Summary
Background
The Aurora kinases control multiple aspects of mitosis, among them centrosome maturation, spindle assembly, chromosome segregation, and cytokinesis. Aurora activity is regulated in part by a subset of Aurora substrates that, once phosphorylated, can enhance Aurora kinase activity. Aurora A substrate activators include TPX2 and Ajuba, whereas the only known Aurora B substrate activator is the chromosomal passenger INCENP.
Results
We report that the C. elegans Tousled kinase TLK-1 is a second substrate activator of the Aurora B kinase AIR-2. Tousled kinase (Tlk) expression and activity have been linked to ongoing DNA replication, and Tlk can phosphorylate the chromatin assembly factor Asf. Here, we show that TLK-1 is phosphorylated by AIR-2 during prophase/prometaphase and that phosphorylation increases TLK-1 kinase activity in vitro. Phosphorylated TLK-1 increases AIR-2 kinase activity in a manner that is independent of TLK-1 kinase activity but depends on the presence of ICP-1/INCENP. In vivo, TLK-1 and AIR-2 cooperate to ensure proper mitotic chromosome segregation.
Conclusions
The C. elegans Tousled kinase TLK-1 is a substrate and activator of the Aurora B kinase AIR-2. These results suggest that Tousled kinases have a previously unrecognized role in mitosis and that Aurora B associates with discrete regulatory complexes that may impart distinct substrate specificities and functions to the Aurora B kinase.
Introduction
The maintenance of genomic integrity is critically important for the health of a cell and the organism as a whole. Defects in chromosome segregation and cytokinesis can result in harmful aneuploidy that can lead to birth defects, tumorigenesis, and lethality.
The highly conserved Aurora kinases regulate multiple events, including mitotic spindle assembly, chromosome segregation, and cytokinesis, that are required to maintain appropriate ploidy [1]. Whereas yeast harbor a single Aurora family member, metazoans have two distinct family members, Aurora A and B. Mammals have a third member, Aurora C, which most closely resembles Aurora B in sequence and subcellular localization [2, 3]. Aurora A is localized to mitotic centrosomes and spindle microtubules and is required for centrosome maturation [4, 5]. Loss of Aurora A leads to defective mitotic spindles and gross errors in chromosome segregation [5]. Aurora B displays a “chromosomal passenger” localization pattern; it associates with kinetochores from prophase to metaphase and then translocates to the central-spindle microtubules at anaphase [6]. This behavior is evolutionarily conserved and is characteristic of other chromosomal passengers, including INCENP, Survivin, and Borealin/Dasra [6–8]. Aurora B is required for chromosome modification (phosphorylation of histone H3), monitoring functional kinetochore/microtubule interactions, and cytokinesis [6].
A paradigm for the regulation of Aurora kinase activity by Aurora substrates has recently emerged. Two substrate activators of mammalian Aurora A have been described, the LIM domain protein Ajuba [9] and the microtubule-associated protein TPX2 [10–13]. Ajuba is phosphorylated by Aurora A at mitotic entry and subsequently enhances Aurora A kinase activity [9]. Depletion of Ajuba resulted in loss of active Aurora A at centrosomes and failure to enter mitosis [9]. TPX2 is required for spindle assembly and has been shown to target Aurora A to mitotic spindle microtubules [14, 15]. Aurora A phosphorylation of TPX2 increases Aurora A activity by inducing a conformational change in the Aurora A kinase; this change protects an autophosphorylation activation site from dephosphorylation by the PP1 phosphatase [16]. Although the function and localization of Aurora A is highly conserved between vertebrates and invertebrates, there are no identifiable homologs of Ajuba or TPX2 in the Drosophila or C. elegans genomes. The presence or identity of similar Aurora A activators in these organisms is currently unknown.
The activity of Aurora B is enhanced by Aurora-B-mediated phosphorylation of the chromosomal-passenger protein INCENP [17, 18]. INCENP targets Aurora B to chromosomes, central-spindle microtubules, and the cleavage furrow [18–20]. Unlike Ajuba or TPX2, there are clear orthologs of vertebrate INCENP in yeast (Sli15) [21], Drosophila [22], and C. elegans (ICP-1) [20]. INCENP can also activate the Aurora C kinase but does not appear to activate Aurora A [2, 3, 17]. Whether INCENP activates Aurora B and C activity in a manner similar to TPX2 activation of Aurora A is not yet clear.
Given that Aurora kinase activity can be regulated by Aurora substrates and that each Aurora kinase has multiple substrates [1], it is likely that there are additional activating substrates of the Aurora kinases. Here, we report that the C. elegans Tousled-like kinase, TLK-1, can act as a substrate and activator of the C. elegans Aurora B kinase AIR-2. Mutations in the founding member of the Tousled family, Arabidopsis thaliana Tousled, resulted in numerous developmental defects that led to a “tousled” appearance of various tissues [23]. Two human homologs (Tlk1 and Tlk2) were subsequently found to be cell-cycle-regulated kinases that display maximum expression and activity during S phase [24]. Human and Drosophila Tousled kinases can phosphorylate the Asf1 chromatin assembly factor in vitro, and Drosophila Tlk interacts genetically with Asf1 in vivo [25, 26]. Although the molecular consequence of Asf1 phosphorylation by Tlk is unknown, it was recently shown that Tlk activity is inhibited in response to S phase DNA damage via the ATM and Chk1 kinase pathways [27, 28]. Altogether, these results suggest that Tousled kinases may play a critical role in chromatin assembly or remodeling during DNA replication and repair.
We have previously shown that the sole C. elegans Tousled kinase, TLK-1, is a nuclear protein that is most highly expressed during interphase, which is in agreement with reports describing human and Drosophila Tlk expression patterns [24, 26, 29]. Depletion of TLK-1 by RNAi resulted in embryonic lethality characterized by a broad reduction in embryonic transcription [29]. Although a previous report suggested that human Tlk1 directly phosphorylates histone H3 at serine 10 [30], we found no evidence that C. elegans TLK-1 directly affects histone H3 phosphorylation in vitro or in vivo [29]. Instead, we report here that C. elegans TLK-1 is a substrate activator of the C. elegans Aurora B kinase AIR-2, a bona fide histone H3 kinase [31]. Furthermore, we present evidence that TLK-1 and AIR-2 functionally cooperate in vivo to regulate mitotic chromosome segregation. These results suggest that the Tousled kinase has a previously unidentified role in mitosis and that Aurora B activity is regulated via association with distinct substrates.
Results
AIR-2 Physically Interacts with the TLK-1 Kinase
We previously reported that the C. elegans Tousled kinase TLK-1 is required for embryonic viability and transcription [29]. We initially identified TLK-1 in a yeast two-hybrid screen of a C. elegans cDNA library with the Aurora B kinase AIR-2 as the bait. An AIR-2 antibody [32] was used to immunoprecipitate AIR-2 from total C. elegans protein extracts to further examine the potential physical interaction between TLK-1 and AIR-2. TLK-1 coimmunoprecipitated with AIR-2 from wild-type (Figure 1, lane 3) but not tlk-1(RNAi) (Figure 1, lane 5) protein extracts. Although air-2(RNAi) (administered by feeding) did not result in complete loss of AIR-2 protein expression, TLK-1 was not detectable in air-2(RNAi) immunoprecipitates (Figure 1, lane 4). Reciprocal experiments with the TLK-1 antibody [29] did not result in immunoprecipitation of TLK-1 and were not pursued (data not shown). Nevertheless, our coimmunoprecipitation results confirm an in vivo interaction between TLK-1 and AIR-2, as predicted from the yeast two-hybrid assay.
Figure 1. TLK-1 Coimmunoprecipitates with AIR-2 from C. elegans Extracts.
AIR-2 was immunoprecipitated from wild-type (lane 3), air-2(RNAi) (lane 4), and tlk-1(RNAi) (lane 5) embryo extracts, and the immune complexes were subjected to SDS/PAGE and Western analysis with TLK-1- and AIR-2-specific antibodies. Protein G beads in the absence of protein extract were used as a negative control (lane 6).
TLK-1 Is Phosphorylated by AIR-2 In Vitro and In Vivo
TLK-1, AIR-2, and kinase-dead versions of both kinases were expressed and purified as recombinant fusion proteins from E. coli to determine whether the TLK-1 and AIR-2 kinases phosphorylate one another. Kinase-dead versions of each kinase were used as substrates to eliminate autophosphorylation. TLK-1 could undergo autophosphorylation and phosphorylate MYBP (see Figure S1, lane 1, in the Supplemental Data available with this article online), but it did not phosphorylate AIR-2KD (Figure S1, lane 2), suggesting that AIR-2 is not a substrate of the TLK-1 kinase in vitro.
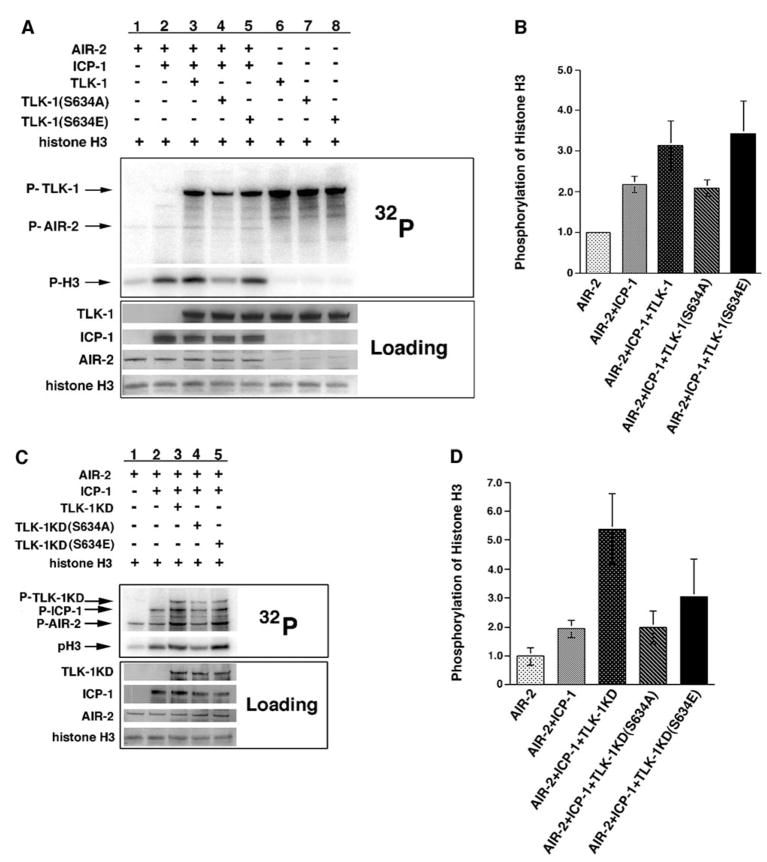
Recombinant AIR-2 underwent autophosphorylation and could phosphorylate ICP-1, a previously identified substrate and activator of the AIR-2 kinase (Figure 2A, lanes 1 and 2) [17, 18, 33]. AIR-2 could not phosphorylate TLK-1KD in the absence of ICP-1 (Figure 2A, lane 3). However, robust AIR-2 phosphorylation of TLK-1KD occurred in the presence of ICP-1 (Figure 2A, lane 4). In addition, a marked increase in AIR-2 autophosphorylation and ICP-1 phosphorylation was seen in kinase reactions with all three fusion proteins (AIR-2, ICP-1, and TLK-1KD) (Figures 2A [lane 4]–2C). This increase in AIR-2 activity will be further addressed below.
Figure 2. AIR-2 Phosphorylates TLK-1 at Serine 634 In Vitro.
(A) AIR-2 was mixed with TLK-1KD in the presence and absence of ICP-1 in kinase buffer supplemented with γ-32P-ATP. 32P incorporation was measured by phosphoimaging, and protein loading was determined by antibody staining. AIR-2 underwent autophosphorylation but did not phosphorylate TLK-1KD in the absence of ICP-1 (lane 3). TLK-1KD was readily phosphorylated by AIR-2 in the presence of ICP-1 (lane 4). AIR-2 autophosphorylation and ICP-1 phosphorylation were increased in the presence of TLK-1KD (compare lanes 2 and 4). ICP-1 and TLK-1KD did not display kinase activity (lanes 5 and 6). * in lanes 2 and 4 denotes phosphorylation of an ICP-1 breakdown product.
(B) Quantitation of AIR-2 autophosphorylation in (A) (lanes 1–4).
(C) Quantitation of ICP-1 phosphorylation in (A) (lanes 2 and 4).
(D) Full-length and various fragments of TLK-1 fused to maltose binding protein (MBP) were assayed for phosphorylation by AIR-2/ICP-1. The full-length TLK-1 protein and all TLK-1 protein fragments except amino acids 651–961 were phosphorylated by AIR-2/ICP-1. Various N-terminal fragments of TLK-1 (from amino acids 1–650) were fused to GST and assayed for phosphorylation by AIR-2/ICP-1 to further map the AIR-2 phosphorylation site. Only TLK-1(454–650) was phosphorylated by AIR-2/ICP-1 in this assay.
(E) AIR-2/ICP-1 was mixed with wild-type TLK-1(454–650) and TLK-1(454–650) in which serine 634 (S634) had been mutated to alanine (S634A) and subjected to kinase assay conditions. 32P incorporation was measured by phosphoimaging, and protein loading was determined by antibody and Ponceau S staining. TLK-1(454–650) was phosphorylated in this assay (lane 2), whereas TLK-1(454–650)(S634A) was not (lane 3).
Various fragments of TLK-1 were expressed and purified as recombinant fusion proteins from E. coli and subjected to phosphorylation by AIR-2/ICP-1 to determine the TLK-1 site that is phosphorylated by AIR-2 in vitro. In TLK-1 fragments that contained the kinase domain, the kinase-dead mutation (D802A) was incorporated to eliminate autophosphorylation. TLK-1 fragments corresponding to amino acids 1–650, 1–800, and 401–961 were phosphorylated by AIR-2/ICP-1, whereas TLK-1 (651–961) was not (Figure 2D). These data placed the AIR-2 phosphorylation site(s) between amino acids 401–650. TLK-1 fusion proteins corresponding to overlapping fragments of the N-terminal 650 amino acids of TLK-1 were expressed and purified from E. coli and incubated under kinase assay conditions with AIR-2/ICP-1 to further map the AIR-2 phosphorylation site. Fragments corresponding to amino acids 1–167, 147–320, and 305–470 were not phosphorylated, whereas fragment 454–650 was readily phosphorylated in this assay (Figures 2D and 2E). Examination of this sequence revealed a motif that loosely matched an Aurora kinase consensus site [17, 34] (Figure 2D). Mutation of TLK-1 Serine 634 to alanine (S634A) abolished AIR-2-mediated phosphorylation of TLK-1 fragment 454–650 (Figure 2E, lanes 2 and 3). However, the S634A mutation did not completely inhibit AIR-2 phosphorylation when incorporated into E. coli fusion proteins corresponding to full-length TLK-1 (data not shown). These results suggest that S634 is phosphorylated by AIR-2, but at least one additional phosphorylation site is present in another portion of the TLK-1 protein.
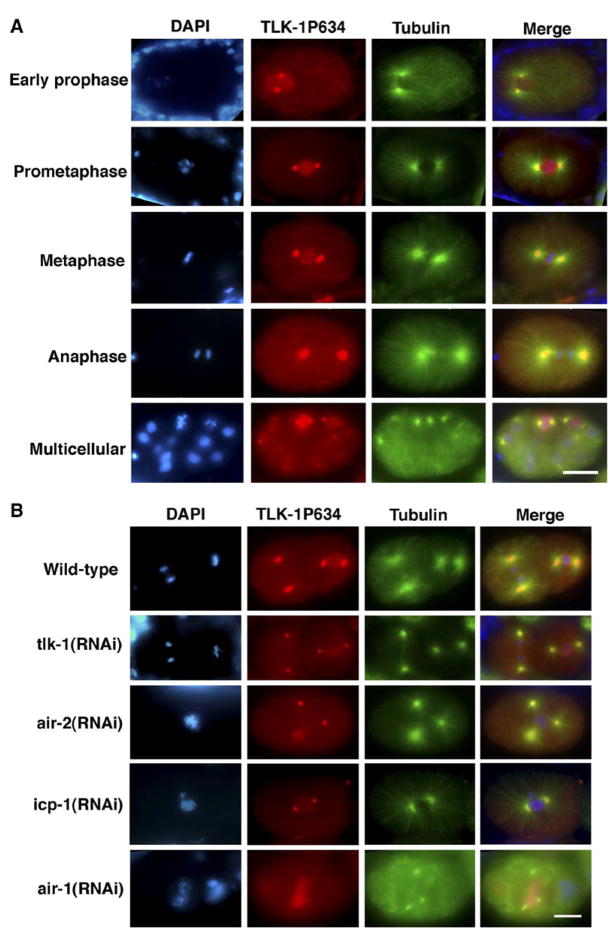
To determine whether TLK-1 S634 is phosphorylated in vivo, we raised a phospho-specific, polyclonal rabbit antibody against a TLK-1 peptide phosphorylated at S634. The antiserum was affinity purified against the TLK-1 phosphopeptide, and ELISA assays confirmed that the purified antibody specifically recognized the phosphopeptide and not the nonphosphorylated peptide (data not shown). Immunostaining of wild-type C. elegans embryos with the purified antibody revealed nuclear staining from early prophase through metaphase (Figure 3A). This staining was dependent on TLK-1 and AIR-2 expression because it was strongly reduced in tlk-1(RNAi) and air-2(RNAi) embryos (Figure 3B). Phospho-TLK-1 staining was also very much reduced in icp-1(RNAi) embryos (Figure 3B), supporting the conclusion that TLK-1 phosphorylation by AIR-2 is greatly enhanced by the presence of ICP-1. Importantly, TLK-1 expression is not dependent on the presence of ICP-1 or the AIR-2 kinase (Figure S2).
Figure 3. In Vivo AIR-2-Dependent Phosphorylation of TLK-1 S634 Is Detectable from Early Prophase through Metaphase.
(A) Wild-type C. elegans embryos were fixed and stained with DAPI (blue) and antibodies specific for α-tubulin (green) and phosphorylated TLK-1S634 (TLK-1P634) (red). Representative 1 cell embryos from early prophase through anaphase of the first mitotic cell cycle and a multicellular embryo are shown. Nuclear and/or chromatin-associated TLK-1P634 staining was present from early prophase through metaphase. Interphase, anaphase, and telophase embryos had much lower levels of nuclear/chromatin-associated TLK-1P634 staining (as seen in the anaphase 1 cell embryo and the multicellular embryo). Centrosome staining appeared to be an artifact and did not correspond to TLK-1 immunostaining (see below). The scale bar represents 10 μm.
(B) Wild-type, tlk-1(RNAi), air-2(RNAi), icp-1(RNAi), and air-1(RNAi) embryos were fixed and stained with DAPI and antibodies specific for α-tubulin and TLK-1P634. Nuclear/chromatin-associated TLK-1P634 staining was dependent on TLK-1, AIR-2, and ICP-1 expression and was not reduced in the absence of the AIR-1 kinase. In contrast, TLK-1P634 centrosome staining was dependent on AIR-1 expression, but not TLK-1, AIR-2, or ICP-1. The scale bar represents 10 μm.
In wild-type embryos, strong mitotic centrosome staining was seen in addition to nuclear staining (Figure 3A). This staining is likely to recognize an antigen other than TLK-1 because centrosome staining is not found with the non-phospho-TLK-1 antibody [29] and was not eliminated in tlk-1(RNAi), air-2(RNAi), or icp-1(RNAi) embryos (Figure 3B). However, we cannot eliminate the possibility that a small fraction of phospho-TLK-1 does localize to centrosomes. Interestingly, the centrosome staining was absent in air-1(RNAi) embryos, raising the intriguing possibility that this centrosome antigen is a direct substrate of the AIR-1 kinase or is dependent on AIR-1 for localization to the centrosome.
TLK-1-, AIR-2-, and ICP-1-dependent nuclear immuno-staining was strongest at prometaphase and persisted as a nuclear “halo” around the metaphase plate despite nuclear-envelope breakdown (Figure 3A). Phospho- TLK-1-specific staining was very low at anaphase and telophase, as well as in interphase cells. These results are in sharp contrast to the high interphase/S phase levels of nuclear TLK-1 staining found with a non-phospho-specific TLK-1 antibody [29]. Altogether, the TLK-1 S634 phospho-specific antibody provides strong evidence that TLK-1 is phosphorylated by the AIR-2 kinase during prophase and prometaphase in vivo.
AIR-2 Phosphorylation of TLK-1 Increases TLK-1 Kinase Activity
Recombinant proteins corresponding to full-length wild-type, S634A mutant, and phospho-mimetic (S634E) mutant TLK-1 were expressed and purified from E. coli to determine the consequence of AIR-2 phosphorylation at TLK-1 S634 with regard to TLK-1 kinase activity. The kinase activity of each TLK-1 protein was examined with MYBP and recombinant ASF-1 as substrates. All three TLK-1 proteins were active kinases in vitro, under-going autophosphorylation and phosphorylating MYBP and ASF-1 (Figure 4A). However, the phospho-mimetic TLK-1(S634E) protein showed consistently higher levels of kinase activity than TLK-1 or TLK-1(S634A) (Figures 4A and 4B).
Figure 4. AIR-2 Phosphorylation Increases the Kinase Activity of TLK-1.
(A) Wild-type TLK-1, TLK-1(S634E), and TLK-1(S634A) were incubated with ASF-1 and MYBP in kinase buffer supplemented with γ-32P-ATP. 32P incorporation was measured by phosphoimaging, and protein loading was determined by antibody and Ponceau S staining. All three TLK-1 fusion proteins underwent autophosphorylation (P-TLK-1) and phosphorylated ASF-1 (P-ASF-1) and MYBP (P-MYBP). However, the relative levels of 32P incorporation differed, with TLK-1(S634E) displaying increased kinase activity with respect to wild-type TLK-1 or TLK-1(S634A).
(B) Quantitation of 32P incorporation into MYBP and ASF-1 in the kinase assays shown in (A). Error bars represent the standard deviation from at least three separate experiments.
(C) Wild-type TLK-1, TLK-1(S634A), and TLK-1(S634E) were incubated with ASF-1 in kinase buffer supplemented with γ-32P-ATP in the presence and absence of AIR-2/ICP-1. All three TLK-1 proteins underwent autophosphorylation (P-TLK-1) and phosphorylated ASF-1 (P-ASF-1) (lanes 2, 4, and 6). However, ASF-1 phosphorylation by wild-type TLK-1 was increased in the presence of AIR-2/ICP-1 (lane 3). Phosphorylation of ASF-1 by TLK-1(S634A) and TLK-1(S634E) did not change in the presence or absence of AIR-2/ICP-1 (lanes 4–7).
(D) Quantitation of 32P incorporation into ASF-1 in the kinase assays shown in (C). Error bars represent the standard deviation from at least three separate experiments.
Phosphorylation of the TLK-1 substrate ASF-1 by wild-type TLK-1, TLK-1(S634A), and TLK-1(S634E) was assayed in the presence and absence of AIR-2/ICP-1 to directly test whether AIR-2 phosphorylation could enhance TLK-1 kinase activity. ASF-1 was not phosphorylated by AIR-2/ICP-1 alone (Figure 4C, lane 1). However, ASF-1 phosphorylation was increased when AIR-2/ICP-1 was added to TLK-1 (Figure 4C, compare lanes 2 and 3, and Figure 4D). This increase was abolished when TLK-1(S634A) was substituted for TLK-1 (Figures 4C, lanes 4 and 5, and 4D). As described above, the TLK-1(S634E) kinase had greater activity against ASF-1 than wild-type TLK-1, but this increase was not augmented by the presence of AIR-2/ICP-1 (Figures 4C, lanes 6 and 7, and 4D). Altogether, these results demonstrate that AIR-2-mediated phosphorylation of TLK-1 at S634 increases TLK-1 kinase activity in vitro.
Phosphorylated TLK-1 Enhances AIR-2 Kinase Activity
Human Tlk1 was previously reported to be a histone H3 kinase [30]. However, we found that C. elegans TLK-1 could not directly phosphorylate histone H3 in vitro, and histone H3 phosphorylation was not affected by loss of TLK-1 expression in vivo [29]. The differing results between human and C. elegans Tousled kinases could be due to species-specific differences, or, alternatively, the histone H3 kinase activity attributed to human Tlk1 could be due to the association of Tlk1 with the Aurora B kinase. Given that our initial AIR-2 kinase assay with TLK-1 suggested that TLK-1 addition might further enhance the activity of the AIR-2/ICP-1 kinase complex (Figure 2A), we examined AIR-2 kinase activity in the presence and absence of ICP-1, wild-type TLK-1, TLK-1(S634A), and TLK-1(S634E) fusion proteins with histone H3 (H3) as a substrate. As a control, the TLK-1 proteins were incubated with H3 in kinase assay buffer without AIR-2/ICP-1. All three TLK-1 proteins underwent robust autophosphorylation but did not phosphorylate H3 (Figure 5A, lanes 6–8). As previously reported, addition of ICP-1 to AIR-2 substantially increased AIR-2 kinase activity, as measured by phosphorylation of histone H3 (Figures 5A [lanes 1 and 2] and 5B) [17, 33]. Addition of wild-type TLK-1 further augmented H3 phosphorylation (Figures 5A [lane 3] and 5B). Interestingly, TLK-1 autophosphorylation appeared to be inhibited by the presence of AIR-2/ICP-1 (Figure 5A, lanes 3–5 versus 6–8). However, we cannot distinguish between AIR-2 phosphorylation and TLK-1 autophosphorylation in this assay. Importantly, TLK-1(S634A) did not enhance H3 phosphorylation (Figures 5A [lane 4] and 5B). In the presence of TLK-1(S634E), H3 phosphorylation was increased to the same extent as addition of wild-type TLK-1 (Figures 5A [lane 5] and 5B). Given that the TLK-1 fusion proteins have no kinase activity against histone H3 on their own, our results suggest that phosphorylation of TLK-1 by AIR-2/ICP-1 (or addition of the phospho-mimic S634E mutation) initiates a feedback loop that augments AIR-2 kinase activity.
Figure 5. Phosphorylated TLK-1 Increases AIR-2 Kinase Activity.
(A) AIR-2 was incubated with histone H3 in the presence and absence of ICP-1 and wild-type TLK-1, TLK-1(S634A), and TLK-1(S634E) in kinase buffer supplemented with γ-32P-ATP. AIR-2 phosphorylation of histone H3 was increased in the presence of ICP-1 (lanes 1 and 2). A further increase in H3 phosphorylation occurred when wild-type TLK-1 or TLK-1(S634E) was incubated with AIR-2/ICP-1 (lanes 3 and 5). Histone H3 phosphorylation did not increase in the presence of TLK-1(S634A) (lane 4). Although all three TLK-1 fusion proteins underwent autophosphorylation, none of them phosphorylated histone H3 in the absence of AIR-2/ICP-1 (lanes 6–8).
(B) Quantitation of histone H3 phosphorylation in the kinase assays shown in (A). Error bars represent the standard deviation from at least three separate experiments.
(C) AIR-2 was incubated with histone H3 in the presence and absence of ICP-1 and kinase-dead versions of TLK-1, TLK-1(S634A), and TLK-1(S634E) in kinase buffer supplemented with γ-32P-ATP. AIR-2 phosphorylation of histone H3 was increased in the presence of ICP-1 (lanes 1 and 2). A further increase in H3 phosphorylation occurred when TLK-1KD or TLK-1KD(S634E) was incubated with AIR-2/ICP-1 (lanes 3 and 5). Histone H3 phosphorylation was not increased in the presence of TLK-1KD(S634A) (lane 4). AIR-2 autophosphorylation (P-AIR-2) was also significantly increased in the presence of TLK-1KD or TLK-1KD(S634E) (lanes 3 and 5).
(D) Quantitation of histone H3 phosphorylation in the kinase assays shown in (C). Error bars represent the standard deviation from at least three separate experiments.
AIR-2 phosphorylation of TLK-1 at S634 appears to increase TLK-1 kinase activity (Figure 4). Thus, an alternative explanation for the H3 phosphorylation increase noted above is that AIR-2 phosphorylation of TLK-1 augments TLK-1 kinase activity or specificity with respect to H3, such that H3 is directly phosphorylated by phosphorylated TLK-1. To test whether TLK-1 kinase activity is required for the increase in H3 phosphorylation, we repeated the above experiment with kinase-dead (KD) forms of TLK-1, TLK-1(S634A), and TLK-1(S634E). H3 phosphorylation was significantly increased when TLK-1KD or TLK-1(S634E)KD was added to AIR-2/ICP-1 kinase assays (Figures 5C [lanes 3 and 5] and 5D), whereas TLK-1(S634A)KD did not enhance H3 phosphorylation (Figures 5C [lane 4] and 5D). AIR-2 autophosphorylation and ICP-1 phosphorylation were affected similarly to H3 phosphorylation (Figure 5C). Identical results were obtained when MYBP was used as a substrate (Figure S3). Thus, TLK-1 kinase activity is not required for the noted increase in H3, AIR-2, ICP-1, and MYBP phosphorylation, eliminating the possibility that TLK-1 is directly phosphorylating H3, AIR-2, or ICP-1. Instead, these data fully support a model in which AIR-2-mediated phosphorylation of TLK-1 increases AIR-2 kinase activity.
Whereas TLK-1 kinase activity was not necessary to augment AIR-2 kinase activity, the presence of ICP-1 was required. Phosphorylation of histone H3 by AIR-2 was not affected by addition of kinase-active (Figures S4A [lanes 2–4] and S4B) or kinase-inactive forms (Figures S4A [lanes 5–7] and S4B) of TLK-1, TLK-1(S634A), or TLK-1(S634E) in the absence of ICP-1. These results suggest that TLK-1’s effect on AIR-2 kinase activity may be mediated in part through ICP-1 or that ICP-1 is absolutely necessary for AIR-2 to phosphorylate TLK-1 and initiate the activity feedback loop.
Loss of TLK-1 Enhances the Chromosome-Segregation Defects of an air-2 Hypomorphic Allele
The experiments described above revealed that TLK-1 is a bona fide in vivo AIR-2 substrate that acts independently of its own kinase activity to augment the kinase activity of the AIR-2/ICP-1 complex. These results suggest that the TLK-1 kinase is likely to play an important role during mitosis. Indeed, in addition to transcription defects [29], tlk-1(RNAi) embryos displayed metaphase chromosome-congression abnormalities, anaphase bridges, and polyploidy (Figure 6). However, these defects were only apparent in later-stage embryos (>30–40 cells) and were not found in young embryos, nor are they characteristic of other mutants that affect embryonic transcription [35, 36].
Figure 6. tlk-1(RNAi) Embryos Have Abnormal Nuclei and Display Mitotic Chromosome Defects.
Wild-type and tlk-1(RNAi) embryos were fixed and stained with DAPI. Although chromosome defects were not apparent in early embryos, the majority of tlk-1(RNAi) embryos past the 30 cell stage exhibited various chromosome defects, including polyploid nuclei (arrows), wide metaphase plates (open arrow heads), and anaphase bridges (closed arrowheads). The scale bar represents 10 μm.
Given that the TLK-1 kinase appears to be an activator of AIR-2, we hypothesized that loss of TLK-1 expression would enhance the mitotic defects seen when AIR-2 function is reduced. tlk-1(RNAi) was performed with wild-type and air-2(or207ts) animals reared at permissive (15°C), semipermissive (20°C), and restrictive (25°C) temperatures to test this hypothesis. The or207ts mutation is due to a missense mutation at a residue in subdomain XI of the predicted AIR-2 kinase domain (P265L) [37]. In vitro, this mutation completely eliminates AIR-2 kinase activity [17]. In vivo, air-2(or207ts) embryos display <15% lethality at 15°C, 60%–95% lethality at 20°C, and 100% lethality at 25°C (T. Furuta and J.S., unpublished data; [37]). Embryos from tlk-1(RNAi), air-2(or207ts), and air-2(o207ts);tlk-1(RNAi) animals reared at 15°C, 20°C, and 25°C were fixed and stained with DAPI and antibodies specific for phospho-histone H3 (pH3) and α-tubulin. At 15°C, all tlk-1(RNAi) and the majority of air-2(or207ts) embryos examined (71%) had no discernible mitotic or cellular defects, and both types of embryos stained strongly with the pH3 antibody (Figure 7; Table 1). However, the majority of air-2(or207ts);tlk-1(RNAi) embryos displayed gross chromosome segregation and cytokinesis defects (Figure 7; Table 1). pH3 staining was also greatly reduced. At 20°C and 25°C, the majority of air-2(or207ts) embryos displayed abnormalities in chromosome segregation and cytokinesis, and the air-2(or207ts);tlk-1(RNAi) embryos had similar defects (data not shown). Altogether, these results indicate that loss of TLK-1 expression enhances the phenotype of the air-2(or207ts) mutation when AIR-2 activity is partially compromised (15°C). However, at higher temperatures, AIR-2 activity is reduced to such a significant extent that loss of TLK-1 has no discernible effect. These results fully support our molecular model, in which the TLK-1 and AIR-2 kinases enhance the activity of one another.
Figure 7. Depletion of TLK-1 Enhances the Chromosome-Segregation Defects of a Hypomorphic air-2 Mutant Allele.
Wild-type, tlk-1(RNAi), air-2(or207ts), and tlk-1(RNAi);air-2(or207ts) embryos isolated from hermaphrodites reared at 15°C were fixed and stained with DAPI (blue) and antibodies specific for phospho-histone H3 (pH3) (red) and α-tubulin (green). 1 cell embryos at anaphase and 2 cell embryos (or embryos with four centrosomes) are shown for each experimental condition. All wild-type and tlk-1(RNAi) and the majority of air-2(or207ts) early embryos (71%) had no discernible defects in chromosome behavior or changes in pH3 staining. However, the majority of tlk-1(RNAi);air-2(or207ts) early embryos (64%) displayed abnormal metaphase chromosome alignment, anaphase bridges, cytokinesis defects, and reduced pH3 staining. The scale bar represents 10 μm.
Table 1.
tlk-1(RNAi) Enhances Chromosome-Segregation Defects of air-2(or207ts) Embryos at 15°C
| Genotype 15°C | Normal Anaphase | Abnormal Anaphase |
|---|---|---|
| Wild-type | 45 (100%) | 0 |
| tlk-1(RNAi) | 72 (100%) | 0 |
| air-2(or207ts) | 135 (71%) | 56 (29%) |
| air-2(or207ts);tlk-1(RNAi) | 42 (36%) | 75 (64%) |
Embryos from the wild-type and tlk-1(RNAi), air-2(or207ts), and air-2(or207ts);tlk-1(RNAi) embryos reared at 15°C were fixed and stained with DAPI and pH3 and α-tubulin antibodies. 1–4 cell embryos with satisfactory tubulin staining were assayed for chromosome-segregation defects at anaphase.
Discussion
Here, we report that the C. elegans Tousled-like kinase TLK-1 is phosphorylated by the Aurora B kinase AIR-2 at a specific residue in vitro and in vivo. This phosphorylation increases the kinase activity of TLK-1 and also initiates a feedback loop that enhances AIR-2 kinase activity. The ability of TLK-1 to augment AIR-2 activity does not require TLK-1 kinase activity but is dependent on the presence of ICP-1, a previously characterized substrate and activator of Aurora B kinases. Furthermore, loss of TLK-1 enhances the chromosome-segregation defects of an air-2 hypomorphic allele. Altogether, our results provide strong in vitro and in vivo evidence for a functional interaction between the Tousled and Aurora B kinases.
Aurora B, Tousled, and Chromosomal Passengers
The highly conserved Aurora B kinases are components of the “chromosomal passenger” complex. In vertebrates, this complex consists of Aurora B, INCENP, Survivin, and the newly characterized Borealin/Dasra protein [6–8]. A similar complex is present in C. elegans and consists of the respective Aurora B, INCENP, and Survivin orthologs AIR-2, ICP-1, and BIR-1 [20, 32, 38]. A fourth component, CSC-1, shares limited homology with Borealin/Dasra [33]. Loss of any one of AIR-2, ICP-1, BIR-1, and CSC-1 results in a similar embryonic lethal phenotype characterized by chromosome segregation and cytokinesis defects [20, 32, 33, 38]. All four proteins colocalize with one another, associating with chromosomes from prophase through metaphase and translocating to the central-spindle microtubules at anaphase [20, 32, 33, 38]. As noted above, ICP-1 is a substrate and activator of the AIR-2 kinase [17, 33]. ICP-1 is also necessary for the interaction of AIR-2 with BIR-1 and CSC-1 [33]. It has been suggested that BIR-1 and CSC-1 localize AIR-2/ICP-1 catalytic activity to subcellular sites requiring the function of the chromosomal passenger complex [33]. Interestingly, when the passenger complex was reconstituted with recombinant proteins coexpressed in insect cells, two distinct complexes were formed, one containing all four proteins and one consisting of just ICP-1 and AIR-2 [33]. These results suggest that AIR-2 and ICP-1 may participate in complexes that are distinct from the chromosomal passenger complex. Each of these putative complexes may have discrete localization patterns, substrate specificities, and functions.
TLK-1 is clearly not a chromosomal-passenger protein because it is most highly expressed during S phase and does not display a chromosomal passenger localization during mitosis [29]. The pattern of phosphorylated TLK-1 is also distinct from that of the chromosomal passengers. Phospho-TLK-1 is first detectable in prophase nuclei and persists as a halo around the chromosomes after nuclear-envelope breakdown. We cannot distinguish whether TLK-1 is phosphorylated early in mitosis and the phosphorylated protein persists until anaphase, or whether TLK-1 is continually phosphorylated from prophase to the metaphase/anaphase transition. Nevertheless, our results suggest that a portion of AIR-2/ICP-1 catalytic activity is distinct from that of the chromosomal passengers or that TLK-1 transiently associates with the chromosomal passenger complex and then disperses from the chromosomes to form a distinctive halo. We are currently testing the hypothesis that AIR-2, ICP-1, and TLK-1 form a complex that has specific substrates and functions that are distinct from those of the chromosomal passenger complex.
Aurora B, Tousled, and Chromatin
To date, the only reported substrates of the Tousled kinases are histone H3 [30] and Asf1 chromatin assembly factors [25, 26]. Although it was suggested that human Tlk1 directly phosphorylates histone H3 [30], we found no evidence that C. elegans TLK-1 could directly phosphorylate histone H3 in vitro or in vivo [29]. Instead, our findings that TLK-1 can activate the histone H3 kinase AIR-2 explain the observations of Li et al., 2001, including that human Tlk1 can enhance histone H3 phosphorylation in and rescue the lethality of Ipl1 mutant yeast [30].
A second Tousled substrate, Asf1, was initially identified in S. cerevisae as a gene that could derepress silenced loci when overexpressed from a plasmid [39, 40]. Subsequently, Asf1 and its partner CAF1 were found to be required for the deposition of newly synthesized acetylated histone H3 and H4 during DNA replication and repair [41–43]. Asf1 histone chaperone activity appears to be negatively regulated by binding of the Rad53 DNA damage-checkpoint kinase (Chk2 in humans) [44, 45]. This inhibition is released when Rad53 is phosphorylated in response to DNA damage, presumably allowing DNA-repair-coupled chromatin assembly to occur.
Asf1 proteins are in vitro substrates of human, Arabidopsis, Drosophila, and C. elegans Tousled-like kinases [25, 26, 46]. Although the molecular and cellular consequences of Asf1 phosphorylation by Tousled kinases are unknown, several lines of evidence support a role for Tlks in the regulation of DNA replication, chromatin assembly, and chromosome structure. Overexpression of Tlk or Asf1 in Drosophila disrupts salivary-gland chromosome architecture and inhibits endoreplication [26]. Interestingly, expression of kinase-dead Tlk has similar defects but can be rescued by increasing Asf1 expression [26]. These experiments suggest that Tlk and Asf1 cooperate in some aspects of chromatin assembly and endoreplication. Human Tlk was recently shown to be transiently inhibited in response to S phase DNA damage via the ATM and Chk1 kinase pathways [27, 28]. As noted above, Asf1 activity is regulated by the S phase checkpoint in a rad53/Chk2-dependent manner [44, 45]. Thus, the inhibition of Tlk activity may halt ongoing DNA replication, allowing Asf1 to participate in DNA repair pathways.
Previously, we showed that C. elegans embryos depleted of TLK-1 are broadly defective in transcription, likely at the level of transcription elongation [29]. We hypothesized that these defects could arise from chromatin-assembly abnormalities that result in a chromatin template that is not amenable to disassembly during transcription elongation. A role for yeast asf1 in chromatin disassembly and transcription has recently been described [47, 48], further supporting a functional relationship between Tlks and Asf1 in chromatin-based aspects of gene expression.
Tlks were previously thought to be S-phase-specific kinases [24]. Our data suggest that Tlks may also have an important role in mitosis. Although early tlk-1(RNAi) embryos did not display chromatin-segregation or chromosome-morphology abnormalities, loss of TLK-1 activity in older embryos (around the 30–40 cell stage) resulted in altered nuclear morphology and anaphase chromosome-segregation defects. Mitotic chromosome segregation defects were also noted in Drosophila embryos harboring loss-of-function mutations in Drosophila Tlk [26]. In addition, the activity of Arabidopsis Tousled (tsl) peaks during G1 and again at the G2/M transition, and tsl mutants have increased expression of cyclin B, suggestive of mitotic delays [46].
Overall, our current results and those described above suggest that Tousled kinases may have two distinct roles in the cell cycle, one that influences DNA replication and chromatin assembly/disassembly in partnership with Asf1 and a second that affects mitotic chromosome dynamics in cooperation with the Aurora B kinase. Alternatively, Tlk-dependent mitotic defects could be a secondary consequence of poor chromosome architecture arising from Tlk’s role in chromatin assembly. Mitotic abnormalities similar to those found in older tlk-1(RNAi) C. elegans embryos were also noted in embryos depleted of C. elegans ASF-1 (J.S., unpublished data). In this model, the Aurora B kinase may serve as bridge between chromatin structure and chromosome segregation. In support of this view, Aurora B/INCENP kinase activity is required for the dissociation of the ISWI chromatin remodeling complex from mitotic chromosomes, perhaps through Aurora-B-mediated histone H3 phosphorylation [49]. Clearly much remains to be learned regarding the relationship between histone H3 phosphorylation, chromosome structure, and cell-cycle-dependent chromosome behavior. Deciphering the emerging role of the Aurora B/INCENP and Tousled kinases in the regulation of these events remains a long-term goal.
Supplementary Material
Supplemental Data including detailed Experimental Procedures and additional figures are available at http://www.current-biology.com/cgi/content/full/15/10/894/DC1/.
Acknowledgments
We thank Y. Kohara for C. elegans cDNA clones, M. Barton and K. Shumway for reagents, B. Bowerman and the C. elegans Genetics Center for strains, R. Haynes for media preparation, X. Chen and R. Jacobson for advice on protein expression and purification, T. Heallen for technical assistance, S. Dent and K. Zhang for helpful discussions, and S. Dent, M. Barton, and P. Amayed for critical reading of the manuscript. DNA sequencing was performed at the M.D. Anderson Cancer Center sequencing core (supported by National Cancer Institute grant CA-16672). This work was supported by National Institutes of Health grants awarded to J.M.S. (R01 GM62181-01) and J.R.S. (K01 DK02966-01A1).
References
- 1.Andrews PD, Knatko E, Moore WJ, Swedlow JR. Mitotic mechanics: The auroras come into view. Curr Opin Cell Biol. 2003;15:672–683. doi: 10.1016/j.ceb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Sakashita G, Matsuzaki H, Sugimoto K, Kimura K, Hanaoka F, Taniguchi H, Furukawa K, Urano T. Direct association with inner centromere protein (INCENP) activates the novel chromosomal-passenger protein, Aurora-C. J Biol Chem. 2004;279:47201–47211. doi: 10.1074/jbc.M403029200. [DOI] [PubMed] [Google Scholar]
- 3.Sasai K, Katayama H, Stenoien DL, Fujii S, Honda R, Kimura M, Okano Y, Tatsuka M, Suzuki F, Nigg EA, et al. Aurora-C kinase is a novel chromosomal-passenger protein that can complement Aurora-B kinase function in mitotic cells. Cell Motil Cytoskeleton. 2004;59:249–263. doi: 10.1002/cm.20039. [DOI] [PubMed] [Google Scholar]
- 4.Hannak E, Kirkham M, Hyman AA, Oegema K. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J Cell Biol. 2001;155:1109–1116. doi: 10.1083/jcb.200108051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ducat D, Zheng Y. Aurora kinases in spindle assembly and chromosome segregation. Exp Cell Res. 2004;301:60–67. doi: 10.1016/j.yexcr.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Vagnarelli P, Earnshaw WC. Chromosomal passengers: The four-dimensional regulation of mitotic events. Chromosoma. 2004;113:211–222. doi: 10.1007/s00412-004-0307-3. [DOI] [PubMed] [Google Scholar]
- 7.Gassmann R, Carvalho A, Henzing AJ, Ruchaud S, Hudson DF, Honda R, Nigg EA, Gerloff DL, Earnshaw WC. Borealin: A novel chromosomal passenger required for stability of the bipolar mitotic spindle. J Cell Biol. 2004;166:179–191. doi: 10.1083/jcb.200404001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampath SC, Ohi R, Leismann O, Salic A, Pozniakovski A, Funabiki H. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 2004;118:187–202. doi: 10.1016/j.cell.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 9.Hirota T, Kunitoku N, Sasayama T, Marumoto T, Zhang D, Nitta M, Hatakeyama K, Saya H. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003;114:585–598. doi: 10.1016/s0092-8674(03)00642-1. [DOI] [PubMed] [Google Scholar]
- 10.Eyers PA, Erikson E, Chen LG, Maller JL. A novel mechanism for activation of the protein kinase Aurora A. Curr Biol. 2003;13:691–697. doi: 10.1016/s0960-9822(03)00166-0. [DOI] [PubMed] [Google Scholar]
- 11.Trieselmann N, Armstrong S, Rauw J, Wilde A. Ran modulates spindle assembly by regulating a subset of TPX2 and Kid activities including Aurora A activation. J Cell Sci. 2003;116:4791–4798. doi: 10.1242/jcs.00798. [DOI] [PubMed] [Google Scholar]
- 12.Tsai MY, Wiese C, Cao K, Martin O, Donovan P, Ruderman J, Prigent C, Zheng Y. A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat Cell Biol. 2003;5:242–248. doi: 10.1038/ncb936. [DOI] [PubMed] [Google Scholar]
- 13.Eyers PA, Maller JL. Regulation of Xenopus Aurora A activation by TPX2. J Biol Chem. 2004;279:9008–9015. doi: 10.1074/jbc.M312424200. [DOI] [PubMed] [Google Scholar]
- 14.Kufer TA, Sillje HH, Korner R, Gruss OJ, Meraldi P, Nigg EA. Human TPX2 is required for targeting Aurora-A kinase to the spindle. J Cell Biol. 2002;158:617–623. doi: 10.1083/jcb.200204155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wittmann T, Wilm M, Karsenti E, Vernos I. TPX2, a novel Xenopus MAP involved in spindle pole organization. J Cell Biol. 2000;149:1405–1418. doi: 10.1083/jcb.149.7.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayliss R, Sardon T, Vernos I, Conti E. Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol Cell. 2003;12:851–862. doi: 10.1016/s1097-2765(03)00392-7. [DOI] [PubMed] [Google Scholar]
- 17.Bishop JD, Schumacher JM. Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B Kinase stimulates Aurora B kinase activity. J Biol Chem. 2002;277:27577–27580. doi: 10.1074/jbc.C200307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honda R, Korner R, Nigg EA. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell. 2003;14:3325–3341. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams RR, Wheatley SP, Gouldsworthy AM, Kandels-Lewis SE, Carmena M, Smythe C, Gerloff DL, Earnshaw WC. INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle, and cleavage furrow. Curr Biol. 2000;10:1075–1078. doi: 10.1016/s0960-9822(00)00673-4. [DOI] [PubMed] [Google Scholar]
- 20.Kaitna S, Mendoza M, Jantsch-Plunger V, Glotzer M. Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr Biol. 2000;10:1172–1181. doi: 10.1016/s0960-9822(00)00721-1. [DOI] [PubMed] [Google Scholar]
- 21.Kang J, Cheeseman IM, Kallstrom G, Velmurugan S, Barnes G, Chan CS. Functional cooperation of Dam1, Ipl1, and the inner centromere protein (INCENP)-related protein Sli15 during chromosome segregation. J Cell Biol. 2001;155:763–774. doi: 10.1083/jcb.200105029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams RR, Maiato H, Earnshaw WC, Carmena M. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, meta-phase chromosome alignment, kinetochore disjunction, and chromosome segregation. J Cell Biol. 2001;153:865–880. doi: 10.1083/jcb.153.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roe JL, Rivin CJ, Sessions RA, Feldmann KA, Zambryski PC. The Tousled gene in A. thaliana encodes a protein kinase homolog that is required for leaf and flower development. Cell. 1993;75:939–950. doi: 10.1016/0092-8674(93)90537-z. [DOI] [PubMed] [Google Scholar]
- 24.Sillje HH, Takahashi K, Tanaka K, Van Houwe G, Nigg EA. Mammalian homologues of the plant Tousled gene code for cell-cycle-regulated kinases with maximal activities linked to ongoing DNA replication. EMBO J. 1999;18:5691–5702. doi: 10.1093/emboj/18.20.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sillje HH, Nigg EA. Identification of human Asf1 chromatin assembly factors as substrates of Tousled-like kinases. Curr Biol. 2001;11:1068–1073. doi: 10.1016/s0960-9822(01)00298-6. [DOI] [PubMed] [Google Scholar]
- 26.Carrera P, Moshkin YM, Gronke S, Sillje HH, Nigg EA, Jackle H, Karch F. Tousled-like kinase functions with the chromatin assembly pathway regulating nuclear divisions. Genes Dev. 2003;17:2578–2590. doi: 10.1101/gad.276703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krause DR, Jonnalagadda JC, Gatei MH, Sillje HH, Zhou BB, Nigg EA, Khanna K. Suppression of Tousled-like kinase activity after DNA damage or replication block requires ATM, NBS1 and Chk1. Oncogene. 2003;22:5927–5937. doi: 10.1038/sj.onc.1206691. [DOI] [PubMed] [Google Scholar]
- 28.Groth A, Lukas J, Nigg EA, Sillje HH, Wernstedt C, Bartek J, Hansen K. Human Tousled like kinases are targeted by an ATM- and Chk1-dependent DNA damage checkpoint. EMBO J. 2003;22:1676–1687. doi: 10.1093/emboj/cdg151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han Z, Saam JR, Adams HP, Mango SE, Schumacher JM. The C. elegans Tousled-like kinase (TLK-1) has an essential role in transcription. Curr Biol. 2003;13:1921–1929. doi: 10.1016/j.cub.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, DeFatta R, Anthony C, Sunavala G, De Benedetti A. A translationally regulated Tousled kinase phosphorylates histone H3 and confers radioresistance when over-expressed. Oncogene. 2001;20:726–738. doi: 10.1038/sj.onc.1204147. [DOI] [PubMed] [Google Scholar]
- 31.Hsu JY, Sun ZW, Li X, Reuben M, Tatchell K, Bishop DK, Grushcow JM, Brame CJ, Caldwell JA, Hunt DF, et al. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell. 2000;102:279–291. doi: 10.1016/s0092-8674(00)00034-9. [DOI] [PubMed] [Google Scholar]
- 32.Schumacher JM, Golden A, Donovan PJ. AIR-2: An Aurora/Ipl1-related protein kinase associated with chromosomes and midbody microtubules is required for polar body extrusion and cytokinesis in Caenorhabditis elegans embryos. J Cell Biol. 1998;143:1635–1646. doi: 10.1083/jcb.143.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romano A, Guse A, Krascenicova I, Schnabel H, Schnabel R, Glotzer M. CSC-1: A subunit of the aurora b kinase complex that binds to the survivin-like protein BIR-1 and the incenp-like protein ICP-1. J Cell Biol. 2003;161:229–236. doi: 10.1083/jcb.200207117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheeseman IM, Anderson S, Jwa M, Green EM, Kang J, Yates JR, 3rd, Chan CS, Drubin DG, Barnes G. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell. 2002;111:163–172. doi: 10.1016/s0092-8674(02)00973-x. [DOI] [PubMed] [Google Scholar]
- 35.Powell-Coffman JA, Knight J, Wood WB. Onset of C. elegans gastrulation is blocked by inhibition of embryonic transcription with an RNA polymerase antisense RNA. Dev Biol. 1996;178:472–483. doi: 10.1006/dbio.1996.0232. [DOI] [PubMed] [Google Scholar]
- 36.Shim EY, Walker AK, Shi Y, Blackwell TK. CDK-9/cyclin T (P-TEFb) is required in two postinitiation pathways for transcription in the C. elegans embryo. Genes Dev. 2002;16:2135–2146. doi: 10.1101/gad.999002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Severson AF, Hamill DR, Carter JC, Schumacher J, Bowerman B. The aurora-related kinase AIR-2 recruits ZEN-4/CeMKLP1 to the mitotic spindle at metaphase and is required for cytokinesis. Curr Biol. 2000;10:1162–1171. doi: 10.1016/s0960-9822(00)00715-6. [DOI] [PubMed] [Google Scholar]
- 38.Speliotes EK, Uren A, Vaux D, Horvitz HR. The survivin-like C. elegans BIR-1 protein acts with the Aurora-like kinase AIR-2 to affect chromosomes and the spindle midzone. Mol Cell. 2000;6:211–223. doi: 10.1016/s1097-2765(00)00023-x. [DOI] [PubMed] [Google Scholar]
- 39.Singer MS, Kahana A, Wolf AJ, Meisinger LL, Peterson SE, Goggin C, Mahowald M, Gottschling DE. Identification of high-copy disruptors of telomeric silencing in Saccharomyces cerevisiae. Genetics. 1998;150:613–632. doi: 10.1093/genetics/150.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le S, Davis C, Konopka JB, Sternglanz R. Two new S-phase-specific genes from Saccharomyces cerevisiae. Yeast. 1997;13:1029–1042. doi: 10.1002/(SICI)1097-0061(19970915)13:11<1029::AID-YEA160>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 41.Verreault A, Kaufman PD, Kobayashi R, Stillman B. Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell. 1996;87:95–104. doi: 10.1016/s0092-8674(00)81326-4. [DOI] [PubMed] [Google Scholar]
- 42.Tyler JK, Adams CR, Chen SR, Kobayashi R, Kamakaka RT, Kadonaga JT. The RCAF complex mediates chromatin assembly during DNA replication and repair. Nature. 1999;402:555–560. doi: 10.1038/990147. [DOI] [PubMed] [Google Scholar]
- 43.Tyler JK, Collins KA, Prasad-Sinha J, Amiott E, Bulger M, Harte PJ, Kobayashi R, Kadonaga JT. Interaction between the Drosophila CAF-1 and ASF1 chromatin assembly factors. Mol Cell Biol. 2001;21:6574–6584. doi: 10.1128/MCB.21.19.6574-6584.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emili A, Schieltz DM, Yates JR, 3rd, Hartwell LH. Dynamic interaction of DNA damage checkpoint protein Rad53 with chromatin assembly factor Asf1. Mol Cell. 2001;7:13–20. doi: 10.1016/s1097-2765(01)00150-2. [DOI] [PubMed] [Google Scholar]
- 45.Hu F, Alcasabas AA, Elledge SJ. Asf1 links Rad53 to control of chromatin assembly. Genes Dev. 2001;15:1061–1066. doi: 10.1101/gad.873201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehsan H, Reichheld JP, Durfee T, Roe JL. TOUSLED kinase activity oscillates during the cell cycle and interacts with chromatin regulators. Plant Physiol. 2004;134:1488–1499. doi: 10.1104/pp.103.038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adkins MW, Tyler JK. The histone chaperone asf1p mediates global chromatin disassembly in vivo. J Biol Chem. 2004;279:52069–52074. doi: 10.1074/jbc.M406113200. [DOI] [PubMed] [Google Scholar]
- 48.Adkins MW, Howar SR, Tyler JK. Chromatin disassembly mediated by the histone chaperone Asf1 is essential for transcriptional activation of the yeast PHO5 and PHO8 genes. Mol Cell. 2004;14:657–666. doi: 10.1016/j.molcel.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 49.MacCallum DE, Losada A, Kobayashi R, Hirano T. ISWI remodeling complexes in Xenopus egg extracts: Identification as major chromosomal components that are regulated by INCENP-aurora B. Mol Biol Cell. 2002;13:25–39. doi: 10.1091/mbc.01-09-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data including detailed Experimental Procedures and additional figures are available at http://www.current-biology.com/cgi/content/full/15/10/894/DC1/.