Abstract
AIM: To investigate the anti-tumor effects of combined cytotoxic drug (gemcitabine) and photodynamic therapy (PDT) on human pancreatic cancer xenograft in nude mice.
METHODS: Human pancreatic cancer cell line SW1990 was used in the investigation of the in vivo effect of combined gemcitabine and PDT on human pancreatic cancer xenograft in mice. Sixty mice were randomly allocated into a control group (without treatment), photosensitizer treatment group (2 mg/kg photosan, without illumination), chemotherapy group (50 mg/kg gemcitabine i.p.), PDT group (2 mg/kg photosan + laser irradiation) and combined treatment group (photosan + chemotherapy), with 12 mice in each group. Tumor size was measured twice every week. Anti-tumor activity in different groups was evaluated by tumor growth inhibition (TGI).
RESULTS: No significant anti-tumor effect was observed either in photosensitizer treatment group or in chemotherapy group. PDT led to necrosis in cancer lesions and significantly reduced tumor volume compared with photosensitizer on day 6 and at the following time points after initialization of therapy (0.24 ± 0.15-0.49 ± 0.08 vs 0.43 ± 0.18-1.25 ± 0.09, P < 0.05). PDT significantly reduced tumor volume in combined treatment group compared with photosensitizer treatment group (0.12 ± 0.07-0.28 ± 0.12 vs 0.39 ± 0.15-1.20 ± 0.11, P < 0.05), small dose chemotherapy group (0.12 ± 0.07-0.28 ± 0.12 vs 0.32 ± 0.14-1.16 ± 0.08, P < 0.05) and control group (0.12 ± 0.07-0.28 ± 0.12 vs 0.43 ± 0.18-1.25 ± 0.09, P < 0.05). TGI was higher in the combined treatment group (82.42%) than in the PDT group (58.18%).
CONCLUSION: PDT has a significant anti-tumor effect, which is maintained for a short time and can be significantly enhanced by small doses of gemcitabine.
Keywords: Pancreatic carcinoma, Nude mice, Animal model, Photodynamic therapy, Gemcitabine
INTRODUCTION
Pancreatic cancer remains a lethal disease. Most pancreatic cancer patients are at advanced stage when apparent symptoms occur. Pancreatic cancer patients undergoing resection at the time of initial diagnosis account for less than 20%[1], and the 5-year survival rates after complete and partial pancreatic resection are 15%-25%[2,3] and 8%-14%[4,5], respectively. Treatment modalities for inoperable patients are largely limited to radiotherapy, chemotherapy, or their combination. Pancreas is a retroperitoneal organ, with stomach, intestine, and spinal cord around it. Since the sensitivity of pancreatic cancer to radiotherapy is poor, and the tolerant dosage of pancreas tissue is low, radiotherapy does not lead to a convincing beneficial effect on survival. Chemotherapy is the main therapeutic method for advanced pancreatic cancer. 5-fluorouacil is probably the most useful single agent for symptomatic relief[6]. Gemcitabine may also have a value for palliation[7–9]. Overall, the prognosis of pancreatic cancer patients is poor with a 1-year survival rate of about 10%[10]. Therefore, new treatment modalities are urgently needed.
Photodynamic therapy (PDT) produces localized tissue necrosis with light (most conveniently from a laser), after administration of a photosensitizing agent in the presence of oxygen[10–12], based on the use of photosensitizing compounds that localize quite selectively in neoplastic/hyperplastic tissues and become cytotoxic when exposed to light[12–14]. In view of the antitumor effect of single treatment, PDT is local. PDT in combination with surgery[15], radiotherapy[16] or chemotherapy[17], has become a subject of research. New photosensitizers with improved spectroscopic, photochemical and tissue-localizing properties and improved laser instrumentation have stimulated attempts to establish clinical protocols for incorporation of PDT into multi-treatment modalities[18–24].
Most studies on PDT to date have been on lesions of the skin or in the wall of hollow organs, but recent interest is more in its potential for treating lesions of solid organs such as the pancreas[10,11,20]. We have undertaken experiments on treating pancreatic cancer of mice with different doses of gemcitabine[25]. The results indicate that the growth of transplanted tumors can be inhibited by gemcitabine at 100 mg/kg, which shows severe side effects such as diarrhea, dehydration and loss of weight[25]. When gemcitabine at 100 mg/kg is used, the growth of transplanted tumors could not be controlled[25]. On the other hand, combined angiogenetic inhibitors can decrease side effects of gemcitabine, meanwhile the growth and metastasis of transplanted tumors are effectively inhibited[25].
In this study, gemcitabine was used as a cytotoxic drug. The cytotoxic and antitumor effects of combined gemcitabine and PDT were evaluated. Human pancreatic cancer cell line SW1990 was used in experiments to assess the effect of gemcitabine or PDT, or their combination, on pancreatic cancer in accordance with institutional guidelines.
MATERIALS AND METHODS
Tumor line, animals and drugs
SW1990, a high transferred human pancreatic cancer cell line (ATCC, Kyriazis MD, USA), was maintained in Laboratory of Sun Yat-Sen Memorial Hospital and serially passed in nude mice. Five- to six-week-old male BALB/c nude mice were obtained from Animal Center Sun Yat-Sen University. Photosan, a second generation of photosensitizer, was provided by Diolitec Pharmaceutical Company (Germany). Gemcitabine was provided by Lilly Pharmaceutical Company (USA).
Animal model and therapeutic group
Two male nude mice (6 wk of age) were inoculated subcutaneously with 0.5 × 107 SW1990 cells. Tumors in subcutaneous tissue were excised and tumor tissue was implanted subcutaneously in 60 nude mice.
A tumor model was established and observed for 10-14 d after implantation of tumor tissue. Tumor-bearing nude mice were divided into control group (group A), photosensitizer group (group B), chemotherapy group (group C), PDT group (group D), and combined group (group E), with 12 mice in each group.
Forty-eight hours after the mice had photosan, tumor masses in mice of the PDT group and combined group were exposed to light (λ = 630 nm, 120 J/cm2) from a PDT630 semiconductor laser (Diolitec Pharmaceutical Company) for 20 min. Gemcitabine (50 mg/kg) was injected into the peritoneal cavity of mice in the combined group 1 h prior to light exposure and on days 3, 6 and 9 after light exposure. The same dose of gemcitabine was given to mice in the chemotherapy group at the same time point as in the combined group.
Data collection
All experimental mice were weighed and tumor diameters were measured with vernier calipers before treatment and twice a week after treatment. On day 21 after treatment, all experimental mice were sacrificed with their tumors removed and weighed to obtain tumor weight (TW). Tumor volume (TV) was determined according to the formula: TV = 3/4 × π × (b/2)2 × a/2, where a is the length and b is the height of the tumor. Antitumor activity was evaluated by tumor growth inhibition (TGI), calculated according to the formula: TGI = (1 - TWT/TWC) × 100% in treated (T) and control (C) mice.
Statistical analysis
Data analysis was performed using SPSS11.0 statistical package (SPSS, Chicago, USA). Tumor response to treatment was compared using two-way ANOVA and Student-Neuman-Keuls test. P < 0.05 was considered statistically significant.
RESULTS
Tumor volume
Tumor volume increased with time after treatment in the photosensitizer group, small dose chemotherapy group and control group (Table 1 and Figure 1). Tumor volume had no significant difference at the same time point in three groups (ANOVA).
Table 1.
Tumor volume in different groups after treatment with PDT and/or gemcitabine (cm3) (mean ± SE)
| Groups | Pre-therapy | 3 d | 6 d | 9 d | 12 d | 15 d | 18 d | 21 d | P |
| A | 0.14 ± 0.09 | 0.26 ± 0.13 | 0.43 ± 0.18 | 0.56 ± 0.23 | 0.66 ± 0.23 | 0.80 ± 0.10 | 1.01 ± 0.12 | 1.25 ± 0.09 | < 0.01 |
| B | 0.12 ± 0.06 | 0.26 ± 0.11 | 0.39 ± 0.15 | 0.51 ± 0.18 | 0.62 ± 0.17 | 0.75 ± 0.09 | 0.93 ± 0.08 | 1.20 ± 0.11 | < 0.01 |
| C | 0.13 ± 0.07 | 0.23 ± 0.10 | 0.32 ± 0.14 | 0.44 ± 0.14 | 0.57 ± 0.12 | 0.72 ± 0.10 | 0.91 ± 0.12 | 1.16 ± 0.08 | < 0.01 |
| D | 0.14 ± 0.08 | 0.22 ± 0.12 | 0.24 ± 0.15 | 0.24 ± 0.16 | 0.28 ± 0.12 | 0.35 ± 0.10 | 0.42 ± 0.12 | 0.49 ± 0.08 | < 0.01 |
| E | 0.12 ± 0.07 | 0.13 ± 0.09 | 0.14 ± 0.10 | 0.15 ± 0.09 | 0.17 ± 0.08 | 0.18 ± 0.10 | 0.22 ± 0.10 | 0.28 ± 0.12 | < 0.01 |
| P | 0.951 | 0.038 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
Variance-test, P > 0.05.
Figure 1.
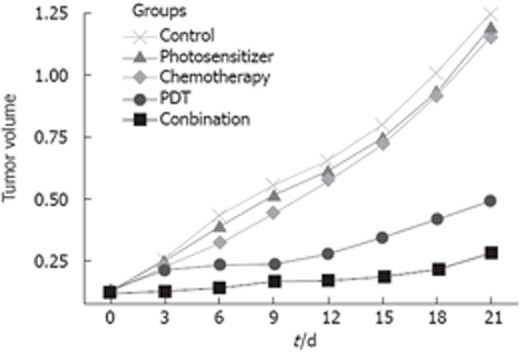
Tumor volume in different groups after treatment with PDT and/or gemcitabine (cm3) (mean ± SE).
PDT led to necrosis in cancer lesions. Partial tumor necrotic tissue was exfoliated and a necrotic edge of volcano-like uplift was formed 1 wk after treatment. Tumor volume was significantly reduced in PDT treatment group compared with the photosensitizer treatment group and control group at different time points after initialization of therapy (ANOVA, P < 0.05). No significant difference in tumor volume was found on days 3, 6, 9 and 12, but tumor volume increased significantly on days 15, 18 and 21 (P < 0.05) in the PDT group after treatment.
Tumor volume was significantly decreased in the combined PDT and gemcitabine treatment group compared with photosensitizer treatment group, small dose chemotherapy group and control group (P < 0.05). Tumor volume was significantly smaller in the combined treatment group than in the PDT group at different time points after treatment.
Tumor weight and TGI
Tumor weight and TGI of mice in five groups are listed in Table 2. Tumor weights of mice in the combination group (0.29 ± 0.20 g) and PDT group (0.69 ± 0.23 g) were significantly lower than those in the photosensitizer treatment group (1.62 ± 0.12 g), chemotherapy group (1.37 ± 0.19 g) and control group (1.65 ± 0.21 g) (P < 0.01). Tumor weights of mice in the combined group were obviously lower than those in the PDT group (P < 0.01). The mean tumor weight of mice in the two groups was significantly different (P < 0.01). Tumor weights were not significantly different in the photosensitizer treatment group, small dose chemotherapy group and control group. TGI (82.42%) was higher in the combined treatment group than in the PDT treatment group (58.18%).
Table 2.
Tumor weight (g) and TGI (%) response to PDT and/or gemcitabine
| Group | Animals (n) | TW (mean ± SE) | TGI |
| A | 12 | 1.65 ± 0.21 | - |
| B | 12 | 1.62 ± 0.12 | 1.80 |
| C | 12 | 1.37 ± 0.19 | 17.00 |
| D | 12 | 0.69 ± 0.23 | 58.18 |
| E | 12 | 0.29 ± 0.20 | 82.42 |
| P | - | < 0.01 | - |
Variance-test, P = 0.1.
Toxicity
The mice in four experimental groups had a loss of weight during the experiments. The weight in the four experimental groups was not significantly different from that in the control group.
Four treatment modalities did not induce signs of toxicity such as diarrhea and vomiting. No treatment-related deaths occurred. Mice in the PDT group and combined treatment group had no skin photosensitivity to light.
DISCUSSION
PDT may be defined as a treatment based on an oxygen-dependent reaction between a photosensitizing dye and light, that is, the light combination of two absolutely non-toxic elements, drug and light, in the presence of oxygen, can result in selective destruction of tissue[11,26]. The technique consists of administration of a tumor-localizing photosensitizing agent, which most often requires metabolic synthesis followed by activation of the agent by light with a specified wavelength. Photosensitizing agents used in PDT are macromolecular materials, which contribute to preferential location in neoplastic tissues and delay clearance of neoplastic tissues[27]. Therefore, PDT aims at a sequence of photochemical and photobiological processes that cause irreversible damage to tumor tissues and little damage to connective tissues, and maintain the mechanical integrity of organs[12,24]. It was reported that PDT has a selective effect on malignant pancreas but no significant effect on normal pancreas, and could well match other treatment modalities, except for radical surgery[28]. In a pilot clinical trail, Bown et al[10] used PDT in the palliative treatment of 16 patients with cancers in the pancreatic head that could not be treated with surgery because of the advanced nature of the disease or the general condition of the patients, and they concluded that PDT may be of value for treating localized cancers in patients who are poor candidates for definitive surgery or in whom the location of tumor makes pancreatic resection inappropriate. Abulafi et al[29] and Tseng et al[30] reported that patients with pancreatic and ampullary carcinoma who are not suitable for surgery should be treated with PDT, since PDT is both feasible and safe for small tumors.
PDT, on the other hand, has some disadvantages and limitations. Little information is currently available concerning the uptake of photosensitizer by pancreas or pancreatic cancer. Local spread of photodynamic agents to vital organs is common, and may cause perforation of the duodenum and jejunum, leading to death after treatment with PDT[11,31]. In addition, large tumor mass limits PDT to penetrate into the effective depth of tissue and needs multiple interstitial optical fibers to increase its volume[32]. Therefore, the ability of chemotherapeutic agents to enhance the effects of PDT in cell culture and transplantable mouse tumors has been studied by several groups[20–22]. Kirveliene et al[20] used murine hepatoma MH-22A to investigate in vitro and in vivo cytotoxic and anti-tumor effects of doxorubicin (Dox), a conventional anticancer drug, and PDT, showing that Dox potentiates therapeutic efficacy of PDT and vice versa, and that the degree of potentiation is influenced by Dox. Peterson et al[21] and Snyder et al[22] reported that combined treatment with PDT and Dox is more effective than treatment with either PDT or Dox alone. In vitro studies have revealed a significant effect of fluoropyrimidines[18] and mitomycin C[19] on the viability of mTHPC-photosensitized cells. Some studies focused on the effects of PDT on pancreas cancer[10,11]. As we know, no study about the effect of therapy with photodynamic- cytotoxic agents on pancreatic cancer has been reported.
Gemcitabine is an active nucleoside analogue against a wide variety of cancers, including non-small cell lung cancer and pancreatic cancer[7–9]. Gemcitabine, acting as an antimetabolite, can inhibit ribonucleotide reductase and DNA synthesis, and induce apoptosis[33]. Gemcitabine today remains a first-line drug for patients with advanced pancreatic cancer[7–9]. However, it has a narrow therapeutic index due to rapid enzyme deamination by deoxytidine deaminase into its corresponding inactive uracil derivative, and can also induce drug resistance[34,35]. Therefore, a high dose of gemcitabine is needed to achieve the desired therapeutic response with different adverse effects[36]. To overcome such drawbacks, based on the relation between quantity and effect of gemcitabine[25], we used photosan and gemcitabine as representatives of photosensitizing and cytotoxic agents to investigate the cytotoxic and antitumor effects of gemcitabine and PDT on pancreatic cancer in vivo. The results indicate that small dose gemcitabine or photosensitizer can not inhibit the growth of pancreatic cancer. PDT had a significant anti-tumor effect, but lasted a short time. Photodynamic-cytotoxic therapy (small dose gemcitabine) could significantly inhibit the growth of pancreatic cancer, and showed a relative long-duration anti-tumor effect compared with PDT. The inhibition rate of photodynamic-cytotoxic agents and PDT for tumors was 82.42% and 58.18%, respectively. The four treatment modalities did not induce any signs of toxicity such as diarrhea and vomiting. No treatment-related death occurred. Animals in the PDT group and combined treatment group had no skin photosensitivity to light.
In conclusion, low dose gemcitabine increases the anticancer effect of PDT with no obvious adverse effects. Combined PDT and gemcitabine can be used in treatment of pancreatic cancer in patients who are poor candidates for surgery.
COMMENT
Background
Most patients with pancreatic cancer are at advanced stage when apparent symptoms occur. Treatment of pancreatic cancer remains a great challenge, and new treatment modalities are urgently needed. In this study, gemcitabine was used as a cytotoxic drug, and cytotoxic and antitumor effects of combined gemcitabine and photodynamic therapy (PDT) were evaluated.
Research frontiers
Gemcitabine may have a value in treatment of pancreatic cancer. Recently, more studies on PDT have been focused on solid pancreatic caner. The prognosis of pancreatic cancer is poor. This is the first report on the synergetic anticancer effect of combined gemcitabine and PDT on pancreatic cancer in vivo.
Innovations and breakthroughs
The results of this study indicate that PDT-cytotoxic therapy (small dose gemcitabine) could significantly inhibit the growth of pancreatic cancer, and showed a relative long duration of anti-tumor effect compared with PDT. This study first demonstrated that low dose gemcitabine could increase the anticancer effect of PDT with no obvious adverse effects.
Applications
Combined PDT and gemcitabine therapy can be used in treatment of patients who are poor candidates for surgery.
Terminology
PDT is a way to produce local tissue necrosis with light (most conveniently from a laser) after administration of a photosensitizing agent in the presence of oxygen. PDT is based on the use of photosensitizing compounds that localize quite selectively in neoplastic/hyperplastic tissues and become cytotoxic when exposed to light.
Peer review
The study revealed that PDT can significantly inhibit the growth of pancreatic cancer, and its effect could be significantly enhanced by a small dose of gemcitabine. The findings are of great interest and provide a foundation for its application in clinical practice. The data are reliable and valuable.
Acknowledgments
The authors thank Wen-Ge Huang and Fen-Fen Guo for their excellent technical assistance.
Footnotes
Peer reviewer: Dr. Terumi Kamisawa, Department of Internal Medicine, Tokyo Metropolitan Komagome Hospital, 3-18-22 Honkomagome, Bunkyo-ku, Tokyo, Japan
S- Editor Tian L L- Editor Wang XL E- Editor Yin DH
References
- 1.Kern S, Hruban R, Hollingsworth MA, Brand R, Adrian TE, Jaffee E, Tempero MA. A white paper: the product of a pancreas cancer think tank. Cancer Res. 2001;61:4923–4932. [PubMed] [Google Scholar]
- 2.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 3.Richter A, Niedergethmann M, Sturm JW, Lorenz D, Post S, Trede M. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg. 2003;27:324–329. doi: 10.1007/s00268-002-6659-z. [DOI] [PubMed] [Google Scholar]
- 4.Dalton RR, Sarr MG, van Heerden JA, Colby TV. Carcinoma of the body and tail of the pancreas: is curative resection justified? Surgery. 1992;111:489–494. [PubMed] [Google Scholar]
- 5.Brennan MF, Moccia RD, Klimstra D. Management of adenocarcinoma of the body and tail of the pancreas. Ann Surg. 1996;223:506–511; discussion 511-512. doi: 10.1097/00000658-199605000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiMagno EP, Reber HA, Tempero MA. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. American Gastroenterological Association. Gastroenterology. 1999;117:1464–1484. doi: 10.1016/s0016-5085(99)70298-2. [DOI] [PubMed] [Google Scholar]
- 7.Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 8.Cardenes HR, Chiorean EG, Dewitt J, Schmidt M, Loehrer P. Locally advanced pancreatic cancer: current therapeutic approach. Oncologist. 2006;11:612–623. doi: 10.1634/theoncologist.11-6-612. [DOI] [PubMed] [Google Scholar]
- 9.Haefner M, Bluethner T, Niederhagen M, Moebius C, Wittekind C, Mossner J, Caca K, Wiedmann M. Experimental treatment of pancreatic cancer with two novel histone deacetylase inhibitors. World J Gastroenterol. 2008;14:3681–3692. doi: 10.3748/wjg.14.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bown SG, Rogowska AZ, Whitelaw DE, Lees WR, Lovat LB, Ripley P, Jones L, Wyld P, Gillams A, Hatfield AW. Photodynamic therapy for cancer of the pancreas. Gut. 2002;50:549–557. doi: 10.1136/gut.50.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan BG, Andren-Sandberg A. Photodynamic therapy for pancreatic cancer. Pancreas. 2007;34:385–389. doi: 10.1097/mpa.0b013e3180439c50. [DOI] [PubMed] [Google Scholar]
- 12.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayaru L, Wittmann J, Macrobert AJ, Novelli M, Bown SG, Pereira SP. Photodynamic therapy using verteporfin photosensitization in the pancreas and surrounding tissues in the Syrian golden hamster. Pancreatology. 2007;7:20–27. doi: 10.1159/000101874. [DOI] [PubMed] [Google Scholar]
- 14.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 15.Herrera-Ornelas L, Petrelli NJ, Mittelman A, Dougherty TJ, Boyle DG. Photodynamic therapy in patients with colorectal cancer. Cancer. 1986;57:677–684. doi: 10.1002/1097-0142(19860201)57:3<677::aid-cncr2820570347>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 16.Kostron H, Fritsch E, Grunert V. Photodynamic therapy of malignant brain tumours: a phase I/II trial. Br J Neurosurg. 1988;2:241–248. doi: 10.3109/02688698808992675. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez S, Arnfield MR, Meeker BE, Tulip J, Lakey WH, Chapman JD, McPhee MS. Treatment of Dunning R3327-AT rat prostate tumors with photodynamic therapy in combination with misonidazole. Cancer Res. 1986;46:2858–2862. [PubMed] [Google Scholar]
- 18.Zimmermann A, Walt H, Haller U, Baas P, Klein SD. Effects of chlorin-mediated photodynamic therapy combined with fluoropyrimidines in vitro and in a patient. Cancer Chemother Pharmacol. 2003;51:147–154. doi: 10.1007/s00280-002-0549-9. [DOI] [PubMed] [Google Scholar]
- 19.van Geel IP, Oppelaar H, Oussoren YG, Schuitmaker JJ, Stewart FA. Mechanisms for optimising photodynamic therapy: second-generation photosensitisers in combination with mitomycin C. Br J Cancer. 1995;72:344–350. doi: 10.1038/bjc.1995.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirveliene V, Grazeliene G, Dabkeviciene D, Micke I, Kirvelis D, Juodka B, Didziapetriene J. Schedule-dependent interaction between Doxorubicin and mTHPC-mediated photodynamic therapy in murine hepatoma in vitro and in vivo. Cancer Chemother Pharmacol. 2006;57:65–72. doi: 10.1007/s00280-005-0006-7. [DOI] [PubMed] [Google Scholar]
- 21.Peterson CM, Shiah JG, Sun Y, Kopeckova P, Minko T, Straight RC, Kopecek J. HPMA copolymer delivery of chemotherapy and photodynamic therapy in ovarian cancer. Adv Exp Med Biol. 2003;519:101–123. doi: 10.1007/0-306-47932-X_7. [DOI] [PubMed] [Google Scholar]
- 22.Snyder JW, Greco WR, Bellnier DA, Vaughan L, Henderson BW. Photodynamic therapy: a means to enhanced drug delivery to tumors. Cancer Res. 2003;63:8126–8131. [PubMed] [Google Scholar]
- 23.Streckyte G, Didziapetriene J, Grazeliene G, Prasmickiene G, Sukeliene D, Kazlauskaite N, Characiejus D, Griciute L, Rotomskis R. Effects of photodynamic therapy in combination with Adriamycin. Cancer Lett. 1999;146:73–86. doi: 10.1016/s0304-3835(99)00241-4. [DOI] [PubMed] [Google Scholar]
- 24.Hopper C. Photodynamic therapy: a clinical reality in the treatment of cancer. Lancet Oncol. 2000;1:212–219. doi: 10.1016/s1470-2045(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 25.Jia L, Zhang MH, Yuan SZ, Huang WG. Antiangiogenic therapy for human pancreatic carcinoma xenografts in nude mice. World J Gastroenterol. 2005;11:447–450. doi: 10.3748/wjg.v11.i3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheng C, Pogue BW, Wang E, Hutchins JE, Hoopes PJ. Assessment of photosensitizer dosimetry and tissue damage assay for photodynamic therapy in advanced-stage tumors. Photochem Photobiol. 2004;79:520–525. doi: 10.1562/mu-03-33.1. [DOI] [PubMed] [Google Scholar]
- 27.Laftavi MR, Lens P, Margonari J, Cathingol D, Dubernard JM, Martin X. Photodynamic therapy can selectively eradicate pancreas exocrine secretion. Transplant Proc. 1998;30:596–598. doi: 10.1016/s0041-1345(97)01422-x. [DOI] [PubMed] [Google Scholar]
- 28.Bown SG, Lovat LB. The biology of photodynamic therapy in the gastrointestinal tract. Gastrointest Endosc Clin N Am. 2000;10:533–550. [PubMed] [Google Scholar]
- 29.Abulafi AM, Allardice JT, Williams NS, van Someren N, Swain CP, Ainley C. Photodynamic therapy for malignant tumours of the ampulla of Vater. Gut. 1995;36:853–856. doi: 10.1136/gut.36.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tseng WW, Saxton RE, Deganutti A, Liu CD. Infrared laser activation of indocyanine green inhibits growth in human pancreatic cancer. Pancreas. 2003;27:e42–e45. doi: 10.1097/00006676-200310000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Schroder T, Chen IW, Sperling M, Bell RH Jr, Brackett K, Joffe SN. Hematoporphyrin derivative uptake and photodynamic therapy in pancreatic carcinoma. J Surg Oncol. 1988;38:4–9. doi: 10.1002/jso.2930380103. [DOI] [PubMed] [Google Scholar]
- 32.Wilson BC. Potential applications of photodynamic therapy in regenerative medicine. J Craniofac Surg. 2003;14:278–283. doi: 10.1097/00001665-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Cappella P, Tomasoni D, Faretta M, Lupi M, Montalenti F, Viale F, Banzato F, D’Incalci M, Ubezio P. Cell cycle effects of gemcitabine. Int J Cancer. 2001;93:401–408. doi: 10.1002/ijc.1351. [DOI] [PubMed] [Google Scholar]
- 34.Reid JM, Qu W, Safgren SL, Ames MM, Krailo MD, Seibel NL, Kuttesch J, Holcenberg J. Phase I trial and pharmacokinetics of gemcitabine in children with advanced solid tumors. J Clin Oncol. 2004;22:2445–2451. doi: 10.1200/JCO.2004.10.142. [DOI] [PubMed] [Google Scholar]
- 35.Moog R, Burger AM, Brandl M, Schuler J, Schubert R, Unger C, Fiebig HH, Massing U. Change in pharmacokinetic and pharmacodynamic behavior of gemcitabine in human tumor xenografts upon entrapment in vesicular phospholipid gels. Cancer Chemother Pharmacol. 2002;49:356–366. doi: 10.1007/s00280-002-0428-4. [DOI] [PubMed] [Google Scholar]
- 36.Reddy LH, Khoury H, Paci A, Deroussent A, Ferreira H, Dubernet C, Decleves X, Besnard M, Chacun H, Lepetre-Mouelhi S, et al. Squalenoylation favorably modifies the in vivo pharmacokinetics and biodistribution of gemcitabine in mice. Drug Metab Dispos. 2008;36:1570–1577. doi: 10.1124/dmd.108.020735. [DOI] [PubMed] [Google Scholar]


