Abstract
Probiotics promote intestinal epithelial integrity and reduce infection and diarrhea. We evaluated the effect of Lactobacillus rhamnosus GG-produced soluble proteins (p40 and p75) on the hydrogen peroxide-induced disruption of tight junctions and barrier function in Caco-2 cell monolayers. Pretreatment of cell monolayers with p40 or p75 attenuated the hydrogen peroxide-induced decrease in transepithelial resistance and increase in inulin permeability in a time- and dose-dependent manner. p40 and p75 also prevented hydrogen peroxide-induced redistribution of occludin, ZO-1, E-cadherin, and β-catenin from the intercellular junctions and their dissociation from the detergent-insoluble fractions. Both p40 and p75 induced a rapid increase in the membrane translocation of PKCβI and PKCε. The attenuation of hydrogen peroxide-induced inulin permeability and redistribution of tight junction proteins by p40 and p75 was abrogated by Ro-32-0432, a PKC inhibitor. p40 and p75 also rapidly increased the levels of phospho-ERK1/2 in the detergent-insoluble fractions. U0126 (a MAP kinase inhibitor) attenuated the p40- and p75-mediated reduction of hydrogen peroxide-induced tight junction disruption and inulin permeability. These studies demonstrate that probiotic-secretory proteins protect the intestinal epithelial tight junctions and the barrier function from hydrogen peroxide-induced insult by a PKC- and MAP kinase-dependent mechanism.
Keywords: intestine, mucosal protection, occludin, zonula occludens-1, protein kinase C, Lactobacillus rhamnosus GG
PROBIOTICS ARE LIVE MICROORGANISMS that confer health benefits to the host including disease prevention and/or treatment (13). Given the substantial increase in probiotic research in basic sciences and clinical studies, Food and Agriculture Organization/World Health Organization define probiotics as “live microorganisms which, when consumed in adequate amounts as part of food, confer a health benefit on the host” (23). Clinical applications of probiotics include treatment or prevention of inflammatory bowel diseases, diarrhea, irritable bowel syndrome, gluten intolerance, gastroenteritis, and Helicobacter pylori infection (4). To exert beneficial roles on the host, probiotics regulate intestinal epithelial homeostasis, such as promotion of cell survival and barrier function (17, 24, 25), improve intestinal microbial ecology, and regulate immune function (26).
Lactobacillus rhamnosus GG (LGG), a natural occurring bacterium originally isolated from the healthy human intestine (8), is one of the best-studied probiotic bacteria in clinical trials for treating and/or preventing several intestinal disorders, including inflammatory bowel diseases and diarrhea (26). Recent mechanistic studies using LGG as a model of probiotic bacterium find that LGG prevent cytokine-induced apoptosis in intestinal epithelial cells through activation of Akt and inhibition of p38 activation. More importantly, constituents recovered from LGG culture broth supernatant stimulate Akt action to prevent cytokine-induced apoptosis in intestinal epithelial cells (24). Furthermore, two LGG-produced soluble proteins, p75 and p40, have been successfully purified and cloned. Both p75 and p40 activate Akt and regulate intestinal epithelial cell antiapoptotic responses (25).
Intestinal epithelial tight junction (TJ) prevents the diffusion of potential injurious factors from the gastrointestinal lumen into the tissue (1). Disruption of TJ and elevated permeability to luminal toxins, allergens, and pathogens play a crucial role in the pathogenesis of a number of gastrointestinal diseases such as inflammatory bowel disease, celiac disease, and alcoholic liver disease. Proinflammatory factors such as reactive oxygen species (3, 14–16, 18), cytokines (5, 22), and toxins (19) disrupt the TJ and compromise the barrier function of the intestinal epithelium. The factors that prevent this inflammation-mediated disruption of the TJ and barrier function may provide potential therapeutic benefit in the treatment of many gastrointestinal diseases. TJ is formed by the organization of a number of specific proteins including occludin, zonula occludens (ZO-1, ZO-2, and ZO-3), claudins, and junctional adhesion molecule (1). Previous studies have demonstrated that hydrogen peroxide (H2O2) disrupts TJs in the Caco-2 cell monolayer by a mechanism involving phosphatidylinositol 3-kinase (18) and c-Src (3). H2O2 induces the redistribution of TJ and adherens junction (AJ) proteins, occludin, ZO-1, E-cadherin, and β-catenin, from the intercellular junctions into the intracellular compartments.
In the present study we evaluated the effect of proteins secreted by probiotic LGG. The results show that 1) pretreatment of Caco-2 cell monolayers with LGG culture supernatant (LGG-s) or LGG-produced soluble proteins (p40 and p75) significantly diminish H2O2-induced disruption of barrier function and increase in paracellular permeability without affecting the H2O2 level, 2) probiotic proteins prevent the H2O2-induced redistribution of TJ and AJ proteins and attenuate the H2O2-induced loss of detergent-insoluble fractions of TJ and AJ proteins, 3) p40 and p75 induce the membrane translocation of PKCβI and PKCε and prevent the H2O2-induced disruption of TJ by a PKC-dependent mechanism, and 4) p40 and p75 rapidly activate ERK1/2 and prevent the H2O2-induced disruption of TJ by a MAP kinase-dependent mechanism.
MATERIALS AND METHODS
Chemicals
Cell culture supplies were obtained from Invitrogen (San Jose, CA), and Transwell inserts and other cell culture plastic wares were purchased from Costar (Cambridge, MA). FITC-inulin, H2O2, and BSA were purchased from Sigma-Aldrich (St. Louis, MO). Ro-32-0432 and U0126 were purchased from EMD Chemicals (San Diego, CA). Other fine chemicals and laboratory supplies were obtained from Fisher Scientific (Pittsburgh, PA) or Sigma-Aldrich.
Antibodies
Mouse monoclonal anti-occludin antibody and rabbit polyclonal anti-occludin and anti-ZO-1 antibodies were purchased from Zymed Laboratories (South San Francisco, CA). Rabbit polyclonal anti-β-catenin, anti-phospho-Akt, anti-actin, anti-PKCε, and anti-PKCβI antibodies were purchased from Upstate USA (Lake Placid, NY), and mouse monoclonal anti-E-cadherin antibody was purchased from BD Transduction Laboratories (Lexington, KY). AlexaFluor 488-conjugated anti-mouse IgG was obtained from Molecular Probes (Eugene, OR) and Cy3-conjugated anti-rabbit IgG was purchased from Sigma Immunochemicals (St. Louis, MO). Rabbit polyclonal anti-phospho-ERK1/2 and anti-phospho-Akt antibodies were obtained from Cell Signaling Technology (Danvers, MA). Polyclonal antibodies against p75 and p40 were generated as described before (25) and conjugated to protein A/G beads (Santa Cruz Bio-technology, Santa Cruz, CA) by incubating antibodies with beads in PBS for 2 h at 4°C.
Preparation of LGG-s and isolation of p40 and p75
LGG (ATCC 531030) were cultured in Lactobacillus MRS broth at 37°C according to ATCC guidelines. Bacteria were harvested from MRS broth by centrifugation and washed twice with phosphate-buffered saline (PBS). Following centrifugation, the bacteria-free supernatant (LGG-s) was passed through a 0.2-μm filter (24). Purification of p40 and p75 from LGG-s has been described before (24). LGG-s was loaded onto UNOsphere S ion-exchange media (Bio-Rad Laboratories, Hercules, CA). Bound proteins were eluted using 30 nM Tris, pH 7.3, containing sequential concentrations of NaCl (100–800 mM). Eluted proteins were then concentrated by using Amicon Ultra-4 centrifugal filter devices (Millipore, Bedford, MA). Protein concentrations were determined by using a DC protein assay (Bio-Rad Laboratories).
For immunodepletion of p40 and p75, LGG-s was incubated with anti-p75 antibody-conjugated beads for 4 h at 4°C. After removal of anti-p75 antibody-conjugated beads, LGG-s was incubated with anti-p40 antibody-conjugated beads for another 4 h. LGG-s incubated with preimmune-IgG on beads was used as the negative control. The amounts of p75 and p40 present in LGG-s or immunodepleted LGG-s were detected by immunoblot analysis.
Cell culture
Caco-2, T84, and HT29 cells were purchased from American Type Culture Collection (Rockville, MD) and grown under standard cell culture conditions as described before (3, 14–17). Cells were grown on polycarbonate membranes in Transwell inserts (6.5, 12, or 24 mm; Costar). The experiments were conducted 11–13 days (6.5 or 12 mm Transwells) or 17–19 days (24 mm Transwell) postseeding.
Cell treatments
H2O2 (10–100 μM) in PBS (Dulbecco’s saline containing 1.2 mM CaCl2, 1 mM MgCl2, and 0.6% BSA) was administered to both the apical and the basal media as previously described (3, 14–16, 18) to Caco-2, T84, or HT29 cell monolayers. Probiotic proteins, LGG-s (1–10 μg/ml), p40 (0.1–1.0 μg/ml), and p75 (0.1–1.0 μg/ml) were administered to the apical, basal, or apical and basal media 30 min prior to H2O2 administration. In some experiments, cells were pretreated with probiotics and washed off prior to H2O2 administration. U0126 (10 μM) or Ro-32-0432 (1 μM) was administered to both the apical and basal media 30 min prior to probiotic administration. Control cell monolayers were incubated in PBS without H2O2 and/or inhibitors.
Measurement of TER
Transepithelial resistance (TER) was measured as described before (11) using a Millicell-ERS Electrical Resistance System (Millipore). The TER varied from 550 to 650 Ω·cm2. TER recorded in unseeded Transwell inserts (usually 50–80 Ω·cm2) was subtracted from all values.
Unidirectional flux of inulin
Unidirectional flux of inulin was measured by incubating cell monolayers in the presence of 0.5 μg/ml of FITC-inulin in the basal well. At different times, 100 μl each of apical and basal media were withdrawn and fluorescence was measured in a microplate fluorescence reader (FLX-800 fluorescence microplate reader; Bio TEK Instruments, Winooski, VT). Flux into the apical well was calculated as a percentage of the total fluorescence administered into the basal well per hour per square centimeter of surface area.
H2O2assay
The levels of H2O2 were measured as described previously (12). Aliquots (100 μl) of samples were incubated with 100 μl of phenol red solution containing 40 U/ml of horseradish peroxidase and 1.16 mM phenol red in PBS in 96-well plates at room temperature for 15 min. Reaction was terminated by adding 10 μl of 1 N sodium hydroxide, and the absorbance was read at 610 nm in an automated plate reader. A standard curve was constructed by use of 2–50 μM H2O2.
Immunofluorescence microscopy
Cell monolayers were fixed in acetone-methanol (1:1) at 0°C for 5 min. The fixed membranes were rehydrated in PBS and permeabilized with 0.2% Triton X-100 in PBS. Cell monolayers were then blocked with 4% nonfat milk in TBST (Tris-buffered saline containing 0.05% Tween-20) and were then stained with a mixture of mouse monoclonal anti-occludin and rabbit polyclonal anti-ZO-1 antibodies or mouse monoclonal anti-E-cadherin antibody and rabbit polyclonal anti-β-catenin antibody. A mixture of AlexaFluor 488-conjugated anti-mouse IgG and Cy3-conjugated anti-rabbit IgG antibodies was used as secondary antibodies. Cells were mounted and the images were collected via a confocal laser scanning microscope (Zeiss LSM510 PASCAL) as a series of images of 1-μm XY sections. The images were stacked by using the Image J software and processed by Adobe Photoshop (Adobe Systems, San Jose, CA).
Preparation of plasma membrane fraction
Plasma membrane fractions were prepared by the method described before (3). Briefly, cell monolayers (24 mm) were washed twice with ice-cold PBS and once with lysis buffer F (PBS containing 10 mM β-glycerophosphate, 2 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml bestatin, 10 μg/ml pepstatin-A, 1 mM benzamidine, and 1 mM PMSF). Cells suspended in lysis buffer F were dispersed by homogenization in a glass/Teflon Dounce homogenizer with 50 strokes in and lysed by sonication at 4°C with two strokes (5 s each) with a 30-s interval between the strokes. The cell lysate was centrifuged first at 3,000 g for 10 min at 4°C to sediment the cell debris and then at 30,000 g for 30 min at 4°C to pellet the plasma membrane. The membrane fraction was dissolved in Laemmli’s sample buffer and heated at 100°C for 5 min.
Preparation of detergent-insoluble fractions
Cell monolayers in Transwell inserts (24 mm) were washed twice with ice-cold PBS and incubated for 15 min at 4°C with lysis buffer-CS (Tris buffer containing 1.0% Triton X-100, 2 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml bestatin, 10 μg/ml pepstatin-A, 1 mM vanadate, and 1 mM PMSF). Cell lysates were scraped from the substratum and passed through a pipette tip five times. It was then centrifuged at 15,600 g for 4 min at 4°C to sediment the high-density actin cytoskeleton. The pellet was suspended in 200 μl of lysis buffer-CS. Protein contents in different fractions were measured by the BCA method (Pierce Bio-technology, Rockford, IL). The Triton-insoluble and Triton-soluble fractions were mixed with equal volume of Laemmli’s sample buffer (2× concentrated) and heated at 100°C for 5 min and stored until immunoblot analysis. Triton-insoluble fractions were immunoblotted for occludin, ZO-1, E-cadherin, and β-catenin. Samples were also immunoblotted for actin as a housekeeping protein. The experiment was repeated at least two times.
Immunoblot analysis
Proteins in the plasma membrane or the detergent-insoluble and -soluble fractions were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. Blots were probed for occludin, ZO-1, E-cadherin, β-catenin, PKCβI, PKCε, phospho-ERK1/2, or actin. Horseradish peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG antibodies were used as secondary antibodies. The blots were developed by the enhanced chemiluminescence method (Amersham, Arlington Heights, IL). Immunoblot analysis for each experiment was repeated at least two times with similar results.
Statistics
Comparison between two groups was made by the Student’s t-tests (unpaired) for grouped data. The significance in all tests was derived at the 95% or greater confidence level.
RESULTS
LGG-s attenuates the H2O2-induced disruption of barrier function
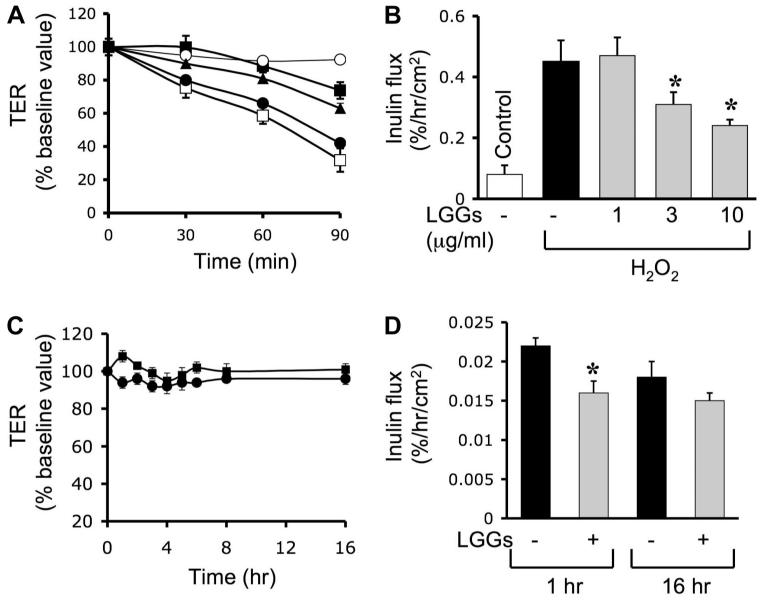
Previous study showed that H2O2 disrupts TJ and increases the paracellular permeability in Caco-2 cell monolayers (3, 14–16, 18). In the present study, we evaluated the effect of unpurified LGG growth medium (LGG-s) and the soluble proteins, p40 and p75, purified from LGG-s, on the H2O2-induced increase in paracellular permeability. Incubation of cell monolayers with H2O2 reduced the TER (Fig. 1A) and increased the inulin permeability (Fig. 1B) in a time-dependent manner. Treatment of cells with LGG-s significantly reduced the H2O2-induced decrease in TER (Fig. 1A) and increase in the inulin permeability (Fig. 1B) in a time- and dose-dependent manner. LGG-s by itself showed a slight increase in TER (Fig. 1C) and a reduction in the inulin flux (Fig. 1D); this effect lasted for at least 16 h. Pretreatment of cell monolayers with p40 (Fig. 2A) or p75 (Fig. 2B) also significantly and dose dependently attenuated the H2O2-induced decrease in TER. Both p40 and p75 significantly reduced H2O2-induced increase in inulin permeability (Fig. 2C). By themselves, p40 and p75 produced no significant effect on the inulin permeability of Caco-2 cell monolayers (Fig. 2D).
Fig. 1.
Lactobacillus rhamnosus GG (LGG) culture supernatant (LGG-s) prevents H2O2-induced paracellular permeability. A and B: Caco-2 cell monolayers were incubated with or without H2O2 (20 μM) in the absence (□) or presence of 1 μg/ml (●), 3 μg/ml (▲), or 10 μg/ml (■) of LGG-s (unpurified LGG growth medium, administered 30 min prior to H2O2). Transepithelial resistance (TER; A) was measured at varying times, and inulin flux (B) was measured at 2 h after H2O2 administration. C: TER was measured in cell monolayers treated with (□) or without (○) probiotics in the absence of H2O2. Values are means ± SE (n = 8; n includes values from 4 independent experiments). D: inulin flux was measured in cell monolayers treated with or without LGG-s in the absence of H2O2 at 1 or 16 h. Values are means ± SE (n = 8). *Significantly (P < 0.05) different from corresponding values for cell monolayers without LGG-s treatment.
Fig. 2.
p40 and p75 prevent H2O2-induced paracellular permeability. A and B: Caco-2 cell monolayers were incubated with or without H2O2 (20 μM) in the absence (■) or presence of 0.1 μg/ml (▲), 0.3 μg/ml (◆), or 1.0 μg/ml (●) of p40 (A) or p75 (B) that were administered 30 min prior to H2O2. TER was measured at varying times. Values are means ± SE (n = 8; values from 3 independent experiments). C: inulin flux was measured in cell monolayers treated with or without varying concentrations of p40 and p75. Inulin permeability was measured at 2 h after H2O2 administration. Values are means ± SE (n = 8). *Significantly (P < 0.05) different from control values; #different from value for H2O2-treated cells without p40 or p75. D: inulin flux was measured in cell monolayers treated with or without p40 or p75 in the absence of H2O2. Values are means ± SE (n = 8). E: Caco-2 cell monolayers were incubated with or without H2O2 (20 μM) in the absence or presence of LGG-s (3 μg/ml), p40 (1.0 μg/ml), or p75 (1.0 μg/ml). Three hours after H2O2 administration the levels of H2O2 were measured in the incubation medium. Values are means ± SE (n = 4).
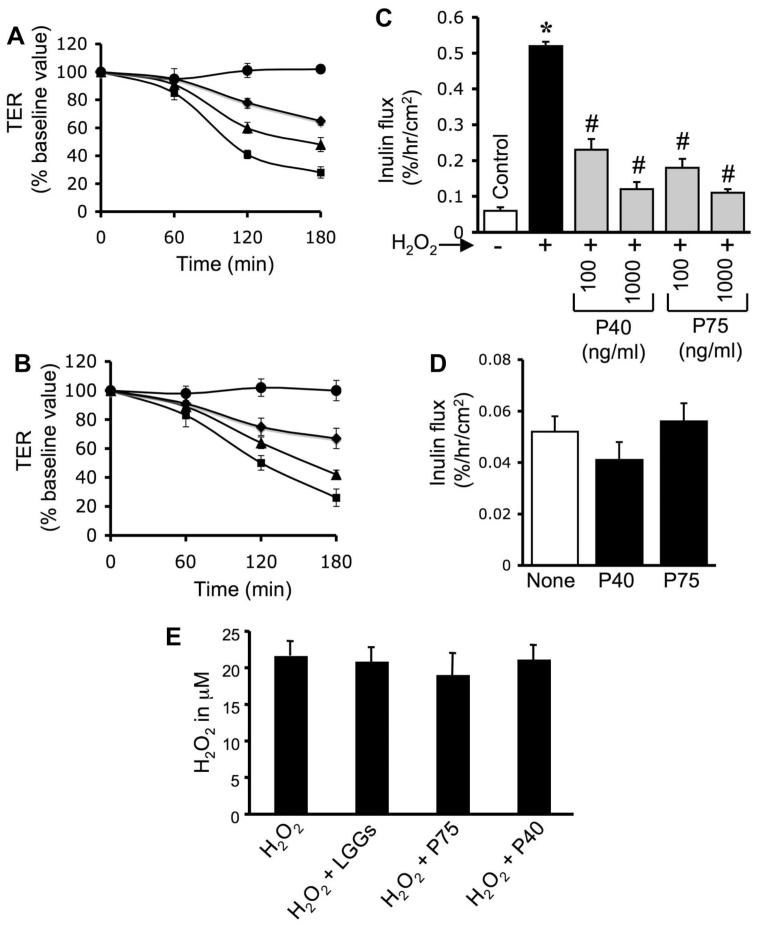
One possible mechanism of probiotic-mediated protection is the degradation of H2O2. To determine the effect of probiotics on the potential H2O2 degradation, we measured the level of H2O2 in the incubation medium after 3 h of treatment with LGG-s, p40, or p75. Results show that p40 and p75 do not change the level of H2O2 (Fig. 2E). Incubation of live and killed LGG also attenuated H2O2-induced decrease in TER (Fig. 3A) and increase in inulin permeability (Fig. 3B). This observation indicates that intact cell is not required for the protective effect of LGG. Incubation of LGG-s samples with anti-p40 and anti-p75 antibodies resulted in depletion of these proteins (Fig. 3C). The immunodepleted LGG-s failed to prevent H2O2-induced changes in TER (Fig. 3A) and inulin flux (Fig. 3B).
Fig. 3.
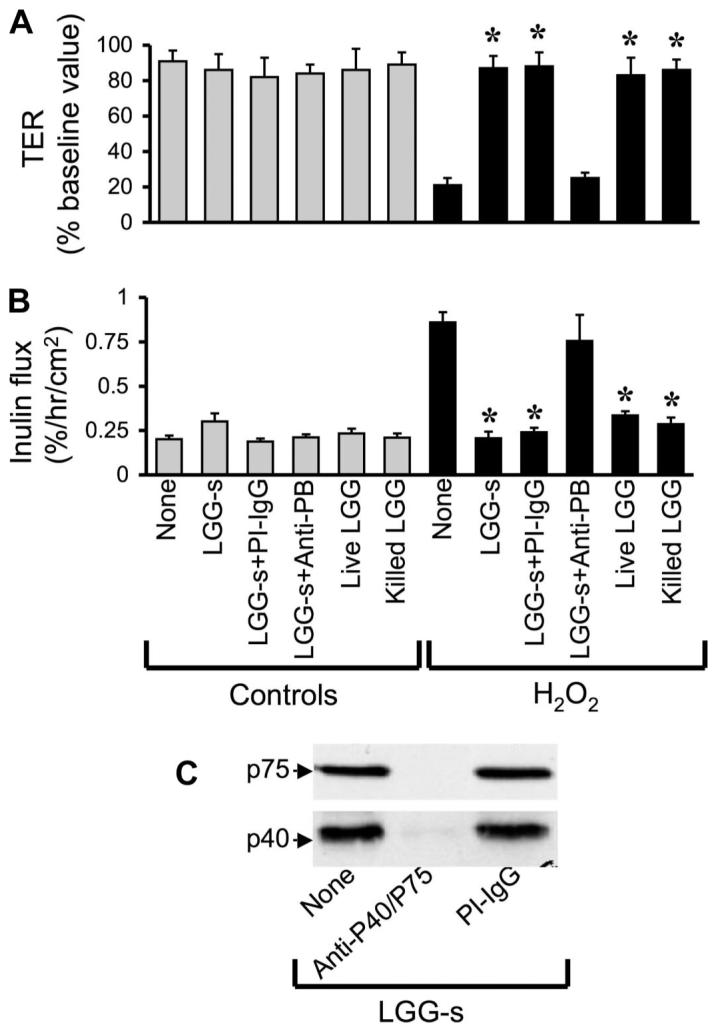
p40 and p75 mediate LGG-s-induced prevention of H2O2-induced paracellular permeability. A and B: Caco-2 cell monolayers were incubated with or without H2O2 (20 μM) in the absence or presence of live or killed LGG (106 cells/ml) or with 10 μg/ml LGG-s (untreated, immune-depleted using anti-probiotic proteins (PB) antibodies, or preimmune-IgG-treated) (administered 30 min prior to H2O2). TER (A) and inulin flux (B) were measured at 2 h after H2O2 administration. Values are means ± SE (n = 5, from 2 independent experiments). *Significantly (P < 0.05) different from corresponding values for H2O2 treatment. C: extracts of LGG-s that was untreated or treated with anti-p40/p75 or preimmune IgG were immunoblotted for p40 and p75.
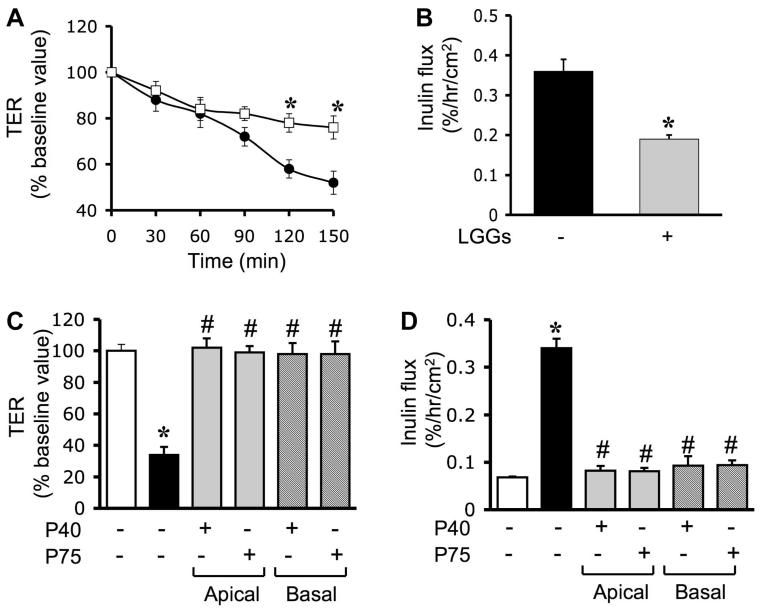
In all the above experiments, probiotics were added 30 min prior to the administration of H2O2, and the presence of probiotics continued during the H2O2 treatment. To determine the protective effect of probiotics in the absence of a direct contact between probiotics and H2O2, we pretreated the cell monolayers with LGG-s for 60 min, and the traces of LGG-s were washed off prior to the H2O2 administration. Results show that pretreatment alone was able to significantly attenuate the H2O2-induced decrease in TER (Fig. 4A) and increase in inulin permeability (Fig. 4B). To determine the polarity of the probiotic effect, cell monolayers were pretreated with p40 or p75 on the apical or basal surface. Results demonstrate that p40 and p75 attenuate the H2O2-induced decrease in TER (Fig. 4C) and the increase in inulin permeability (Fig. 4D) when they were administered to either the apical surface or the basal surface. Administration of H2O2 at varying concentrations induced dose-dependent increase in inulin permeability in both T84 (Fig. 5A) and HT29 (Fig. 5B) cell monolayers. Pretreatment with p75 significantly reduced H2O2-induced inulin permeability (Fig. 5) in both T84 and HT29 cell monolayers.
Fig. 4.
Probiotic factors at both apical and basal surfaces prevent H2O2-induced paracellular permeability. A and B: Caco-2 cell monolayers were incubated with ([□] and solid bar) or without (● and shaded bar) LGG-s for 60 min and washed twice to remove traces of probiotics prior to administration of H2O2. TER (A) was measured at varying times, and inulin flux (B) was measured at 2 h after H2O2 administration. Values are means ± SE (n = 6; values from 3 independent experiments). *Significantly (P < 0.05) different from corresponding values for cell monolayers without LGG-s treatment. C and D: Caco-2 cell monolayers were incubated with p40 or p75 (1.0 μg/ml) on the apical or basal surfaces for 30 min prior to administration of H2O2. TER (C) and inulin flux (D) were measured at 2 h after H2O2 administration. Values are means ± SE (n = 6). *Significantly (P < 0.05) different from corresponding control values; #different from corresponding values for H2O2-treated cells without p40 or p75.
Fig. 5.
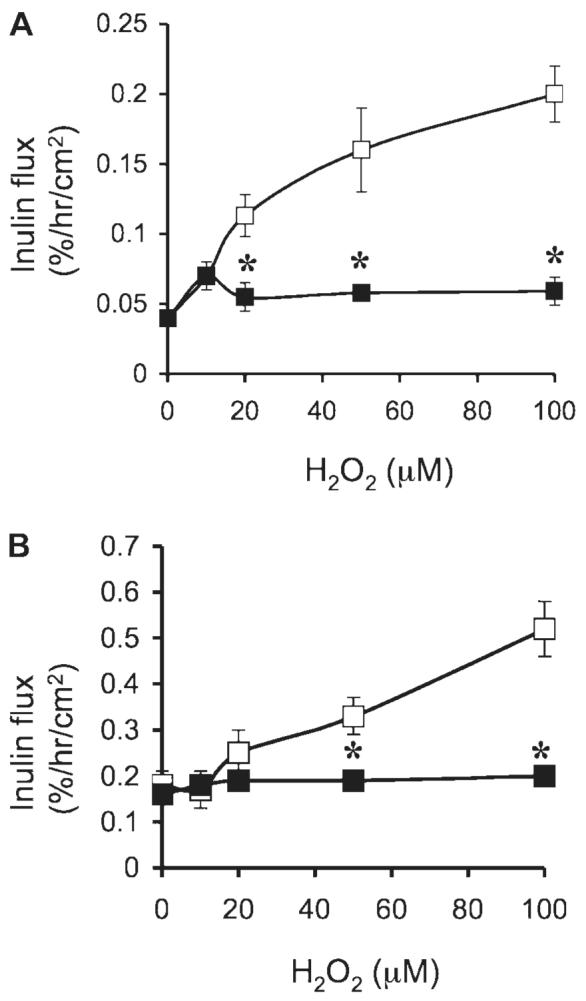
Probiotic proteins prevent H2O2-induced paracellular permeability in T84 and HT29 cell monolayers. T84 (A) and HT29 (B) cell monolayers were incubated with or without H2O2 (10–100 μM) in the absence ([□) or presence of 1 μg/ml (■) of p75 (administered 30 min prior to H2O2). Inulin flux was measured at 2 h after H2O2 administration. Values are means ± SE (n = 6 from 3 independent experiments). *Significantly (P < 0.05) different from corresponding values for cell monolayers without p75 treatment.
p40 and p75 prevent H2O2-induced disruption of TJ and AJ
To determine the protective effect of probiotic proteins on the H2O2-induced disruption of TJ, we analyzed the cellular distribution of the TJ and AJ proteins by immunofluorescence staining and confocal microscopy. Results show that the H2O2 treatment resulted in a redistribution of occludin and ZO-1 from the intercellular junctions into the intracellular compartment (Fig. 6). This H2O2-induced redistribution of occludin and ZO-1 was attenuated by the pretreatment of cells with p40 and p75.
Fig. 6.
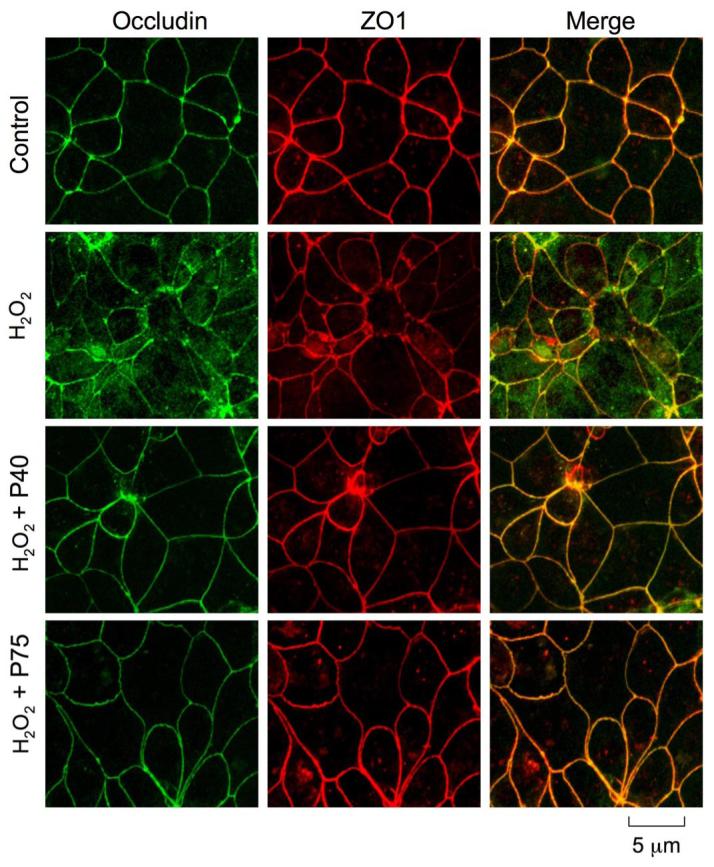
Probiotics prevent H2O2-induced redistribution of tight junction (TJ) proteins. Caco-2 cell monolayers were incubated with p40 or p75 for 30 min prior to the administration of H2O2. At 2 h after H2O2 administration cell monolayers were fixed in acetone-methanol and labeled for occludin (green) and zonula occludens (ZO)-1 (red) by immunofluorescence staining. Images were collected by using a confocal laser-scanning microscope.
Although AJ does not form a physical barrier to the diffusion of macromolecules across the epithelium, it is known to indirectly influence the integrity of TJ (10). Therefore, we investigated the effect of probiotics on H2O2-induced disruption of AJ. H2O2 treatment induced a redistribution of AJ proteins, E-cadherin, and β-catenin from the intercellular junctions into the intracellular compartments (Fig. 7). Pretreatment of cell monolayers with p40 or p75 attenuated the H2O2-induced redistribution of both E-cadherin and β-catenin.
Fig. 7.
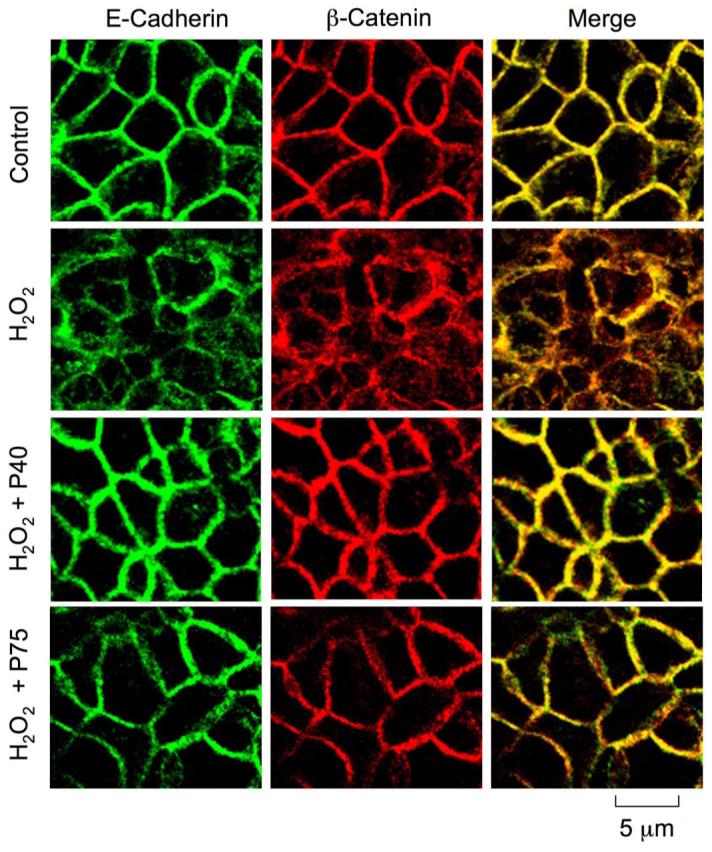
Probiotics attenuate H2O2-induced redistribution of adherens junction (AJ) proteins. Caco-2 cell monolayers were incubated with p40 or p75 for 30 min prior to the administration of H2O2. At 2 h after H2O2 administration cell monolayers were fixed in acetone-methanol and labeled for E-cadherin (green) and β-catenin (red) by immunofluorescence staining. Images were collected by using a confocal laser-scanning microscope.
Probiotic secretory proteins prevent H2O2-induced dissociation of TJ and AJ proteins from the detergent-insoluble fractions
TJ and AJ protein complexes interact with the actin cytoskeleton to anchor these protein complexes at the apical end of the lateral membranes (6). Our previous studies indicate that the detergent-insoluble fraction of TJ and AJ proteins are more relevant to the integrity of junctional complexes than the membrane-associated or the soluble pools of these proteins (16). Association of occludin and ZO-1 with the detergent-insoluble fraction was reduced during the H2O2-induced disruption of TJs, which was prevented by EGF (2). In the present study, we determined the effect of probiotics on the H2O2-induced reduction in detergent-insoluble fractions of TJ and AJ proteins. Both p40 and p75 attenuated the H2O2-induced reduction of occludin, ZO-1, E-cadherin, and β-catenin in the Triton-insoluble fractions (Fig. 8).
Fig. 8.
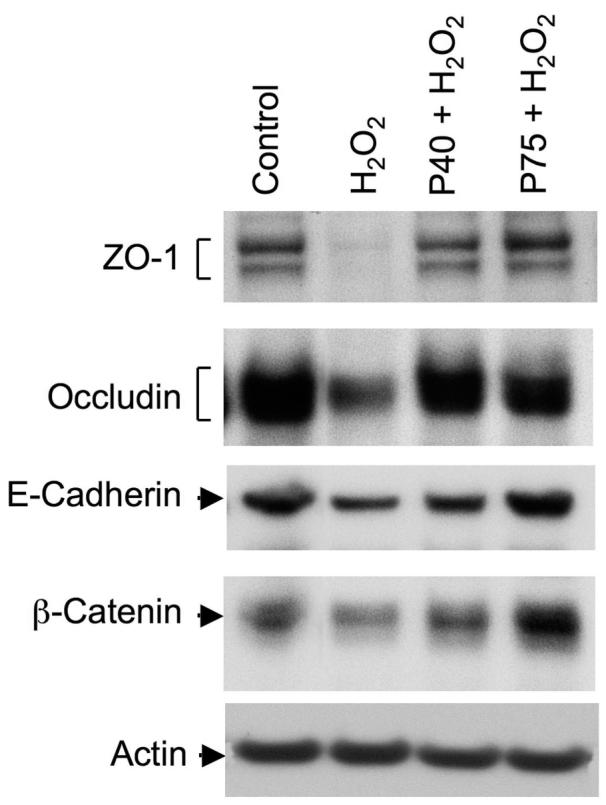
Probiotics ameliorate the H2O2-induced loss of detergent-insoluble fractions of TJ and AJ proteins. Caco-2 cell monolayers were incubated with p40 or p75 for 30 min prior to the administration of H2O2. At 2 h after H2O2 administration, Triton-insoluble fractions were prepared and immunoblotted for TJ and AJ proteins.
PKC activity is involved in the prevention of H2O2-induced TJ disruption by probiotic secretory proteins
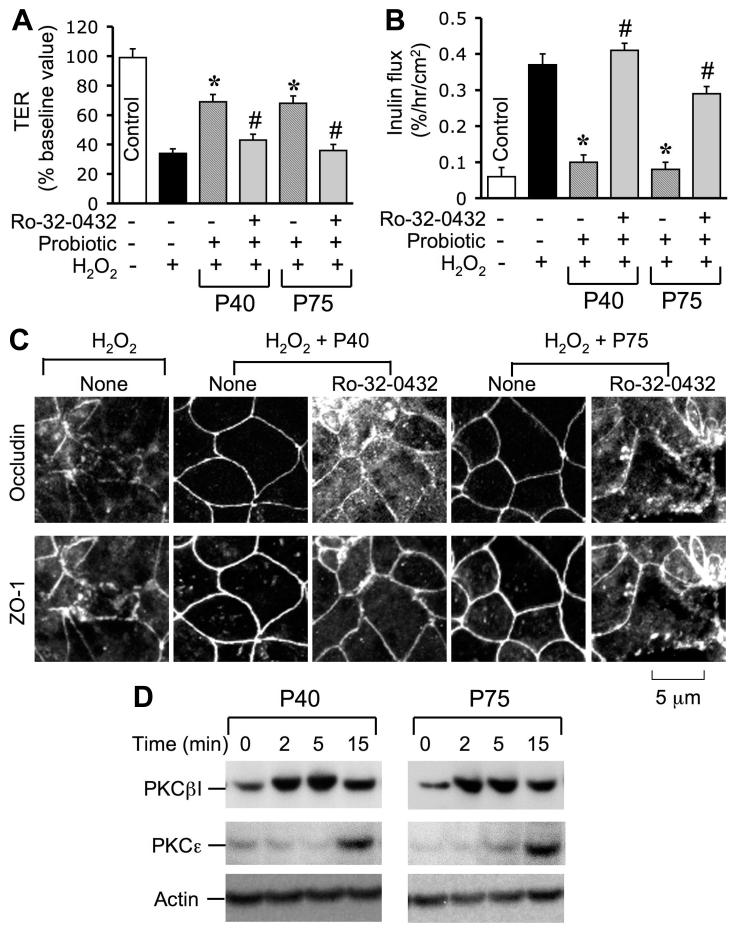
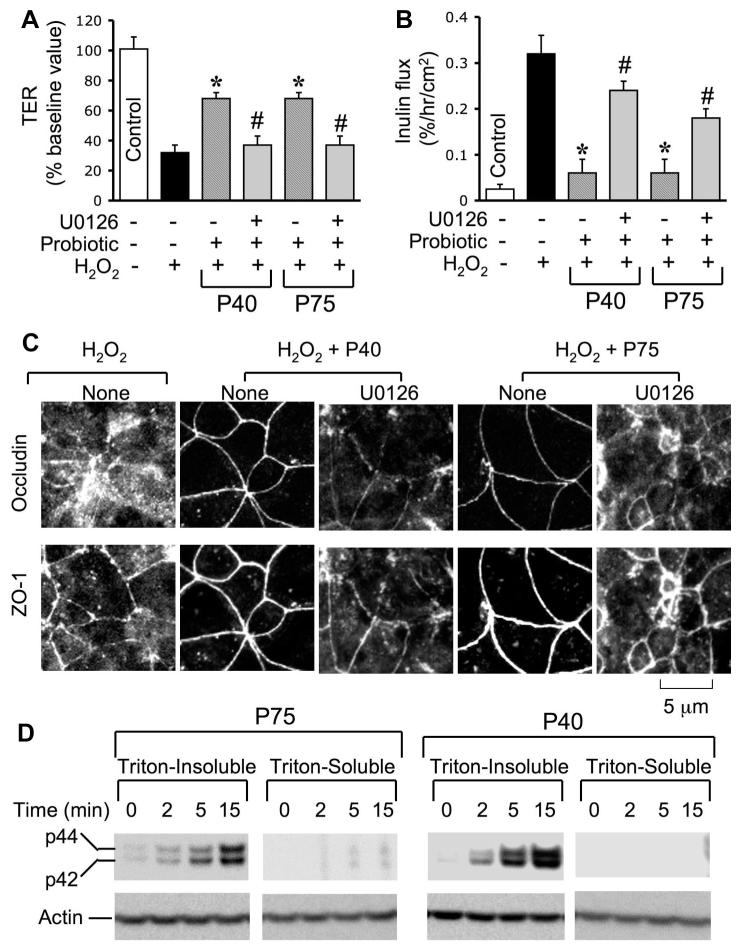
Evidence indicates that PKC activity is involved in the EGF-mediated protection of TJ from oxidative stress (7). To determine the mechanism involved in the probiotic-mediated protection of TJ from H2O2, we investigated the role of PKC activity in probiotic-mediated protection of TJ. Pretreatment of cell monolayers with Ro-32-0432 (a PKC-selective inhibitor) attenuated the p40- and p75-mediated prevention of H2O2-induced decrease in TER (Fig. 9A) and increase in inulin permeability (Fig. 9B). Ro-32-0432 also blocked p40- and p75-mediated prevention of H2O2-induced redistribution of occludin and ZO-1 from the intercellular junctions (Fig. 9C). p40 and p75 rapidly increased the translocation of PKCε and PKCβI into the plasma membrane fractions of the cell (Fig. 9D). p40 or p75 increased the plasma membrane-associated PKCβI at 2 min, whereas increase in membrane PKCε was detectable only at 15 min after probiotic administration.
Fig. 9.
PKC activity is involved in probiotics-mediated prevention of H2O2-induced disruption of TJ. A and B: Caco-2 cell monolayers were incubated with or without 20 μM H2O2 in the absence or presence of p40 or p75 (administered 30 min prior to H2O2) for 2 h. In some groups, cell monolayers were pretreated with Ro-32-0432 (1 μM) for 30 min prior to the administration of probiotics. TER (A) and inulin flux (B) were recorded at 2 h after H2O2 administration. Values are means ± SE (n = 6). *Significantly (P < 0.05) different from values for H2O2; #significantly different from values for probiotics + H2O2 group. C: Caco-2 cell monolayers were incubated with H2O2 in the absence or presence of probiotics and in the absence or presence of Ro-32-0432. Two hours after H2O2 treatment, cell monolayers were fixed and double labeled for occludin and ZO-1 by immunofluorescence staining. Fluorescence images were collected by confocal microscopy. D: Caco-2 cell monolayers were incubated with p40 or p75 for varying times. Plasma membrane fraction were prepared and immunoblotted for PKCε, PKCβI, and actin.
MAP kinase activity is required for the p40- and p75-mediated prevention of H2O2-induced disruption of TJ
Recent studies showed that ERK interacts directly with occludin and mediates the EGF-induced protection of TJ from H2O2 in Caco-2 cell monolayer (2). In the present study, we evaluated the role of MAP kinase in the p40- and p75-mediated prevention of H2O2-induced disruption of TJ. Pretreatment of cell monolayers with U0126 (a selective inhibitor of MEK) attenuated both p40- and p75-mediated prevention of H2O2-induced decrease in TER (Fig. 10A) and increase in inulin flux (Fig. 10B). U0126 also blocked the p40- and p75-mediated prevention of H2O2-induced redistribution of occludin and ZO-1 from the intercellular junctions (Fig. 10C). Probiotic proteins, p40 and p75, rapidly increased the levels of phospho-ERK1/2, the activated ERK1/2, predominantly in the Triton-insoluble fractions (Fig. 10D).
Fig. 10.
MAP kinase activity is involved in probiotics-mediated prevention of H2O2-induced disruption of TJ. A and B: Caco-2 cell monolayers were incubated without or with 20 μM H2O2 in the absence or presence of p40 or p75 (administered 30 min prior to H2O2) for 2 h. In some groups, cell monolayers were pretreated with U0126 (10 μM) for 30 min prior to the administration of probiotics. TER (A) and inulin flux (B) were recorded at 2 h after H2O2 administration. Values are means ± SE (n = 6). *Significantly (P < 0.05) different from values for H2O2; #significantly different from values for probiotics + H2O2 group. C: Caco-2 cell monolayers were incubated with H2O2 in the absence or presence of probiotics and in the absence or presence of U0126. Two hours after H2O2 treatment, cell monolayers were fixed and double labeled for occludin and ZO-1 by immunofluorescence staining. Fluorescence images were collected by confocal microscopy. D: Caco-2 cell monolayers were incubated with p40 or p75 for varying times. Triton-insoluble and soluble fractions were prepared and immunoblotted for phospho-ERK1/2. Blots were reblotted for actin as a housekeeping protein.
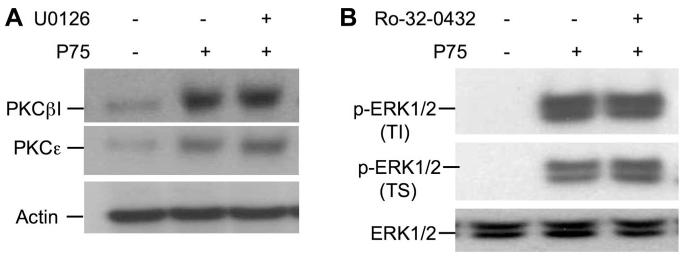
p75-induced membrane translocation of PKCε and PKCβI was unaffected by the pretreatment of cells with U0126 (Fig. 11A). Similarly, p75-induced phosphorylation of ERK1/2 was unaffected by Ro-32-0432 (Fig. 11B). Ro-32-0432 had no effect on the total level of ERK1/2.
Fig. 11.
EGF independently activates ERK1/2 and PKC isoforms. Caco-2 cell monolayers were preincubated with or without U0126 or Ro-32-0432 for 1 h prior to administration of p75 (1 μg/ml). After 15 min incubation with p75, plasma membrane (A) or detergent-insoluble fractions (B) were prepared and immunoblotted for PKCε, PKCβI, ERK, phospho-ERK1/2, and β-actin.
DISCUSSION
Probiotics protect the intestinal mucosa from numerous insults (9, 21, 24, 25, 27); however, the mechanism involved is not well understood. Recent studies have begun to shed some light on the mechanisms involved in the beneficial effects of probiotics in the gastrointestinal tract (24, 25). Probiotics may enhance the defense mechanisms in the gastrointestinal mucosa and prevent mucosal injury from various insults. In the present study, we show that the proteins secreted by a probiotic, LGG, protect the intestinal epithelium from oxidative stress. Secretory proteins, p40 and p75, attenuate the H2O2-induced disruption of TJ and AJ in Caco-2 cell monolayers, a well-established model of the intestinal epithelium. This effect of probiotic secretory proteins is mediated by the activation of PKC isoforms PKCε and PKCβI and by MAP kinases ERK1 and 2.
As previously demonstrated (3, 14–16, 18), H2O2 increases the paracellular permeability in Caco-2 cell monolayers. The attenuation of H2O2-induced decrease in TER and increase in inulin permeability by LGG-s, p40, and p75 indicated that the proteins secreted by LGG ameliorate the oxidative stress-induced increase in paracellular permeability. A previous study demonstrated that the probiotics protect the intestinal epithelial cells from TNF-α-induced apoptosis (24). Two different secretory proteins, p40 and p75, were isolated from LGG-s, and they promote cell survival and growth in the rat intestine (25). H2O2 at low concentration (20 μM) was previously shown to disrupt the TJ and increase the paracellular permeability without inducing apoptosis or cell necrosis (3, 14–16, 18). The present study shows that p40 and p75 induce a significant reduction in the H2O2-induced redistribution of occludin and ZO-1 from the intercellular junctions. Therefore, in addition to their antiapoptotic effect, probiotic proteins protect the TJ from H2O2-induced insult. Although ZO-1 and occludin levels in detergent-insoluble fractions are dramatically reduced by H2O2 treatment, confocal microscopy showed that redistribution of occludin from the junctions was much more pronounced compared with the redistribution of ZO-1. This may be explained by proposing a possibility that H2O2 had only minimal effect on the interaction between ZO-1 and claudins, and therefore a significant portion of ZO-1 remains to be localized at the intercellular junctions.
The AJ lies beneath the TJ and is formed by the organization of E-cadherin and β-catenin. Evidence suggests that the AJ indirectly regulates the integrity of TJ, and the present study shows that the probiotic proteins (p40 and p75) also prevent H2O2-induced redistribution of E-cadherin and β-catenin. Therefore, probiotics may stabilize both TJ and AJ and preserve the integrity of barrier function.
Probiotic secretory proteins, LGG-s, p40, and p75, did not change the level of H2O2, indicating that the effect of probiotic was not mediated by an antioxidant effect. This was further supported by the observation that LGG-s effectively prevents H2O2-induced disruption of barrier function when the cells were pretreated with LGG-s and removed before the administration of H2O2. p40 and p75 were effective in preventing H2O2-induced disruption of TJ even when they were administered only to the apical surface or the basal surface. This indicates that the necessary membrane receptors required for their protective effects are present in both the apical and the basolateral membranes of Caco-2 cell monolayers. Probiotic protein, p75, also prevented H2O2-induced disruption of barrier function in T84 and HT29 cell monolayers, indicating that this effect is not confined to one cell line.
Both p40 and p75 are novel bacterial proteins, which induce intestinal epithelial cell signal transduction and antiapoptotic responses (25). The deduced full-length p40 sequence is 79% identical to the sequence of a 396-amino acid protein of unknown function in L. casei 334 (NCBI GeneBank COG3883), and the partial sequence of p75, which is most closely related to a 493-amino-acid cell wall-associated hydrolase of L. casei 334 (NCBI GeneBank COG0791). The predicted molecular mass of the full-length cell wall-associated hydrolase of L. casei 334 (49 kDa) differs substantially from the molecular mass of the LGG p75 protein. The p40 gene sequence and the partial p75 gene sequence do not show significant similarity, and the experimentally determined NH2-terminal amino acid sequences of these two proteins are not related. Thus, on the basis of the available sequence data, there is no evidence to suggest that p40 is a degradation product of p75. However, it is possible that there could be sequence similarity between p40 and the uncharacterized COOH-terminal portion of p75. The mechanisms of p40 and p75 regulating cell signaling are an area of active investigation including identification of potential interacting proteins and potential receptor(s); however, no candidates have been clearly identified so far.
Both p40 and p75 effectively prevented H2O2-induced loss of TJ and AJ proteins from the detergent-insoluble fractions. Previous studies showed that the TJ and AJ proteins occludin, ZO-1, E-cadherin, and β-catenin are associated with the F-actin-rich Triton-insoluble fractions in an intact epithelium, and these fractions of the TJ and AJ proteins correlate well with the integrity of TJ (16). H2O2 caused a dissociation of these TJ and AJ proteins from the detergent-insoluble fractions, suggesting that the dissociation of TJ and AJ proteins from the actin cytoskeleton is one of the mechanisms involved in this TJ disruption. Probiotic proteins somehow prevent H2O2-induced loss of interaction between the actin cytoskeleton and the TJ and AJ proteins. Therefore, the protective effects of p40 and p75 are likely to be mediated by specific cellular mechanisms. Previous studies have shown that p40 and p75 activate Akt in intestinal epithelial cells (24). Therefore, we evaluated the effect of p40 and p75 in activation of Akt by immunoblot analysis of phospho-Akt. Results showed that phospho-Akt was undetectable in p40- or p75-treated Caco-2 cells. Therefore, Akt does not play a role in the protection of TJ from H2O2(data not shown).
Evidence suggests that PKC activity may be involved in EGF-mediated protection of the intestinal epithelial barrier function from oxidative stress (7). Therefore, we evaluated the role of PKC activity in the p40- and p75-mediated protection of TJ from H2O2. Attenuation of the p40- and p75-mediated protection of barrier function and TJ from H2O2 by Ro-32-0432 (a PKC-selective inhibitor) indicates that PKC activity is involved in the TJ protection by probiotic secretory proteins. Ro-32-0432 is known to selectively inhibit the activities of PKCα, PKCε, and PKCβI. Both p40 and p75 rapidly increase the membrane translocation of PKCε and PKCβI; membrane localization of PKCα was unaltered. This indicates that p40 and p75 induce the activation of PKCε and PKCβI, and this activation of the PKC isoforms may be required for the protection of TJ from H2O2. Maximal activation of PKCβI by EGF was achieved by 2 min, whereas PKCε translocation was detectable only at 15 min after EGF administration. This suggests that PKCβI activation may be one of the initial events in the mechanism of probiotic-mediated protection of TJ and AJ. PKCε may play a role in the downstream events of the signaling pathway involved in this process.
The present study also shows that MAP kinase activity is involved in the p40- and p75-mediated prevention of the H2O2-induced disruption of TJ and increase in paracellular permeability. U0126 (a MEK-selective inhibitor) attenuates the protective effect of p40 and p75 on TJ, indicating that these probiotic proteins activate ERK1/2 via MEK activity. This is confirmed by the demonstration that both p40 and p75 rapidly increase the level of phospho-ERK in Caco-2 cells. A recent study demonstrated that activation of ERK plays a crucial role in the EGF-mediated prevention of H2O2-induced disruption of TJ and the increase in paracellular permeability (2). Phospho-ERK directly interacted with occludin and prevented H2O2-induced dephosphorylation of occludin on Thr residues. A similar mechanism may play a role in the probiotic-mediated protection of the TJ from H2O2-induced insult.
PKC inhibitors failed to prevent probiotic-induced activation of ERK1/2, and, similarly, MEK inhibitor failed to prevent probiotic-induced membrane translocation of PKCε or PKCβI. These data suggested that probiotic-induced activation of MAP kinase and PKC signaling pathways are independent of one another. Therefore, it is likely that probiotic proteins activate multiple signaling pathways, and coordination these signaling pathways is required for the probiotic-mediated protection of intestinal epithelial tight junctions. The precise mechanism involved in PKC and MAP kinase activation in tight junction regulation is not known. Our recent study suggested that PKC activation may be involved in stabilization of perijunctional actomyosin ring (20). MAP kinase activation may regulate the Thr-phosphorylation of occludin.
In summary, this study shows that probiotic secretory proteins ameliorate the oxidative stress-induced disruption of intestinal epithelial TJ and increase in paracellular permeability by a PKC- and MAP kinase-dependent mechanism.
GRANTS
This study was supported by National Institutes of Health Grants DK55532 (R. K. Rao), DK065788 (D. B. Polk), DK54993 (F. Yan), and AA12307 (R. K. Rao).
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anderson JM, Van Italie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol Gastrointest Liver Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- 2.Basuroy S, Seth A, Elias B, Naren AP, Rao RK. MAP kinase interacts with occludin and mediates EGF-induced protection of tight junctions from hydrogen peroxide. Biochem J. 2006;393:69–77. doi: 10.1042/BJ20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basuroy S, Sheth P, Kuppuswamy D, Balasubramanian S, Ray RM, Rao RK. Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J Biol Chem. 2003;278:11916–11924. doi: 10.1074/jbc.M211710200. [DOI] [PubMed] [Google Scholar]
- 4.Brown AC, Valiere A. Probiotics and medical nutrition therapy. Nutr Clin Care. 2004;7:56– 68. [PMC free article] [PubMed] [Google Scholar]
- 5.Clayburgh DR, Musch MW, Leitgs M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest. 2006;116:2682–2694. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 7.Farhadi A, Keshavarzian A, Ranjbaran Z, Fields JZ, Banan A. The role of protein kinase C isoforms in modulating injury and repair of the intestinal barrier. J Pharmacol Exp Ther. 2006;316:1–7. doi: 10.1124/jpet.105.085449. [DOI] [PubMed] [Google Scholar]
- 8.Gorbach SL. The discovery of Lactobacillus GG. Nutr Today. 1996;31:2S–4S. [Google Scholar]
- 9.Gorbach SL, Chang TW, Goldin B. Successful treatment of relapsing Clostridium difficile colitis with Lactobacillus GG. Lancet. 1987;2:1519. doi: 10.1016/s0140-6736(87)92646-8. [DOI] [PubMed] [Google Scholar]
- 10.Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–749. [PubMed] [Google Scholar]
- 12.Kettle AJ, Carr AC, Winterbourn CC. Assays using horseradish peroxidase and phenolic substrates require superoxide dismutase for accurate determination of hydrogen peroxide production by neutrophils. Free Radic Biol Med. 1994;17:161–164. doi: 10.1016/0891-5849(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 13.Lilly DM, Stillwell RH. Probiotics: growth-promoting factors produced by microorganisms. Science. 1965;147:747–748. doi: 10.1126/science.147.3659.747. [DOI] [PubMed] [Google Scholar]
- 14.Rao RK, Baker RD, Baker SS, Gupta A, Holycross M. Oxidant-induced disruption of intestinal epithelial barrier function: role of protein tyrosine phosphorylation. Am J Physiol Gastrointest Liver Physiol. 1997;273:G812–G823. doi: 10.1152/ajpgi.1997.273.4.G812. [DOI] [PubMed] [Google Scholar]
- 15.Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. Tyrosine phosphorylation and dissociation of E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao RK, Li L, Baker SS, Baker RD, Gupta A. Glutathione oxidation and inhibition of protein tyrosine phosphatase in hydrogen peroxide-induced increase in paracellular permeability. Am J Physiol Gastrointest Liver Physiol. 2000;279:G332–G340. doi: 10.1152/ajpgi.2000.279.2.G332. [DOI] [PubMed] [Google Scholar]
- 17.Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNFα and IFNγ-induced dysfunction in human intestinal epithelial cells. Gastroenterology. 2006;130:731–746. doi: 10.1053/j.gastro.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Sheth P, Basuroy S, Li C, Naren AP, Rao RK. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem. 2003;278:49239–49245. doi: 10.1074/jbc.M305654200. [DOI] [PubMed] [Google Scholar]
- 19.Shifflett DE, Clayburgh DR, Koutsouris A, Turner JR, Hecht DA. Enteropathogenic E. coli disrupts tight junction barrier function and structure in vivo. Lab Invest. 2005;85:1308–1324. doi: 10.1038/labinvest.3700330. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki T, Seth A, Rao RK. Role of phospholipase Cγ-induced activation of protein kinase Cε (PKCε) and PKCβI in epidermal growth factor-mediated protection of tight junctions from acetaldehyde in Caco-2 cell monolayers. J Biol Chem. 2008;283:3574–3583. doi: 10.1074/jbc.M709141200. [DOI] [PubMed] [Google Scholar]
- 21.Szajewska H, Kotowska M, Mrukowicz JZ, Armanska M, Mikolajczyk W. Efficacy of Lactobacillus GG in prevention of nosocomial diarrhea in infants. J Pediatr. 2001;138:361–365. doi: 10.1067/mpd.2001.111321. [DOI] [PubMed] [Google Scholar]
- 22.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16:5040–52. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker WA, Goulet O, Morelli I, Antoine JM. Progress in science of probiotics: from cellular microbiology and applied immunology to clinical nutrition. Eur J Nutr. 2006;45:1–18. [Google Scholar]
- 24.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959–50965. doi: 10.1074/jbc.M207050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal cell survival and growth. Gastroenterology. 2007;132:562–575. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan F, Polk DB. Probiotics as functional food in the treatment of diarrhea. Curr Opin Nutr Metab Care. 2006;9:717–721. doi: 10.1097/01.mco.0000247477.02650.51. [DOI] [PubMed] [Google Scholar]
- 27.Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Soderholm JD, Perdue M, Sherman PM. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following psychological stress. Gut. 2006;55:1553–1560. doi: 10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]