Abstract
Transcranial magnetic stimulation (TMS) has quickly progressed from a technical curiosity to a bona-fide tool for neurological research. The impetus has been due to the promising results obtained when using TMS to uncover neural processes in normal human subjects, as well as in the treatment of intractable neurological conditions, such as stroke, chronic depression and epilepsy. The basic principle of TMS is that most neuronal axons that fall within the volume of magnetic stimulation become electrically excited, trigger action potentials and release neurotransmitter into the postsynaptic neurons. What happens afterwards remains elusive, especially in the case of repeated stimulation. Here we discuss the likelihood that certain TMS protocols produce long-term changes in cortical synapses akin to long-term potentiation and long-term depression of synaptic transmission. Beyond the synaptic effects, TMS might have consequences on other neuronal processes, such as genetic and protein regulation, and circuit-level patterns, such as network oscillations. Furthermore, TMS might have non-neuronal effects, such as changes in blood flow, which are still poorly understood.
Introduction
Transcranial magnetic stimulation (TMS) is a technique for studying brain function, with advantages that have become apparent to neuroscientists, neurologists, clinical psychologists and therapists. TMS is non-invasive, causes negligible discomfort to subjects, does not require anaesthesia, and can be applied with exquisite temporal precision by using the appropriate magnetic coils [1,2]. As a result, TMS has been embraced by an expanding community of researchers and has led to a surge of publications. The recent handbooks by Pascual-Leone et al [3], Walsh and Pascual-Leone [4], and Wasserman et al [5] are recommended for the interested parties.
TMS is an emergent technology and, as such, it has many hurdles to overcome [3-5]. Obvious limitations include the relatively low spatial resolution (~1 cm) and the inability to stimulate at high frequencies (over 50 pulses per sec). Another drawback is the rapid decay of the electric field from the source; a pulse given at the scalp's level reaches only ~2 cm in depth [5]. Therefore, TMS can readily activate superficial regions (such as cerebral cortex, cerebellum and spinal cord), but it cannot reach deeper brain regions (such as hippocampus, amygdala, striatum, thalamus and brainstem). It is foreseeable that technical improvements, such as novel magnetic coils with active cooling, deeper penetrating power and more focal spatial resolution, will help overcome the current restrictions. An inherent limitation of TMS, however, is the nonspecific nature of the neural activation that follows a pulse. The activated volume of brain tissue contains excitatory, inhibitory and neuromodulatory neuronal compartments, all with the potential of being concurrently stimulated. Therefore, caution should be exercised when interpreting TMS studies.
In this review, we discuss the neural mechanisms underlying TMS. This topic has not been studied as thoroughly as expected, probably because most investigators are still determining the full range of applications for this emergent technique [6]. It is widely accepted, however, that TMS involves a range of neuronal processes such as synaptic excitation, synaptic inhibition and synaptic plasticity [2,3,6-9]. Moreover, TMS seems to affect circuit-level patterns, such as network oscillations, as well as non-neuronal effects, such as changes in blood flow [10,11].
A detailed understanding of the neural mechanisms at work in TMS is highly desirable because of the steady rise in studies attempting to use TMS in therapeutic settings [12]. For instance, researchers have reasoned that TMS could help awaken dormant cortical areas in individuals who had recently suffered a stroke. However, it has taken several years of dedicated effort to implement stimulation protocols that produce reliable, albeit minor, beneficial effects [2,12-14].
The effect of a single TMS pulse
In 1831, Faraday demonstrated that a rapidly changing magnetic field could induce an electrical current in a nearby conductor. In 1985 this principle was applied successfully to the cerebral cortex of the human brain [1]. This organ works as a conductor because the cells that reside within it maintain electrochemical gradients through a variety of ion channels and ion transporters. Therefore, when a single magnetic field is pulsed directly over the subject's head, via a specialized coil, it induces electrical currents across the different layers of the cerebral cortex (Fig. 1). A standard pulse lasts ~10-5 sec and induces a magnetic field reaching up to 2 Tesla [2]. The magnitude of the pulse directly determines the volume of cortical tissue that is stimulated. Detailed simulations show that a 2 Tesla pulse activates a cylindrical volume (~1 cm radius, ~2 cm height), with an exponential decay from the central activation axis [5,15]. Because neuronal axons have the highest density of ion channels, they become preferentially activated during a weak magnetic pulse. When an axon becomes electrically active, an action potential travels along its axis until it reaches the presynaptic axon terminal. At this point, neurotransmitter is released onto the postsynaptic neuron. Most cortical neurons use the neurotransmitter glutamate and are classified as excitatory neurons. A smaller fraction of cortical neurons release γ-aminobutyric acid (GABA) and are classified as inhibitory neurons. Yet another group of neurons send long axonal projections from different brain nuclei to the cortex and release neuromodulators, such as acetylcholine, dopamine, norepinephrine, and serotonin. Therefore, even a weak TMS pulse always activates a mixture of excitatory and inhibitory neurons and has the potential to activate neuromodulatory pathways. Also, given the dense connectivity of cortical circuits, a TMS pulse potentially activates a chain of neurons, generating feed-forward and feedback loops of excitation and inhibition.
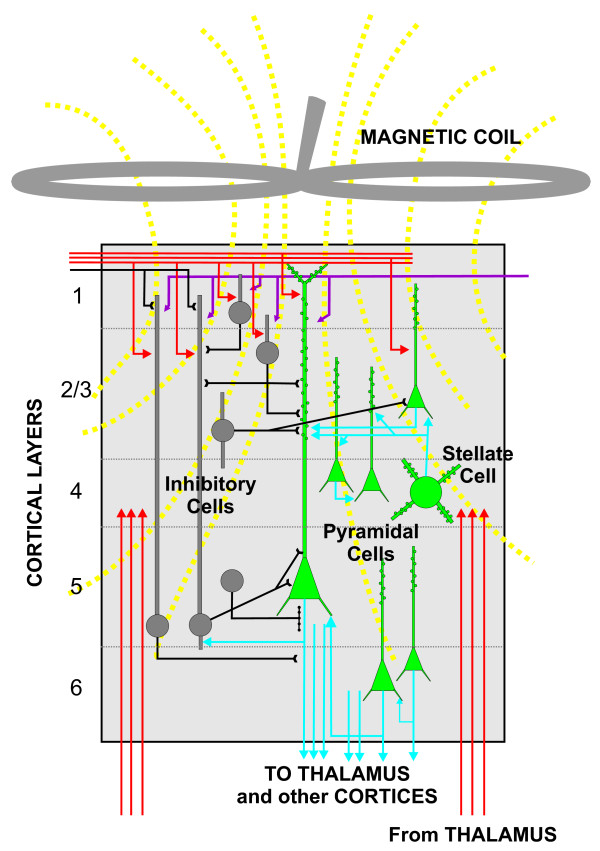
Figure 1.
Schematic representation of the human cerebral cortex. The magnetic coil, represented as a figure-of-eight device, is placed on top of the cerebral cortex and pulses a magnetic field that induces electrical currents across the six layers of the cerebral cortex (indicated by numbers at left). The excitatory cells (green with blue axons) and the inhibitory cells (gray with black axons) have the potential to be activated at the level of their axons, which contain the highest density of ion channels. The incoming axons from other cortical areas and the thalamus (indicated in red) are also activated. The end result of the magnetic pulse is the synaptic activation of a chain of neurons, which generate feed-forward and feedback loops of excitation and inhibition.
The behavioural response elicited by a single TMS pulse depends on the exact cortical area that is stimulated. When a pulse is given over the primary motor cortex (at the top of the head), it can induce twitches in the subject's muscles. In fact, a precisely localized magnetic pulse can lead to movement of a single finger. Similarly, a single pulse directed to the primary visual cortex (at the back of the head) can induce the sensation of seeing light, even when the eyes are closed, an experience known as a phosphene. In this sense, TMS is reminiscent of other techniques (such as electrical brain stimulation, positron emission tomography, and functional magnetic resonance imaging) that allow investigators to study specific cortical areas within dedicated sensory and motor modalities. Given the low spatial resolution of TMS, the technique does not allow for précised mapping of cortical areas.
The primary motor cortex (M1) constitutes the best-examined cortical region in terms of the effect of TMS [1-6]. One of the main reasons for this focused attention is the practical matter that even a weak, single TMS pulse applied over M1 can produce a muscle response, called a motor evoked potential (MEP), that is technically simple to measure. Indeed, the bulk of the TMS studies on M1 use the amplitude of the MEP as the single measure of TMS output. This potential is, however, separated by three synapses from the TMS source (1, synapses onto corticospinal neurons; 2, synapses onto motor neurons in the spinal cord and; 3, neuromuscular synapses). Nevertheless, careful studies have convincingly shown that a TMS pulse over M1 initiates a chain of events that begins with the stimulation of multiple axons distributed across the different cortical layers (Fig. 1). The axons of interneurons show the shortest latency to respond, which is followed by axonal activation of thalamo-cortical inputs and cortico-cortical inputs. The axonal activities of all these cells are synaptically integrated by the corticospinal pyramidal neurons in layer 5 and eventually lead to the generation of action potentials by the output cells (Fig. 1). These action potentials can be measured from the epidural space of the cervical spinal cord in conscious humans; they occur ~5–10 ms after the TMS pulse and have been termed indirect waves to emphasize the fact that they are the product of synaptic activation [2,5]. Once the corticospinal action potentials reach the spinal cord, they activate motor neurons. These cells in turn generate action potentials, which lead to the synaptic activation of muscles, ~20 ms after TMS. It is this activity that is measured as the MEP.
Interestingly, when a magnetic pulse is applied over a cortical area that is involved in cognition, it does not typically elicit an effect by itself. However, if the pulse is given when the person is involved in a cognitive task, it can greatly interfere with proper performance [3,15]. For instance, a single TMS pulse given over Broca's language area (located in the left hemisphere in most people) as the subject verbalizes can produce speech interference. Conversely, a single TMS pulse can have a facilitatory effect when it is applied shortly before a cognitive task. For example, a subject displays a shorter latency for naming an object when a single TMS pulse is given over Wernicke's language area 500–1000 ms before the subject is shown the object [16]. These results indicate that even a single TMS pulse can generate differential consequences depending on the activation state of the cerebral cortex at the moment of applying the pulse [17]. They also call attention to the importance of timing when TMS is used in respect to a particular external stimulus.
Repetitive TMS and synaptic plasticity
TMS protocols that include multiple pulses are known as repetitive TMS. These protocols consist of precisely structured patterns that are characterized by the number of pulses, the frequency with which they are given, and the intensity of each stimulus. It has been determined that repetitive TMS engages a variety of neuronal mechanisms, besides axonal activation, as well as non-neuronal processes that might be collectively responsible for the range of observed effects [4,11].
Remarkably, some protocols of repetitive TMS can elicit residual effects that persist for many minutes. In a serendipitous manner, the TMS patterns that produce long-lasting changes tend to emulate, in the stimulation regimens at least, the patterns that trigger synaptic plasticity in the hippocampus. This suggests that, at minimum, repetitive TMS harnesses the neural processes responsible for triggering changes among synaptic connections in cortical networks. Therefore, we will briefly describe the principles of synaptic plasticity and local inhibition in the rodent hippocampus before scrutinizing to what extent repetitive TMS might engage cortical synaptic plasticity.
Synaptic plasticity in the hippocampus
From the wealth of information available [18,19], we will focus on the synaptic molecules and the patterns of electric stimulation that trigger synaptic plasticity in the CA1 region of the rodent hippocampus (Fig. 2). The excitatory synapses between the axons of CA3 neurons (the inputs) and the dendritic spines of CA1 pyramidal neurons (the targets) have been intensely studied [18,19]. The CA3 axon terminals release glutamate while the CA1 neurons express three types of glutamatergic receptors: alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), N-methyl-D-aspartate receptor (NMDAR), and metabotropic receptor (mGluR). The AMPAR and the NMDAR function as ion channels that permeate positively charged ions when they are activated, depolarizing the neuron.
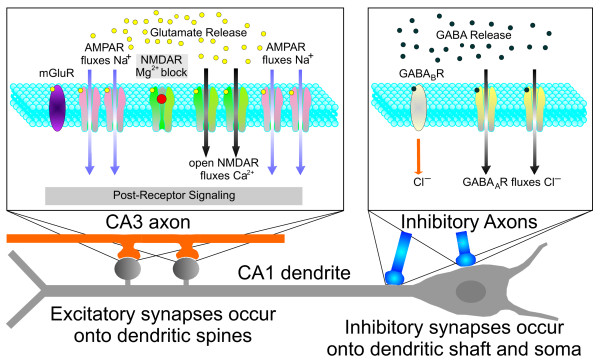
Figure 2.
Schematic representation of the glutamatergic and GABAergic receptors in a CA1 pyramidal neuron. The left box represents a CA3-CA1 synapse. The CA3 axon (orange) releases glutamate from the presynaptic terminals. The postsynaptic CA1 neuron expresses three types of glutamatergic receptors: metabotropic receptor (mGluR), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), and N-methyl-D-aspartate receptor (NMDAR). The AMPARs are represented in their active state, as they allow Na+ to enter onto the dendritic spine. The NMDARs are represented both in the closed state (leftmost NMDAR, with the Mg2+ block seen as a red ball in the mouth of the receptor) and in the open state, when the NMDARs allow Ca2+ to enter onto the spine (notice the absence of the Mg2+ block). The right box represents a synapse between an inhibitory interneuron and the CA1 cell. The interneuron releases γ-aminobutyric acid (GABA) onto the CA1 pyramidal neuron, which expresses GABAA receptors (yellow) and GABAB receptors (gray), leading to inhibition of the target cell. The GABAA receptors are represented in the open state when they allow Cl- to enter onto the CA1 dendrite.
The strength of hippocampal synapses can increase dramatically following high frequency stimulation (HFS) of the inputs. Since the synaptic enhancement may persist for hours, or even days, it is called long-term potentiation or LTP [18,20,21]. There are several HFS patterns that can elicit LTP, but the most common consists of a single train of 100 Hz for 1 sec (100 pulses with 10-ms intervals). Another HFS protocol is called theta burst stimulation (TBS) and consists of 10 bursts (each burst is 4 pulses at 100 Hz) that are separated by an interval of 200 ms from each other [22]. The term theta refers to the fact that 200 ms is the main periodicity of the theta rhythm, a network oscillation that occurs during periods of heightened attention, such as when an animal explores a new environment [18]. Another HFS protocol is called primed burst stimulation [23], and consists of a single pulse that is followed by a burst (4 pulses at 100 Hz) with an interval of 200 ms. Indeed, even a non-primed burst can induce LTP by itself, if it occurs at the peak of a wave in theta rhythm [24]. This paradigm exploits the association of a network oscillation with a finely timed stimulating burst. Another associative protocol for LTP induction is called spike-timing-dependent plasticity [25-27]. It relies on the delivery of two pulses; the first triggers an action potential (or spike) in the input axon, while the second triggers an action potential in the target neuron. To elicit LTP, the input spike must precede the target spike (by 5–50 ms) and the pairing must occur many times. A typical protocol repeats the two pulses (input 10 ms before target) for 50 times at a frequency of 10 Hz [25].
The sequence of events underlying LTP induction is clearly understood [18,28]. When glutamate binds to the AMPAR, this receptor opens its pore for a brief period (10–20 ms), allowing Na+ to enter into the dendritic spine, resulting in a small degree of depolarization. The NMDAR does not open immediately because its pore is blocked by Mg2+ ions. HFS seems to be essential for removing the Mg2+ block of the NMDAR, probably because HFS activates numerous AMPARs thus generating a large depolarization in the dendritic spine. When the NMDAR opens, it permeates Na+ and Ca2+ ions for hundreds of milliseconds. The resting Ca2+ concentration in the cell's cytoplasm is very low (~10-9 M) but when many NMDARs open during HFS, Ca2+ reaches a high concentration (~10-3 M) within the spine that activates several kinases, particularly calcium-calmodulin kinase II [29], and leads to phosphorylation and upregulation of the AMPAR.
The strength of hippocampal synapses can decrease persistently following low frequency stimulation (LFS), a process that has been termed long-term depression or LTD [30]. The most frequent LFS protocol is a single train of 1 Hz for 10 min (600 pulses) or for 15 min (900 pulses). Another effective protocol is paired pulse LFS [31,32] consisting of a train of paired pulses (2 pulses with a 200-ms interval) at 1 Hz for 15 min (1800 pulses). LTD can also be elicited by spike-timing-dependent plasticity [25-27], in which the target spike precedes the input spike (by 5–50 ms) and both spikes occur many times. Remarkably, this LTD induction protocol simply reverses the order of the target and the input spikes from the LTP induction protocol. Surprisingly, LTD induction also depends on the NMDAR. It is generally accepted that during LFS the NMDAR is mildly stimulated, producing an intermediate Ca2+ elevation (~10-6 M) that activates protein phosphatases and leads to dephosphorylation and down-regulation of the AMPAR [18,33].
Inhibition in the hippocampus
The local interneurons in the CA1 region release GABA onto the CA1 pyramidal neurons, which express GABAA receptors and GABAB receptors, leading to inhibition of these target cells (Fig. 2) [34,35]. Since the CA3 axons have synaptic connections with the local interneurons, the activation of CA3 axons results in initial excitation of CA1 pyramidal cells (via the glutamatergic synapses) that is followed by feed-forward inhibition from the interneurons. Furthermore, the axons of CA1 pyramidal neurons themselves connect to the interneurons, so that when a CA1 pyramidal cell generates an action potential, it leads to rapid feedback inhibition. In this manner, the local interneurons are extremely effective in dampening excessive excitation of the CA1 pyramidal cells through the activation of feed-forward and feedback inhibitory loops.
Notably, the local interneurons express GABAB autoreceptors in their presynaptic terminals that stop the release of GABA after ~200 ms [18,36]. This fact explains the tremendous efficacy of TBS and primed burst stimulation for inducing LTP, as well as paired pulse LFS for inducing LTD. In each of these protocols, one of the consequences of the first pulse is to trigger GABA release from the interneuronal terminals, which then blocks its own release at the exact time (200 ms) that the second stimulus occurs. If the second stimulus is a single pulse, it triggers mild NMDAR activation that leads to LTD. If the second stimulus is a burst of pulses, it elicits strong NMDAR activation and subsequent LTP.
Lessons from the hippocampus applied to repetitive TMS
Many reports have demonstrated that the principles of synaptic plasticity that were first uncovered in the hippocampus can be extended to the cerebral cortex [37-46]. In particular, NMDARs and AMPARs seem to play similar roles in the long-term plasticity of cortical synapses as they do in the hippocampus [37]. Moreover, the local interneurons in the cerebral cortex exert strong inhibitory influences over the pyramidal and stellate neurons [47]. However, a crucial difference between these brain regions is that the cortical networks are structurally much more complex than the hippocampal circuits. Cortical neurons are placed in multilayered arrangements (the canonical six layers), with copious synaptic connections within each functional module and with numerous axons running from each module to its connected counterparts (Fig. 1). Also, cortical neurons receive massive inputs from the thalamus and, in turn, project heavily to the same structure. Therefore, there are vast recursive loops of excitation and inhibition between the cortex and the thalamus, as well as between the different areas of the cortex, including loops between both cerebral hemispheres.
Given the structural complexity of the cerebral cortex, it might be surprising that TMS protocols that emulate the induction paradigms for LTP and LTD (in rodents) would be successful in modifying the efficacy of cortical networks in humans. A parsimonious explanation is that patterned TMS can trigger changes in the human cortical synapses that are similar, at the mechanistic level, to the plasticity that occurs in rodent cortical synapses when they undergo LTP or LTD. Although this is a tentative proposal, it is supported by the observation that the most effective TMS protocols (for producing long-term change) mirror closely the protocols used for inducing LTP and LTD in rodent preparations. Two straightforward predictions of this conjecture are: (i) minor deviations from the prescribed LTP and LTD induction protocols would be much less efficient in producing TMS-induced plasticity, (ii) pharmacological agents that block LTP and LTD induction in rodents would be effective in blocking the TMS-induced plasticity.
Thus far, M1 has been the most investigated cortical region with regards to TMS-induced plasticity [2,6,15]. The current evidence highlights the critical effectiveness of TMS protocols that mimic the induction paradigms for LTD and LTP. These TMS protocols invariably produce changes in MEP amplitude that outlast the TMS application [5,12]. It must be noted, however, that using the MEP as the sole readout of TMS-induced plasticity is problematic because the MEP is removed by three synapses from the source of TMS (as detailed above), whereas LTP and LTD are monosynaptic events. It would thus be highly desirable to monitor a cortical readout that is linked by a single synapse to the TMS pulse. Studies in which TMS is coupled with recording techniques such as high-density electroencephalography have the potential to provide such direct monosynaptic readout.
When a train of TMS pulses is applied at 1 Hz, it leads to lasting decrease of the MEP [5,48-51]. In one of the original reports, Chen et al [48] showed that repetitive TMS at 0.9 Hz applied for 15 min (810 pulses), with a stimulation intensity set at 115% of the resting motor threshold, produced 20% decrease of the MEP that lasted for ~15 min. Touge et al [49] used repetitive TMS at 1 Hz, with an intensity of 95% of resting threshold, applied for 25 min (1500 pulses) and obtained a 50% decrease of the MEP that returned to the pre-TMS baseline in ~30 min. Thus, the application of a longer 1-Hz train was able to induce a stronger depression that persisted for a somewhat longer period. These results are in line with the LTD studies in rodents.
It has been shown that high frequency patterns of TMS given over M1 can increase cortical efficacy. In a pioneer study, Pascual-Leone et al [52] used a train of 10 pulses of TMS at 20 Hz, with an intensity of 150% of resting threshold, and obtained a 50% increase of the MEP that lasted for ~5 min. This result is reminiscent of the rodent studies in which an induction protocol of intermediate frequency (i.e., 20 Hz) produces a transient synaptic enhancement that is called short-term potentiation.
Unfortunately, overheating of the magnetic coils prevents investigators from using the classical protocol for inducing LTP (100 Hz for 1 sec). Moreover, there is a nontrivial possibility that such high frequency stimulation may lead to seizures in susceptible individuals. Given these caveats, some studies have used trains of lower frequency in an attempt to enhance efficacy. For example, modest increases of the MEP are obtained following TMS trains at 5 Hz [53,54]. It is important to realize that in rodent studies of synaptic plasticity, a 5-Hz protocol does not fall within the frequency range that would induce LTP. If anything, it might be easier to induce LTD because single pulses at 5 Hz are very effective in mildly activating NMDAR and in suppressing GABA release (through activation of the GABAB auto-receptors). In fact, the landmark study by Allen et al [55] in the cat primary visual cortex clearly demonstrated that TMS trains of 1–8 Hz for 1–4 sec were all capable of depressing visually evoked responses, which were quantified as the rate of action potentials of the cortical neurons that were triggered by a visual stimulus. For example, following a brief TMS train of 4 Hz for 2 sec (8 pulses), the rate of action potentials was greatly depressed for more than 5 min. A visual stimulus that before TMS produced ~80 action potentials per sec was unable to trigger a single event during the initial 2 min post-TMS. The cortical activity slowly recovered to 40 action potentials per sec in response to the visual stimulus 5 min after TMS.
An exciting development in the search for TMS protocols that enhance cortical efficacy has occurred recently. Several investigators have demonstrated that the TBS protocol used for LTP induction can produce a lasting increase in cortical activity [56-59]. Huang et al [56] measured a 50% increase in the MEP, that lasted ~20 min, following a protocol they called intermittent TBS. Their protocol consisted of 600 pulses, with an intensity of 80% of resting threshold, that were distributed in 20 episodes according to the following scheme: each episode consisted of a burst of three TMS pulses (at 50 Hz, 20 ms between each pulse) that was repeated at 5 Hz for 2 sec (for a total of 10 bursts). A silent interval of 8 sec followed and then a new episode was applied. Interestingly, when the 50-Hz bursts were applied in a continuous fashion (that is, the bursts were repeated at 5 Hz with no intervening silent period), the MEP was depressed. Esser et al [57] combined an intermittent TBS protocol with high-density electroencephalographic measurements and found that intermittent TBS over M1 in the left hemisphere enhanced the MEP in the right hand, as expected, but it also increased neural responses in the premotor cortex bilaterally. Therefore, the intermittent TBS protocol was not only able to affect the motor output, but also the efficacy of cortical areas closely related to M1.
The question of whether the post-TBS enhancement displays the NMDAR dependence that would be expected of an LTP mechanism has been recently addressed with the use of the NMDAR antagonists memantine (uncompetitive antagonist) and D-cycloserine (competitive antagonist at high doses) [60,61]. A small amount of memantine (4 doses of 5 mg each, over 2 days) given before TMS, can completely block the facilitatory effect of intermittent TBS and, also, the suppressive effect of continuous TBS [61]. Critically, memantine blocks training-induced motor cortex plasticity, does not commonly produce side effects, and has good blood-brain barrier penetrating rate [62-66]. A dose of D-cycloserine (100 mg, taken 2 hours before TMS) can turn the facilitatory effect of intermittent TBS into a depressive effect [62]. These results are encouraging and, together with the bulk of the TMS studies tend to support the conjecture that synaptic plasticity might mediate the long-term changes in cortical efficacy generated by TMS protocols that mimic LTP and LTD induction paradigms.
Recent studies have explored associative protocols in which TMS is combined with peripheral nerve stimulation to generate plasticity [67-71]. It has been proposed that these protocols follow the association principles of spike-timing-dependent plasticity. For instance, the pioneer study by Stefan et al [67] delivered an electrical stimulus to the right median nerve in the wrist that was followed (25 ms later) by a TMS pulse over the left hemisphere at the optimal site for activating the abductor pollicis brevis muscle. This paired stimulation was repeated 90 times, with an interval of 20 sec, and produced a 55% increase in MEP amplitude that returned to baseline in ~1 hour. To explain this result in terms of spike-timing-dependent plasticity, one needs to argue that the medial nerve stimulation provides the presynaptic spike, whereas the TMS pulse provides a precisely timed postsynaptic spike. Indeed, medial nerve stimulation triggers an action potential that takes ~20 ms to travel from the wrist to the somatosensory cortex and ~3 ms for propagating from the somatosensory cortex to M1. This means that the TMS pulse (given 25 ms after medial nerve stimulation) occurs ~2 ms after the input arriving from the somatosensory cortex. It is therefore possible that the presynaptic spike and the postsynaptic spike occur with the precise timing required for LTP. Although other conceptual scenarios might be able to explain the results obtained with the associative protocols, they could feasibly represent a genuine realization of the principles of spike-timing-dependent plasticity in the human cortex.
TMS and network oscillations
The analysis of how TMS might influence circuit-level events, such as network oscillations, constitutes an emerging area of research. A vast body of work has shown that cortical oscillations represent a signature of ongoing operations occurring in intrinsic cortico-cortical loops and cortico-thalamic circuits [72]. At every moment in time, there is a discrete ensemble of cortical neurons that is active and, when this ensemble becomes silent, it is instantly replaced by a new set of active neurons. This constant wave of neural activation and silencing all over the cortical mantle gives rise to short-lived oscillations that wax and wane according to the brain's internal dynamics [73]. Notably, the cortical ensembles generate oscillatory bands that cover an enormous range of frequencies (0.02 Hz to 600 Hz). In the waking brain, when attending to external stimuli, many cortical ensembles synchronize in the gamma frequency range (30–80 Hz). Therefore, it has been suggested that gamma oscillations reflect the binding (putting together) of the features of external stimuli [72,74]. In the absence of sensory inputs, the most prominent oscillations in the waking brain are in the alpha range (8–12 Hz), and it is thought that alpha oscillations reflect partial disengagement from the environment or internal mental processing [72]. During deep sleep, several slow waves occur, such as the slow 1 oscillation (0.5–0.7 Hz) and the delta oscillation (1.5–4 Hz). It has been suggested that these sleep waves are involved in the process of memory consolidation, although the exact mechanisms have not been identified [75].
Recent TMS studies have measured the consequences of TMS on network oscillations, with the use of concomitant high-density electroencephalography [76-82]. For example, Massimini et al [76] have found that, during quiet wakefulness, a TMS pulse over the premotor cortex (in the right hemisphere) induces a sequence of time-locked gamma oscillations (20–35 Hz) in the first 100 ms, followed by a few slower (8–12 Hz) components that persist until 300 ms. These travelling waves propagate to connected cortical areas, even several centimetres away. Remarkably, during deep sleep, the response to the TMS pulse is radically different, consisting of a single wave of high amplitude in the premotor site that lasts for ~200 ms and does not propagate to the connected areas. In another study, Massimini et al [81] have shown that a TMS pulse over the sensorimotor cortex can trigger a high-amplitude slow wave during sleep that spreads over the whole cortical mantle, and it is reminiscent of the naturally occurring slow oscillation. Since this type of oscillation has been postulated to play a role in memory consolidation, this study opens the possibility of examining this elusive process with TMS technology.
The work by Allen et al [55] in the cat visual cortex represents the most throughout mechanistic study of multiple effects of TMS. The authors measure robust decreases in action potentials, but they also investigate the consequences of TMS on the local network oscillations and the local blood flow. Immediately after TMS, the spontaneous local field oscillations show a great increase in the high frequency band (oscillations between 50–150 Hz) that lasts for ~60 sec. This is consistent with the idea that inhibitory loops are recruited. Moreover, the spontaneous local field oscillations in the lower band (<40 Hz) show a sustained reduction, suggesting an effect on the oscillatory processes that participate in sensory binding. In a technical tour de force, Allen et al [55] also report the levels of tissue oxygen in the visual cortex and find that oxygen is well correlated with the occurrence of action potentials. In fact, the lowest levels of oxygen are recorded after the 8-Hz protocol that also elicits the strongest decrease in the number of action potentials in response to a visual stimulus.
Current encephalographic analysis is a robust methodology with multiple applications in basic and clinical neuroscience. It is expected that the studies that combine high-density electroencephalography with TMS will continue to illuminate the role of network oscillations in the cerebral cortex, as they represent unique markers of neural processes such as sensory binding, memory consolidation and mental ideation. TMS can easily add the much-needed predictive component to these investigations [82].
Other effects of TMS
TMS seems to have several consequences that are not directly related to synaptic plasticity and neuronal excitability. Such effects are just starting to be examined experimentally. The results thus far suggest that repetitive TMS protocols can trigger the activation of neuromodulators, such as acetylcholine, dopamine, norepinephrine and serotonin [83-89]. Presumably, these substances would be released during the TMS protocols and would continue to exert their modulatory effects after TMS has terminated. In fact, neuromodulators are constantly released onto the cerebral cortex in coordination with certain behavioural states. It would be expected that weak TMS protocols, such as single-pulse TMS, would have only minor influences over the ongoing release of neuromodulators. Conversely, patterned TMS paradigms (lasting for several minutes) would be expected to facilitate the release of at least some neuromodulators. Preliminary experiments in rats tend to agree with this premise [83,84], but much work remains to be done. Recently, it has been shown that TMS can trigger the expression of brain-derived neurotrophic factor and plasticity-related genes [90-92]. Moreover, TMS could help in phenotyping individuals with genetic mutations that affect cortical excitability, such as a mutation affecting the gene encoding the GABAA receptor [93], serotonergic gene polymorphisms [94], and the D90A superoxide dismutase-1 gene mutation [95].
TMS has already been incorporated to the arsenal of therapeutic tools that are used to mitigate the negative effects of neurological conditions, but these novel results open the exciting possibility that TMS also becomes a tool for manipulating the release and expression of endogenous trophic factors and beneficial gene products. This topic needs to be investigated further, but its high relevance makes it an attractive research focus for clinical researchers.
Conclusion
We have discussed the biological mechanisms that are most likely to be engaged when TMS is applied over the cerebral cortex. It is clear that TMS can activate a host of neural phenomena, at different levels of organization, from synaptic plasticity to circuit-level oscillations. We have proposed that only a handful of the TMS protocols that are currently used for producing changes in cortical efficacy have the credentials for generating synaptic plasticity, similar to LTP and LTD. We have also mentioned that TMS may influence a large variety of non-neuronal processes that have yet to be fully elucidated.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
Acknowledgements
We are grateful to Eric H. Chang and Thomas Faust for suggestions on the manuscript. This work is supported by grants from the Alliance for Lupus Research, the Burke Foundation and the U.S. National Institutes of Health to P.T.H. and B.T.V.
Contributor Information
Patricio T Huerta, Email: pato.huerta@gmail.com.
Bruce T Volpe, Email: btv3@cornell.edu.
References
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/S0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Davey N, Rothwell J, Wassermann EM, Puri BK. Handbook of Transcranial Magnetic Stimulation. London: Hodder Arnold; 2002. [Google Scholar]
- Walsh V, Pascual-Leone A. Transcranial Magnetic Stimulation: A Neurochronometrics of Mind. Cambridge: The MIT Press; 2005. [Google Scholar]
- Wassermann E, Epstein C, Ziemann U. Oxford Handbook of Transcranial Stimulation. Oxford: Oxford University Press; 2008. [Google Scholar]
- Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Valero-Cabré A, Payne BR, Rushmore J, Lomber SG, Pascual-Leone A. Impact of repetitive transcranial magnetic stimulation of the parietal cortex on metabolic brain activity: a 14C-2DG tracing study in the cat. Exp Brain Res. 2005;163:1–12. doi: 10.1007/s00221-004-2140-6. [DOI] [PubMed] [Google Scholar]
- Allen EA, Pasley BN, Duong T, Freeman RD. Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science. 2007;317:1918–1921. doi: 10.1126/science.1146426. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci. 2007;8:559–67. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65:466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJ, Wagner T, Fecteau S, Rigonatti SP, Riberto M, Freedman SD, Pascual-Leone A. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37:2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- Cowey A. The Ferrier Lecture 2004 what can transcranial magnetic stimulation tell us about how the brain works? Philos Trans R Soc Lond B Biol Sci. 2005;360:1185–205. doi: 10.1098/rstb.2005.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Töpper R, Mottaghy FM, Brügmann M, Noth J, Huber W. Facilitation of picture naming by focal transcranial magnetic stimulation of Wernicke's area. Exp Brain Res. 1998;121:371–378. doi: 10.1007/s002210050471. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21:1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Morris RMR, Amaral D, Bliss T, O'Keefe J. The Hippocampus Book. Oxford: Oxford University Press; 2008. [Google Scholar]
- Kandel ER. Cellular mechanisms of learning and the biological basis of individuality. In: Kandel ER, Schwartz JH, Jessell TM, editor. Principles of Neural Science. Fourth. New York: McGraw-Hill; 2000. pp. 1247–1279. [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Rose GM, Dunwiddie TV. Induction of hippocampal long-term potentiation using physiologically patterned stimulation. Neurosci Lett. 1986;69:244–248. doi: 10.1016/0304-3940(86)90487-8. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron. 1995;15:1053–1063. doi: 10.1016/0896-6273(95)90094-2. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3:1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation – a decade of progress? Science. 1999;285:1870–18874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp N, McQueen J, Faulkes S, Bashir ZI. Different forms of LTD in the CA1 region of the hippocampus: role of age and stimulus protocol. Eur J Neurosci. 2000;12:360–366. doi: 10.1046/j.1460-9568.2000.00903.x. [DOI] [PubMed] [Google Scholar]
- Chang EH, Savage MJ, Flood DG, Thomas JM, Levy RB, Mahadomrongkul V, Shirao T, Aoki C, Huerta PT. AMPA receptor downscaling at the onset of Alzheimer's disease pathology in double knockin mice. Proc Natl Acad Sci USA. 2006;103:3410–3415. doi: 10.1073/pnas.0507313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci USA. 1989;86:9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABA autoreceptors regulate the induction of LTP. Nature. 1991;349:609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Dudek SM, Gold JT, Aizenman CD, Bear MF. Common forms of synaptic plasticity in the hippocampus and neocortex in vitro. Science. 1993;260:1518–1521. doi: 10.1126/science.8502997. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994;14:1634–1645. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF. A synaptic basis for memory storage in the cerebral cortex. Proc Natl Acad Sci USA. 1996;93:13453–13459. doi: 10.1073/pnas.93.24.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Connors BW. Short-term synaptic enhancement and long-term potentiation in neocortex. Proc Natl Acad Sci USA. 1996;93:1335–1339. doi: 10.1073/pnas.93.3.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G, Aizenman CD, Donoghue JP. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol. 1996;75:1765–1778. doi: 10.1152/jn.1996.75.5.1765. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Fox K. Anatomical pathways and molecular mechanisms for plasticity in the barrel cortex. Neuroscience. 2002;111:799–814. doi: 10.1016/S0306-4522(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Wang XF, Daw NW. Long term potentiation varies with layer in rat visual cortex. Brain Res. 2003;989:26–34. doi: 10.1016/S0006-8993(03)03321-3. [DOI] [PubMed] [Google Scholar]
- Werk CM, Chapman CA. Long-term potentiation of polysynaptic responses in layer V of the sensorimotor cortex induced by theta-patterned tetanization in the awake rat. Cereb Cortex. 2003;13:500–507. doi: 10.1093/cercor/13.5.500. [DOI] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, Kaang BK, Zhuo M. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Touge T, Gerschlager W, Brown P, Rothwell JC. Are the after-effects of low-frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clin Neurophysiol. 2001;112:2138–2145. doi: 10.1016/S1388-2457(01)00651-4. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Hallett M. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin Neurophysiol. 2000;111:1002–1007. doi: 10.1016/S1388-2457(00)00284-4. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000;133:425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Morgante F, Sant'angelo A, Battaglia F, Messina C, Siebner HR, Girlanda P. Distinct changes in cortical and spinal excitability following high-frequency repetitive TMS to the human motor cortex. Exp Brain Res. 2005;161:114–124. doi: 10.1007/s00221-004-2052-5. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, Mentschel C, Münchau A, Conrad B, Siebner HR. Subthreshold 5-Hz repetitive transcranial magnetic stimulation of the human primary motor cortex reduces intracortical paired-pulse inhibition. Neurosci Lett. 2000;296:21–24. doi: 10.1016/S0304-3940(00)01616-5. [DOI] [PubMed] [Google Scholar]
- Allen EA, Pasley BN, Duong T, Freeman RD. Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science. 2007;317:1918–1921. doi: 10.1126/science.1146426. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Esser SK, Huber R, Massimini M, Peterson MJ, Ferrarelli F, Tononi G. A direct demonstration of cortical LTP in humans: a combined TMS/EEG study. Brain Res Bull. 2006;69:86–94. doi: 10.1016/j.brainresbull.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Matsunaga K, Nakanishi R, Kawahira K, Murayama N, Tsuji S, Huang YZ, Rothwell JC. Effect of theta burst stimulation over the human sensorimotor cortex on motor and somatosensory evoked potentials. Clin Neurophysiol. 2007;118:1033–1043. doi: 10.1016/j.clinph.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Oliviero A, Mazzone P, Insola A, Ranieri F, Meglio M, Tonali PA, Rothwell JC. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J Physiol. 2008;586:3871–3879. doi: 10.1113/jphysiol.2008.152736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenkreis P, Witscher K, Pleger B, Malin JP, Tegenthoff M. The NMDA antagonist memantine affects training induced motor cortex plasticity – a study using transcranial magnetic stimulation. BMC Neurosci. 2005;6:35. doi: 10.1186/1471-2202-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Chen RS, Rothwell JC, Wen HY. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118:1028–1032. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Teo JT, Swayne OB, Rothwell JC. Further evidence for NMDA-dependence of the after-effects of human theta burst stimulation. Clin Neurophysiol. 2007;118:1649–1651. doi: 10.1016/j.clinph.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P, Witscher K, Janssen F, Addo A, Dertwinkel R, Zenz M, Malin JP, Tegenthoff M. Influence of the N-methyl-d-aspartate antagonist memantine on human motor cortex excitability. Neurosci Lett. 1999;270:137–140. doi: 10.1016/S0304-3940(99)00492-9. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, Quack G. Cerebrospinal fluid and serum concentrations of the N-methyl-d-aspartate (NMDA) receptor antagonist memantine in man. Neurosci Lett. 1995;195:137–139. doi: 10.1016/0304-3940(95)11785-U. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-d-aspartate (NMDA) receptor antagonist – a review of preclinical data. Neuropharmacology. 1999;38:735–767. doi: 10.1016/S0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Edwards MJ, Chen RS. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex. 2008;18:563–570. doi: 10.1093/cercor/bhm087. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Wolters A, Schmidt A, Schramm A, Zeller D, Naumann M, Kunesch E, Benecke R, Reiners K, Classen J. Timing-dependent plasticity in human primary somatosensory cortex. J Physiol. 2005;565:1039–1052. doi: 10.1113/jphysiol.2005.084954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior MM, Stinear JW. Phasic spike-timing-dependent plasticity of human motor cortex during walking. Brain Res. 2006;1110:150–158. doi: 10.1016/j.brainres.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the Brain. Oxford: Oxford University Press; 2006. [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- Funk AP, Epstein CM. Natural rhythm: evidence for occult 40 Hz gamma oscillation in resting motor cortex. Neurosci Lett. 2004;371:181–184. doi: 10.1016/j.neulet.2004.08.066. [DOI] [PubMed] [Google Scholar]
- Werf YD Van Der, Paus T. The neural response to transcranial magnetic stimulation of the human motor cortex. I. Intracortical and cortico-cortical contributions. Exp Brain Res. 2006;175:231–245. doi: 10.1007/s00221-006-0551-2. [DOI] [PubMed] [Google Scholar]
- Werf YD Van Der, Sadikot AF, Strafella AP, Paus T. The neural response to transcranial magnetic stimulation of the human motor cortex. II. Thalamocortical contributions. Exp Brain Res. 2006;175:246–255. doi: 10.1007/s00221-006-0548-x. [DOI] [PubMed] [Google Scholar]
- Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ, Tononi G. TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS ONE. 2007;2:e276. doi: 10.1371/journal.pone.0000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Esser SK, Riedner BA, Huber R, Murphy M, Peterson MJ, Tononi G. Triggering sleep slow waves by transcranial magnetic stimulation. Proc Natl Acad Sci USA. 2007;104:8496–8501. doi: 10.1073/pnas.0702495104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Määttä S, Esser SK, Sarasso S, Ferrarelli F, Watson A, Ferreri F, Peterson MJ, Tononi G. Measures of cortical plasticity after transcranial paired associative stimulation predict changes in electroencephalogram slow-wave activity during subsequent sleep. J Neurosci. 2008;28:7911–7918. doi: 10.1523/JNEUROSCI.1636-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar D, Gazawi H, Riboyad-Levin J, Klein E. Chronic repetitive transcranial magnetic stimulation alters beta-adrenergic and 5-HT2 receptor characteristics in rat brain. Brain Res. 1999;816:78–83. doi: 10.1016/S0006-8993(98)01119-6. [DOI] [PubMed] [Google Scholar]
- Zangen A, Hyodo K. Transcranial magnetic stimulation induces increases in extracellular levels of dopamine and glutamate in the nucleus accumbens. Neuroreport. 2002;13:2401–2405. doi: 10.1097/00001756-200212200-00005. [DOI] [PubMed] [Google Scholar]
- Gerdelat-Mas A, Loubinoux I, Tombari D, Rascol O, Chollet F, Simonetta-Moreau M. Chronic administration of selective serotonin reuptake inhibitor (SSRI) paroxetine modulates human motor cortex excitability in healthy subjects. Neuroimage. 2005;27:314–322. doi: 10.1016/j.neuroimage.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Korchounov A, Ilic TV, Schwinge T, Ziemann U. Modification of motor cortical excitability by an acetylcholinesterase inhibitor. Exp Brain Res. 2005;164:399–405. doi: 10.1007/s00221-005-2326-6. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Ridel KR, Sallee FR, Zhang J, Lipps TD, Wassermann EM. Comparison of the inhibitory and excitatory effects of ADHD medications methylphenidate and atomoxetine on motor cortex. Neuropsychopharmacology. 2006;31:442–449. doi: 10.1038/sj.npp.1300806. [DOI] [PubMed] [Google Scholar]
- Korchounov A, Ilić TV, Ziemann U. TMS-assisted neurophysiological profiling of the dopamine receptor agonist cabergoline in human motor cortex. J Neural Transm. 2007;114:223–229. doi: 10.1007/s00702-006-0523-5. [DOI] [PubMed] [Google Scholar]
- Lang N, Speck S, Harms J, Rothkegel H, Paulus W, Sommer M. Dopaminergic potentiation of rTMS-induced motor cortex inhibition. Biol Psychiatry. 2008;63:231–233. doi: 10.1016/j.biopsych.2007.04.033. [DOI] [PubMed] [Google Scholar]
- Zhang X, Mei Y, Liu C, Yu S. Effect of transcranial magnetic stimulation on the expression of c-Fos and brain-derived neurotrophic factor of the cerebral cortex in rats with cerebral infarct. J Huazhong Univ Sci Technolog Med Sci. 2007;27:415–418. doi: 10.1007/s11596-007-0416-3. [DOI] [PubMed] [Google Scholar]
- Lang UE, Hellweg R, Gallinat J, Bajbouj M. Acute prefrontal cortex transcranial magnetic stimulation in healthy volunteers: no effects on brain-derived neurotrophic factor (BDNF) concentrations in serum. J Affect Disord. 2008;107:255–258. doi: 10.1016/j.jad.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, Houlden H, Bhatia K, Greenwood R, Rothwell JC. A common polymorphism in the brain derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedi M, Berkovic SF, Macdonell RA, Curatolo JM, Marini C, Reutens DC. Intracortical hyperexcitability in humans with a GABAA receptor mutation. Cereb Cortex. 2008;18:664–669. doi: 10.1093/cercor/bhm100. [DOI] [PubMed] [Google Scholar]
- Zanardi R, Magri L, Rossini D, Malaguti A, Giordani S, Lorenzi C, Pirovano A, Smeraldi E, Lucca A. Role of serotonergic gene polymorphisms on response to transcranial magnetic stimulation in depression. Eur Neuropsychopharmacol. 2007;17:651–657. doi: 10.1016/j.euroneuro.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Turner MR, Osei-Lah AD, Hammers A, Al-Chalabi A, Shaw CE, Andersen PM, Brooks DJ, Leigh PN, Mills KR. Abnormal cortical excitability in sporadic but not homozygous D90A SOD1 ALS. J Neurol Neurosurg Psychiatry. 2005;76:1279–11285. doi: 10.1136/jnnp.2004.054429. [DOI] [PMC free article] [PubMed] [Google Scholar]