residues are not accessible to the solvent (Table II and Fig. 5). It is interesting to note that the Jun a 1 sequence of the helix-turn-helix moiety is ~90% conserved in the Pel of the higher plants. Whether or not the covering of the aWiDH region is a feature common to the higher plants and is an inhibitor of an as yet unidentified biological activity are matters for future investigation.
The invariant sequence of RXPXX(R/Q), which has been shown to be required for Pnl or Pel activity (40), is also present in Jun a 1 as well as the other plant Pel-like molecules that have been sequenced. The RXPXXR active sites of the microbial Pnl and Pel are in an unobstructed cleft (Fig. 6A). Because the natural substrate is a polysaccharide embedded in a plant cell wall, it follows that a large linear space on the surface is required, as is seen for Pel C and the R218K Pel C mutant. None of the crystal structures of the Pnl and Pel indicate any interactions across the cleft, which could disrupt the concavity of the region.
The equivalent region in Jun a 1 is not as open (Fig. 6B). A comparison of Fig. 6, A and B clearly shows that the galacturonopentaose substrate cannot fit into the Jun a 1 active site. Not only does the His203 side chain occupy the area where the non-reducing terminus of the substrate would bind, but Lys197, Asn249, and Ile250 would also interfere with substrate binding, as would Glu273 and Tyr275 located further along the cavity. Clearly, steric hindrance is the primary reason for the lack of pectolytic activity by Jun a 1.
Homology modeling studies predicted that the four linear IgE epitopes we identified for Jun a 1 would be on the surface of the molecule and accessible to the solvent (22). The three-dimensional structure of Jun a 1 described here confirmed the location of the four linear epitopes on the surface of the molecule. The finding that the Jun a 1 epitopes are part of the β-helical core of Jun a 1 challenges the concept that epitopes are often located in more flexible regions of the allergens. Furthermore, the Jun a 1 structural and enzymatic activity data indicate that pectolytic activity is not a determinant of the allergenicity of the group I plant allergens, nor do these data exclude a role for some unknown biological activity of Jun a 1 and its plant homologues. The structural analysis of Jun a 1 reported here also suggests that both the gross molecular conformation and fine structures of the Pel and Pnl enzymatic sites are highly conserved in plant molecules, although their biological functions remain to be elucidated.
Acknowledgement
We are grateful to Prof. F. Jurnak, University of California, Irvine, for the coordinates of the Pel C R218K mutant complexed with galacturonopentaose as a plant cell wall fragment.
Footnotes
This research was supported by NICHD, National Institutes of Health-sponsored Child Health Research Center New Project Award Grant P30 HD27841 (to T. M.-H.), NIAID, National Institutes of Health Grant R01 AI052428 (to R. M. G.), funds from the Advanced Technology Program from the Texas Higher Education Coordinating Board (to R. M. G.), funds from the Parker B. Francis Fellowship in Pulmonary Research from the Francis Families Foundation (to T. M.-H.), NIEHS, National Institutes of Health Center Grant P30 ES006676, and funds from the Sealy-Smith Foundation (to E. W. C. and M. A. W.). We declare that we have no competing financial interests.
The atomic coordinates and structure factors (code 1PXZ) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
The abbreviations used are: Jun a 1, major allergen from pollen of mountain cedar Juniperus ashei; PB, parallel β-helical sheet; Pel, pectate lyase; BsPel, Pnl, pectin lyase.
E. Czerwinski, T. Midoro-Horuti, M. White, E. Brooks, and R. Goldblum, unpublished observations.
REFERENCES
- 1.Schwietz LA, Goetz DW, Whisman BA, Reid MJ. Ann. Allergy Asthma Immunol. 2000;84:87–93. doi: 10.1016/S1081-1206(10)62746-9. [DOI] [PubMed] [Google Scholar]
- 2.Sato K, Nakazawa T, Sahashi N, Kochibe N. Ann. Allergy Asthma Immunol. 1997;79:57–61. doi: 10.1016/S1081-1206(10)63085-2. [DOI] [PubMed] [Google Scholar]
- 3.Sone T, Komiyama N, Shimizu K, Kusakabe T, Morikubo K, Kino K. Biochem. Biophys. Res. Commun. 1994;199:619–625. doi: 10.1006/bbrc.1994.1273. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki M, Komiyama N, Itoh M, Itoh H, Sone T, Kino K, Takagi I, Ohta N. Mol. Immunol. 1996;33:451–460. doi: 10.1016/0161-5890(95)00147-6. [DOI] [PubMed] [Google Scholar]
- 5.Aceituno E, Del Pozo V, Minguez A, Arrieta I, Cortegano I, Cardaba B, Gallardo S, Rojo M, Palomino P, Lahoz C. Clin. Exp. Allergy. 2000;30:1750–1758. doi: 10.1046/j.1365-2222.2000.00949.x. [DOI] [PubMed] [Google Scholar]
- 6.Ford SA, Baldo BA, Panzani R, Bass D. Int. Arch. Allergy Appl. Immunol. 1991;95:178–183. doi: 10.1159/000235426. [DOI] [PubMed] [Google Scholar]
- 7.Midoro-Horiuti T, Goldblum RM, Kurosky A, Goetz DW, Brooks EG. J. Allergy Clin. Immunol. 1999;104:608–612. doi: 10.1016/s0091-6749(99)70331-3. [DOI] [PubMed] [Google Scholar]
- 8.Midoro-Horiuti T, Goldblum RM, Brooks EG. Clin. Exp. Allergy. 2001;31:771–778. doi: 10.1046/j.1365-2222.2001.01079.x. [DOI] [PubMed] [Google Scholar]
- 9.Barras F, Vangijsegem F, Chatterjee AK. Annu. Rev. Phytopathol. 1994;32:201–234. [Google Scholar]
- 10.Collmer A, Keen NT. Annu. Rev. Phytopathol. 1986;24:383–409. [Google Scholar]
- 11.Pilnik W, Rombouts FM. In: Enzymes and Food Processing. Birch GG, Blakebrough N, Parker KJ, editors. London: Applied Science Publishers Ltd.; 1981. pp. 105–128. [Google Scholar]
- 12.Carpita N, McCann M. In: Biochemistry and Molecular Biology of Plants. Buchanan BB, Gruissem W, Jones RL, editors. Rockville, MD: American Society of Plant Physiologists; 2000. pp. 52–108. [Google Scholar]
- 13.Marin-Rodriguez MC, Smith DL, Manning K, Orchard J, Seymour GB. Plant Mol. Biol. 2003;51:851–857. doi: 10.1023/a:1023057202847. [DOI] [PubMed] [Google Scholar]
- 14.Midoro-Horiuti T, Goldblum RM, Kurosky A, Wood TG, Schein CH, Brooks EG. J. Allergy Clin. Immunol. 1999;104:613–617. doi: 10.1016/s0091-6749(99)70332-5. [DOI] [PubMed] [Google Scholar]
- 15.Yoder MD, Lietzke SE, Jurnak F. Structure. 1993;1:241–251. doi: 10.1016/0969-2126(93)90013-7. [DOI] [PubMed] [Google Scholar]
- 16.Mayans O, Scott M, Connerton I, Gravesen T, Benen J, Visser J, Pickersgill R, Jenkins J. Structure. 1997;5:677–689. doi: 10.1016/s0969-2126(97)00222-0. [DOI] [PubMed] [Google Scholar]
- 17.Thomas LM, Doan CN, Oliver RL, Yoder MD. Acta Crystallogr. Sect. D Biol. Crystallogr. 2002;58:1008–1015. doi: 10.1107/s0907444902005851. [DOI] [PubMed] [Google Scholar]
- 18.Akita M, Suzuki A, Kobayashi T, Ito S, Yamane T. Acta Crystallogr. Sect. D Biol. Crystallogr. 2001;57:1786–1792. doi: 10.1107/s0907444901014482. [DOI] [PubMed] [Google Scholar]
- 19.Pickersgill R, Jenkins J, Harris G, Nasser W, Robert-Baudouy J. Nat. Struct. Biol. 1994;1:717–723. doi: 10.1038/nsb1094-717. [DOI] [PubMed] [Google Scholar]
- 20.Scavetta RD, Herron SR, Hotchkiss AT, Kita N, Keen NT, Benen JA, Kester HC, Visser J, Jurnak F. Plant Cell. 1999;11:1081–1092. doi: 10.1105/tpc.11.6.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoder MD, Keen NT, Jurnak F. Science. 1993;260:1503–1507. doi: 10.1126/science.8502994. [DOI] [PubMed] [Google Scholar]
- 22.Midoro-Horiuti T, Mathura V, Schein CH, Braun W, Yu S, Watanabe M, Lee JC, Brooks EG, Goldblum RM. Mol. Immunol. 2003;40:555–562. doi: 10.1016/s0161-5890(03)00168-8. [DOI] [PubMed] [Google Scholar]
- 23.Liu D, Midoro-Horiuti T, White MA, Brooks EG, Goldblum RM, Czerwinski EW. Acta Crystallogr. Sect. D Biol. Crystallogr. 2003;59:1052–1054. doi: 10.1107/s0907444903005778. [DOI] [PubMed] [Google Scholar]
- 24.Otwinowski Z, Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 25.Terwilliger TC, Berendzen J. Acta Crystallogr. Sect. D Biol. Crystallogr. 1999;55:849–861. doi: 10.1107/S0907444999000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Acta Crystallogr. Sect. D Biol. Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 27.Collaborative Computational Project No. 4. Acta Crystallogr. Sect. D Biol. Crystallogr. 1994;50:760–763. [Google Scholar]
- 28.McRee DE. J. Struct. Biol. 1999;125:156–165. doi: 10.1006/jsbi.1999.4094. [DOI] [PubMed] [Google Scholar]
- 29.Sheldrick GM, Schneider TR. In: Methods in Enzymology: Macromoecular Crystallography, Part B. Carter J, CW, Sweet RM, editors. Vol. 277. San Diego: Academic Press; 1997. pp. 319–343. [Google Scholar]
- 30.Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. J. Biomol. NMR. 1996;8:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez R, Chinea G, Lopez N, Pons T, Vriend G. Comput. Appl. Biosci. 1998;14:523–528. doi: 10.1093/bioinformatics/14.6.523. [DOI] [PubMed] [Google Scholar]
- 32.Krissinel E, Henrick K. In: Proceedings of the 5th International Conference on Molecular Structural Biology, Vienna, September 3–7, 2003. Kungl AJ, Kungl PJ, editors. Vienna: Biochemistry Subgroup of the Austrian Chemical Society; 2003. p. 88. [Google Scholar]
- 33.Boutselakis H, Dimitropoulos D, Fillon J, Golovin A, Henrick K, Hussain A, Ionides J, John M, Keller PA, Krissinel E, McNeil P, Naim A, Newman R, Oldfield T, Pineda J, Rachedi A, Copeland J, Sitnov A, Sobhany S, Suarez-Uruena A, Swaminathan J, Tagari M, Tate J, Tromm S, Velankar S, Vranken W. Nucleic Acids Res. 2003;31:458–462. doi: 10.1093/nar/gkg065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lietzke SE, Yoder MD, Keen NT, Jurnak F. Plant Physiol. 1994;106:849–862. doi: 10.1104/pp.106.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vitali J, Schick B, Kester HC, Visser J, Jurnak F. Plant Physiol. 1998;116:69–80. doi: 10.1104/pp.116.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brèandāen C-I, Tooze J. Introduction to Protein Structure. 2nd Ed. New York: Garland Publishing; 1999. pp. 84–86. [Google Scholar]
- 37.Ring CS, Kneller DG, Langridge R, Cohen FE. J. Mol. Biol. 1992;224:685–699. doi: 10.1016/0022-2836(92)90553-v. [DOI] [PubMed] [Google Scholar]
- 38.Richardson JS, Richardson DC. Proc. Natl. Acad. Sci. U. S. A. 2002;99:2754–2759. doi: 10.1073/pnas.052706099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilmot CM, Thornton JM. Protein Eng. 1990;3:479–493. doi: 10.1093/protein/3.6.479. [DOI] [PubMed] [Google Scholar]
- 40.Kita N, Boyd CM, Garrett MR, Jurnak F, Keen NT. J. Biol. Chem. 1996;271:26529–26535. doi: 10.1074/jbc.271.43.26529. [DOI] [PubMed] [Google Scholar]
- 41.Herron SR, Benen JA, Scavetta RD, Visser J, Jurnak F. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8762–8769. doi: 10.1073/pnas.97.16.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henrissat B, Heffron SE, Yoder MD, Lietzke SE, Jurnak F. Plant Physiol. 1995;107:963–976. doi: 10.1104/pp.107.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lietzke SE, Scavetta RD, Yoder MD, Jurnak F. Plant Physiol. 1996;111:73–92. doi: 10.1104/pp.111.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]