Abstract
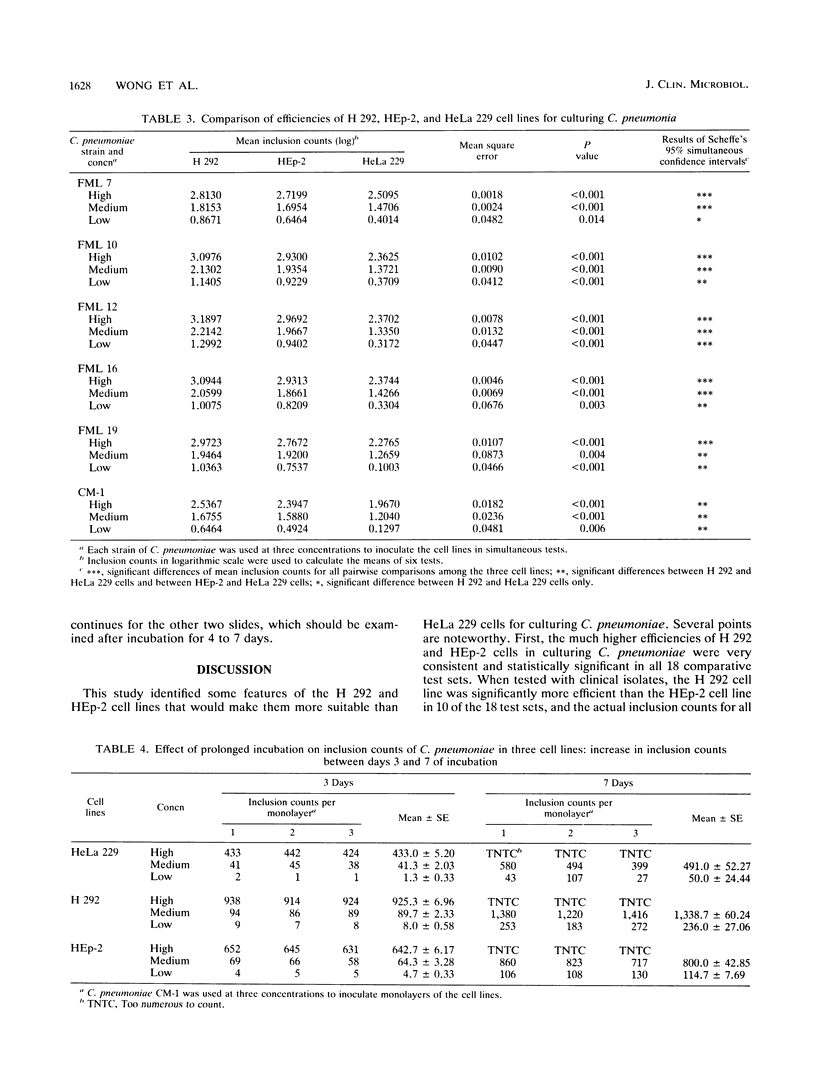
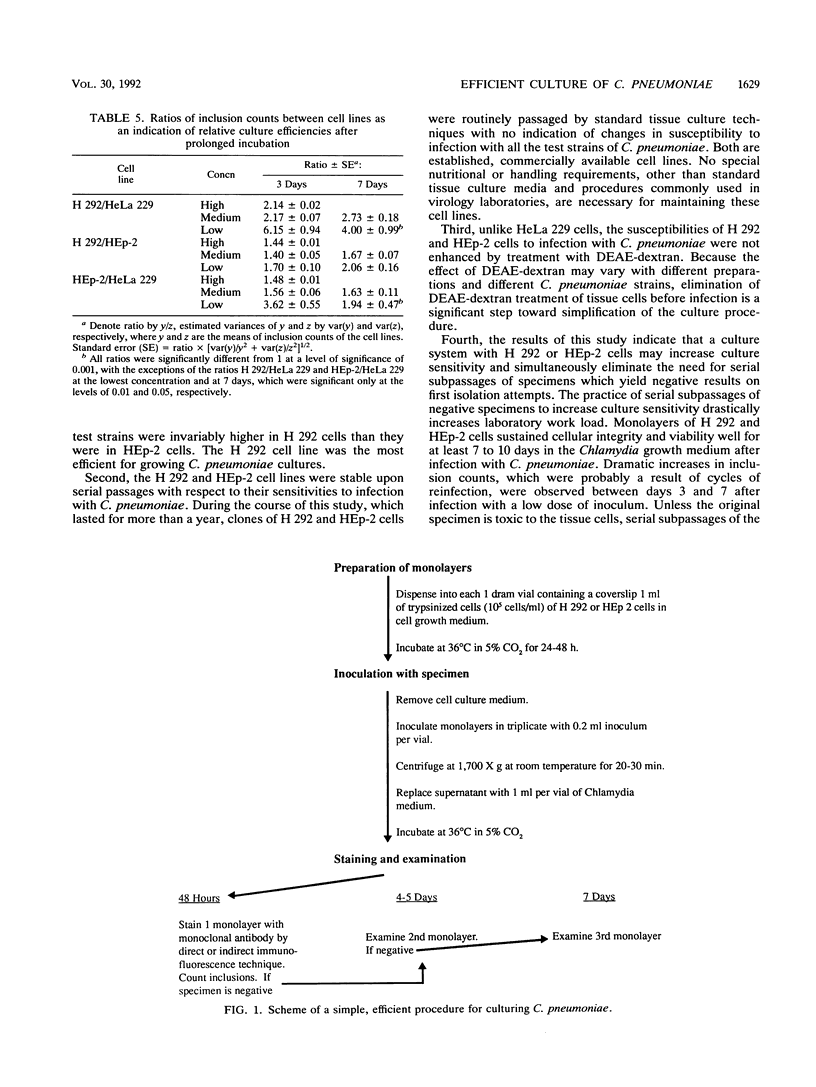
Two established cell lines, H 292 and HEp-2, originating from the human respiratory tract, were found to be significantly more efficient and practical than the currently used HeLa 229 cells for growth of Chlamydia pneumoniae. Six strains of C. pneumoniae recently isolated from patients with respiratory ailments were used as test cultures. The H 292 and HEp-2 cells yielded much higher inclusion counts for all the test strains than did HeLa 229 cells. When they were compared with each other, H 292 cells yielded more inclusions than did HEp-2 cells, and the differences were statistically significant in 10 of 18 test sets. A simple system with these two cell lines appeared to be very efficient for culturing C. pneumoniae. It does not require treatment of tissue cells with DEAE-dextran before infection, and it may eliminate the need for serial subpassages of specimens to increase culture sensitivity. Monolayers of these cells remained intact and viable in the Chlamydia growth medium so that reinfection could take place, resulting in greatly increased inclusion counts for specimens containing few infectious units. This system may make it more practical for laboratories to culture for C. pneumoniae for treatment of infections and outbreak intervention and will facilitate studies on this recently recognized pathogen.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell J. F., Barnes R. C., Kozarsky P. E., Spika J. S. Culture-confirmed pneumonia due to Chlamydia pneumoniae. J Infect Dis. 1991 Aug;164(2):411–413. doi: 10.1093/infdis/164.2.411. [DOI] [PubMed] [Google Scholar]
- Cles L. D., Stamm W. E. Use of HL cells for improved isolation and passage of Chlamydia pneumoniae. J Clin Microbiol. 1990 May;28(5):938–940. doi: 10.1128/jcm.28.5.938-940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. P., Kuo C. C., Campbell L. A. Current knowledge on Chlamydia pneumoniae, strain TWAR, an important cause of pneumonia and other acute respiratory diseases. Eur J Clin Microbiol Infect Dis. 1989 Mar;8(3):191–202. doi: 10.1007/BF01965260. [DOI] [PubMed] [Google Scholar]
- Hahn D. L., Dodge R. W., Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. JAMA. 1991 Jul 10;266(2):225–230. [PubMed] [Google Scholar]
- Kuo C. C., Grayston J. T. A sensitive cell line, HL cells, for isolation and propagation of Chlamydia pneumoniae strain TWAR. J Infect Dis. 1990 Sep;162(3):755–758. doi: 10.1093/infdis/162.3.755. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Grayston J. T. Factors affecting viability and growth in HeLa 229 cells of Chlamydia sp. strain TWAR. J Clin Microbiol. 1988 May;26(5):812–815. doi: 10.1128/jcm.26.5.812-815.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Wang S. P., Grayston J. T. Effect of polycations, polyanions and neuraminidase on the infectivity of trachoma-inclusin conjunctivitis and lymphogranuloma venereum organisms HeLa cells: sialic acid residues as possible receptors for trachoma-inclusion conjunction. Infect Immun. 1973 Jul;8(1):74–79. doi: 10.1128/iai.8.1.74-79.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R. D. A short-duration polyethylene glycol fusion technique for increasing production of monoclonal antibody-secreting hybridomas. J Immunol Methods. 1985 Aug 2;81(2):223–228. doi: 10.1016/0022-1759(85)90207-8. [DOI] [PubMed] [Google Scholar]
- Miller S. T., Hammerschlag M. R., Chirgwin K., Rao S. P., Roblin P., Gelling M., Stilerman T., Schachter J., Cassell G. Role of Chlamydia pneumoniae in acute chest syndrome of sickle cell disease. J Pediatr. 1991 Jan;118(1):30–33. doi: 10.1016/s0022-3476(05)81839-6. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Fujisawa T., Kazuyama Y. Isolation of Chlamydia pneumoniae from middle ear aspirates of otitis media with effusion: a case report. J Infect Dis. 1990 Oct;162(4):1000–1001. doi: 10.1093/infdis/162.4.1000. [DOI] [PubMed] [Google Scholar]
- Saikku P., Leinonen M., Mattila K., Ekman M. R., Nieminen M. S., Mäkelä P. H., Huttunen J. K., Valtonen V. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988 Oct 29;2(8618):983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]


