Abstract
Background
Germline mutations in BRCA1 or BRCA2 genes have been demonstrated to increase the risk of developing breast cancer. Conversely, the impact of BRCA mutations on prognosis and survival of breast cancer patients is still debated. In this study, we investigated the role of such mutations on breast cancer-specific survival among patients from North Sardinia.
Methods
Among incident cases during the period 1997–2002, a total of 512 breast cancer patients gave their consent to undergo BRCA mutation screening by DHPLC analysis and automated DNA sequencing. The Hakulinen, Kaplan-Meier, and Cox regression methods were used for both relative survival assessment and statistical analysis.
Results
In our series, patients carrying a germline mutation in coding regions and splice boundaries of BRCA1 and BRCA2 genes were 48/512 (9%). Effect on overall survival was evaluated taking into consideration BRCA2 carriers, who represented the vast majority (44/48; 92%) of mutation-positive patients. A lower breast cancer-specific overall survival rate was observed in BRCA2 mutation carriers after the first two years from diagnosis. However, survival rates were similar in both groups after five years from diagnosis. No significant difference was found for age of onset, disease stage, and primary tumour histopathology between the two subsets.
Conclusion
In Sardinian breast cancer population, BRCA2 was the most affected gene and the effects of BRCA2 germline mutations on patients' survival were demonstrated to vary within the first two years from diagnosis. After a longer follow-up observation, breast cancer-specific rates of death were instead similar for BRCA2 mutation carriers and non-carriers.
Background
The breast cancer is a complex disease with high biological heterogeneity and wide spectrum of responsiveness to different treatments. The well-established prognostic factors currently used into the management of patients with breast carcinoma include the disease stage (which takes into account tumour size, axillary lymph node involvement, and distant tumour dissemination) as well as the histological type, the degree of differentiation (tumour grade), the proliferation index, and the receptor status [estrogen receptor (ER), progesterone receptor (PR), and, recently, HER2] of the primary tumours [1]. Among them, the expression levels of hormone receptors seem to better predict the breast cancer response to different therapeutic strategies. More in general, the assessment of some molecular mechanisms responsible for the mammary tumourigenesis and studies on molecular profiling allowed to identify several biomarkers which may be helpful to pathologically classify breast cancer lesions into subtypes with different prognostic and clinico-pathologic behaviours [2,3].
Mutations in the BRCA1 and BRCA2 tumour suppressor genes have been associated with the breast cancer risk among families with strong recurrence of the disease [4-9]. Vast majority of studies has shown a highly increased risk of developing breast cancer in BRCA1 or BRCA2 mutation carriers [4-7] with also a greater incidence of a second contra-lateral tumour [4,5,10]. However, majority of breast cancers occur sporadically in individuals with little or no family history, for whom no clear role of the mutations in BRCA genes has emerged. Overall, BRCA mutations are responsible for 30–60% of the hereditary cases and have a prevalence of about 5% in the general population and about 25% in the families with history of breast cancer [9].
Analogously, several studies have investigated the possible effects of BRCA mutations on clinical and pathologic characteristics of breast cancer as well as on prognosis and survival rates of the patients, but the results were inconclusive. Some of these studies have demonstrated that BRCA1 mutation carriers develop cancer with a high proliferation index and low expression of estrogen receptors [11,12]. Moreover, a higher proliferation index has been reported in all breast cancer sporadic cases carrying a BRCA germline mutation (regardless of the gene involved) in comparison to the patients with wild-type BRCA [13-15]. Conversely, other authors observed no difference in histological tumour features among BRCA2-positive familial cases and sporadic cases [16]. Regarding the relationship with the survival, BRCA1 mutation carriers showed either a poor prognosis in patients with negative lymph nodes [17] or a worse outcome in comparison with BRCA2-positive cases [18,19]. Other investigators did not find any significant survival difference in BRCA mutation carriers compared with non-carrier cases [20,21]; moreover, breast cancer specific mortality rates have been found similar for BRCA mutation carriers and non-carriers in Jewish population [22]. Nevertheless, a better assessment of the role on survival and prognosis could be also important for women with a BRCA mutation who face a decision between preventive surgery and intensive surveillance.
In Sardinia, whose population is genetically homogeneous due to the fact that it is relatively isolated and with high rate of inbreeding, the contribution of BRCA mutations to the population incidence of breast cancer has been evaluated by our group in recent past years [23-25]. Three deleterious BRCA germline mutations have been observed in about 15% families and in about 3% non-familial breast cancer patients from North Sardinia (BRCA2 mutations were the most prevalent BRCA sequence variations and a single variant, BRCA2-8765delAG, was the most recurrent mutation with a founder effect in our population) [23-25]. In North Sardinia, breast cancer represents the principal death-causing malignancy, with an incidence rate quite comparable with that observed in Western countries (standardized rate, 95 per 100.000 inhabitants per year); the median age of onset for breast cancer among Sardinian women is 65 years [26]. Based on the existence of an official cancer registry, which has recorded all malignancies diagnosed into the population of the province of Sassari from 1992 to 2003 [26], the objective of the present study was to investigate the relationship between the occurrence of BRCA mutations and the main standardized prognostic factors as well as the overall survival rates among breast cancer patients from North Sardinia. Specifically, we compared survival rates between BRCA2 mutation carriers and non-carriers while adjusting for demographic and clinically-recognized prognostic factors in a homogenous group of Sardinian breast cancer patients.
Methods
Patients' selection
Among 1,835 incident cases during the period 1997–2002 [with 140 (8%) tumour-specific deaths], we selected all consecutive patients with histologically-proven diagnosis of malignant breast cancer (regardless of factors which may influence prognosis: age, family history, disease stage, or type of treatment). Among them, 512 patients gave their consent to undergo genetic analysis for detection of BRCA mutations on germline DNA from peripheral blood. For such cases, the collected information included the disease stage at diagnosis, the expression levels of estrogen and progesterone receptors, and the occurrence of a second cancer. All information have been verified through analysis of the hospital records; all cancer diagnoses were confirmed by pathology reports.
The study was reviewed and approved by the ethical review boards of both Institutions (University of Sassari and A.S.L.1 of Sassari).
Mutation screening
For the BRCA1 and BRCA2 genetic testing, all patients were informed about the aims and limits of the mutation analysis and blood samples were collected after obtaining a patient's written consent (in any case, documentation of counselling was carefully evaluated prior to genetic testing). As previously described [23,24], genomic DNA samples were screened for mutations in BRCA1 and BRCA2 genes by a sequential combination of denaturing high-performance liquid chromatography (DHPLC) analysis and sequencing approach using an automated fluorescence-cycle sequencer (ABIPRISM 3100, Applied Biosystems, Foster City, CA).
Statistical analysis
The following variables and categories were defined and included in our analyses: pathological primary tumor size (pT), pathological nodal status (pN), presence of distant metastases (M), estrogen and progesteron receptor (ER and PR, respectively) status, age at diagnosis, and overall survival (calculated starting from the time of diagnosis to the date of death or to the end of our follow-up observation on December 31, 2004). Receptor status was not known in a fraction (about 30%) of the patients included into the study.
The general mortality data were provided from official regional sources, and in some cases were drawn from the municipality rosters. The death probability was calculated on the mortality rate basis and expressed as the probability that an individual has, at beginning of the age class considered, to die before going to the next age class. The formula from life-table that assume a constant mortality rate within a given period was applied [27]. In this case, the age class was equal to one year, as required by the Hakulinen method for calculation of relative survival [28].
The five-year relative survival figures were also computed following the Hakulinen method [28]; the 95% confidence limits were calculated using the "eurocare " confidence interval algorithm [29]. For the comparison of survival probabilities within the various subgroups, the cumulative relative survival adjusted for age was estimated using the technique proposed by Brenner and colleagues [29].
The role of familiarity and BRCA status as genetic marker in cause-specific survival was investigated by Kaplan-Meier and Cox regression methods. All tests were computed by Stata Software.
Results
Among the 512 breast cancer patients who gave their consent to participate to the study, 103 (20%) had a family history of breast cancer. Cases were classified as familial when at least three affected members (considering first- and second-degree relatives) were diagnosed with breast cancer.
Mutation analysis for all coding regions and splice boundaries of BRCA1 and BRCA2 genes was performed as previously described [24]. Briefly, germline DNA from breast cancer patients was screened by DHPLC analysis; all PCR products presenting an abnormal denaturing profile in comparison to the normal controls were sequenced using an automated approach. Taking into consideration the 103 familial cases, 2 (2%) and 20 (19%) presented a germline mutation in BRCA1 and BRCA2 genes, respectively. Among the remaining 409 patients classified as sporadic cases, 2 (0.5%) and 24 (6%) were found to carry BRCA1 and BRCA2 mutations, respectively. Overall, patients carrying a BRCA mutation were 48/512 (9%). In particular, BRCA1 mutations were detected in only 4/48 (8%) carriers, while BRCA2 mutations were identified in vast majority of them (44/48; 92%), with the BRCA2-8765delAG variant acting as a founder mutation [23-25]. Taking into account such a high preponderance of germline mutations, only BRCA2-positive cases were considered for statistical correlations in our series.
The age of breast cancer onset was evaluated on the basis of the mutation status; 23/44 (52%) BRCA2 mutation positive and 188/464 (41%) BRCA2 mutation negative patients were 50 years or younger at the time of diagnosis (Table 1). Although the average age at diagnosis was younger in patients carrying BRCA2 mutations [23/44 (52%) ≤ 50 years vs. 21/44 (48%) > 50 years] than in cases with no detectable mutation [188/464 (41%) ≤ 50 years vs. 276/464 (59%) > 50 years], such a difference was not statistically significant. Using Pearson's Chi-Squared test, the occurrence of a BRCA2 mutation was evaluated for association with several pathological parameters: pT, pN, M, or, when available, ER and PR. As shown in Table 2, distribution of BRCA2 mutation carriers and non-carriers was quite identical in the different subsets of patients according to such pathological parameters (thus, no statistically significant correlation was observed – not shown).
Table 1.
Distribution of patients according to BRCA2 mutation status and age of onset
| Age Class | BRCA2 mutation negative | BRCA2 mutation positive | Total |
|---|---|---|---|
| 20 | 1 | 1 | |
| 25 | 2 | 1 | 3 |
| 30 | 21 | 2 | 23 |
| 35 | 27 | 3 | 30 |
| 40 | 38 | 7 | 45 |
| 45 | 54 | 6 | 60 |
| 50 | 45 | 4 | 49 |
| 55 | 57 | 8 | 65 |
| 60 | 47 | 3 | 50 |
| 65 | 50 | 5 | 55 |
| 70 | 56 | 1 | 57 |
| 75 | 36 | 2 | 38 |
| 80 | 21 | 1 | 22 |
| 85 | 9 | 1 | 10 |
| 464 | 44 | 508 | |
Table 2.
Distribution of BRCA2 cases according to the TNM and receptor status
| Primary tumour size | |||
|---|---|---|---|
| BRCA2 mutation | T1-2 | T3-4 | Total |
| Negative | 421 91% | 43 9% | 464 |
| Positive | 41 93% | 3 7% | 44 |
| Total | 462 91% | 46 9% | 508 |
| Lymph node metastasis | |||
| BRCA2 mutation | Negative | Positive | Total |
| Negative | 263 57% | 201 43% | 464 |
| Positive | 24 55% | 20 45% | 44 |
| Total | 287 56% | 221 44% | 508 |
| Distant metastasis | |||
| BRCA2 mutation | Absent | Present | Total |
| Negative | 434 94% | 30 6% | 464 |
| Positive | 41 93% | 3 7% | 44 |
| Total | 475 94% | 33 6% | 508 |
| Estrogen receptor | |||
| BRCA2 mutation | Negative | Positive | Total |
| Negative | 91 26% | 257 74% | 348 |
| Positive | 5 29% | 12 71% | 17 |
| Total | 96 26% | 269 74% | 365 |
| Progesterone receptor | |||
| BRCA2 mutation | Negative | Positive | Total |
| Negative | 150 44% | 189 56% | 339 |
| Positive | 6 46% | 7 54% | 13 |
| Total | 156 44% | 196 56% | 352 |
Taking into consideration the primary tumour morphology, 348 (68%) ductal carcinomas, 62 (12%) lobular carcinomas, and 102 (20%) other histological types were registered. The BRCA2 mutations were more prevalent in lobular (7/62; 11%) than in ductal carcinomas (20/348; 6%); again, differences were not statistically significant.
The five-year survival rate was 81% (80%, adjusted by age) among BRCA2 mutation carriers and 91% (92%, adjusted by age) among patients negative for BRCA2 mutations. Overall, the five-year relative survival rate for breast cancer cases from our series was 85%. Evaluation of the overall survival curves using the Kaplan-Meier method indicated that patients carrying BRCA2 mutations presented a lower breast cancer-specific survival in comparison with those resulted negative for BRCA2 mutations, within the first two years from diagnosis (Figure 1). Considering the entire observation period of five years from diagnosis, the survival curves tended to merge with no significant difference in outcome between the two groups (Figure 1). Furthermore, no difference in survival among familial and sporadic BRCA2 mutated cases was observed (not shown). As estimated by Cox regression analysis, the hazard ratio of patients positive for BRCA2 mutations was found to be 0.7 (95% CI, 0.46–1.37), after adjustment by age (Figure 2), and about 0.8 (95% CI, 0.48–1.62), after adjustment by disease stage (Figure 3). Hazard ratios were quite identical for both subsets when adjusted for tumour grade (0.82; 95% CI, 0.53–0.98) and receptor status (0.85; 95% CI, 0.64–0.97) (not shown).
Figure 1.
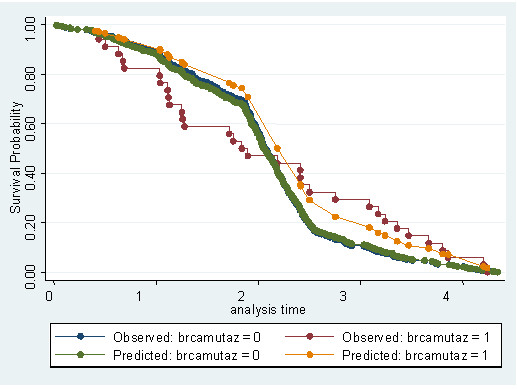
Overall survival curves based on the Kaplan-Meier method. Comparison between observed and predicted survival data for each subset of patients (with or without BRCA2 mutations) is reported.
Figure 2.
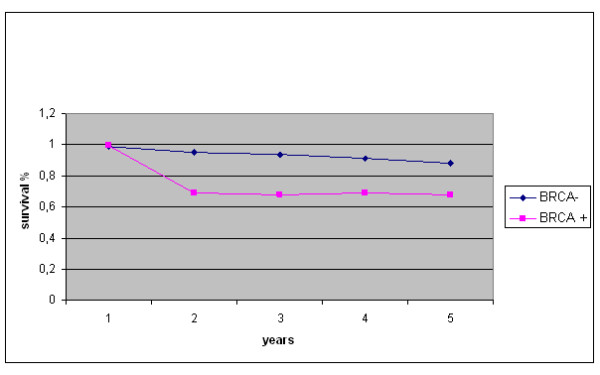
Relative five-year survival for breast cancer patients with or without BRCA2 mutations (BRCA+/-), adjusted by age according to Brenner.
Figure 3.
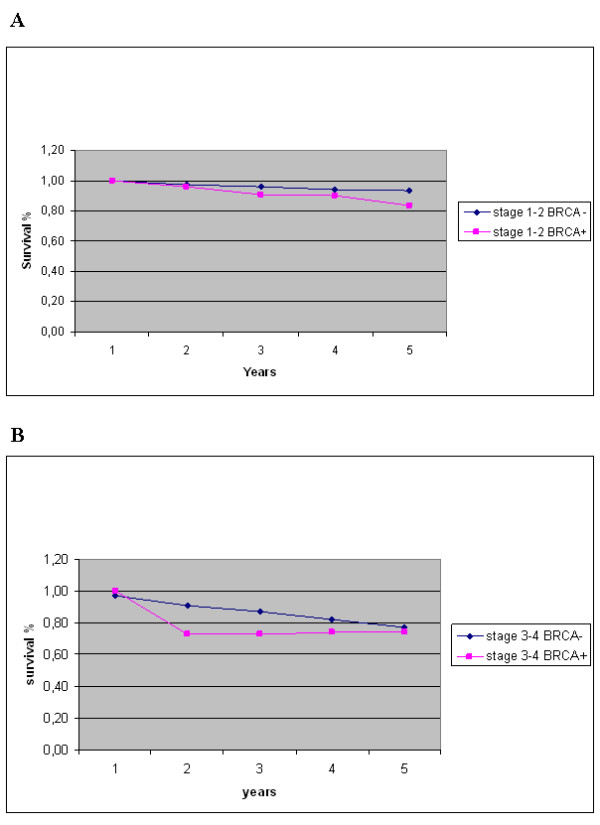
Relative five-year survival for breast cancer patients according to (A) localized disease (stage 1–2) or (B) metastatic disease (stage 3–4), and presence or absence of BRCA2 mutations (BRCA+/-).
Finally, multivariate Cox regression analysis was performed considering all variables (pT, pN, M, ER, PR, and BRCA2 mutations). The presence of metastases was the only parameter with a significant impact on prognosis (p < 0.001; hazard ratio, 8.939; 95% CI, 4.68–17.1). However, such a prognostic factor was not able to exert a confounding effect on mutation-based survival curves due to the low number of patients with distant metastases (33/508; 6%), who even showed a similar prevalence of BRCA2 mutation-negative (30/464; 6%) and mutation-positive (3/44; 7%) cases (see Table 2). No other association between BRCA2 mutation status and overall survival was observed for the remaining variables.
Discussion and conclusion
Breast cancers carrying BRCA1 or BRCA2 germline mutations often occur in younger women as well as present a high tumour grade and/or lack of expression of estrogen/progesterone receptors (mostly, among BRCA1-positive tumours) [11-16,30-32]. Although these features have been associated with a poor prognosis, the relationship between the occurrence of a BRCA1 or BRCA2 mutation and the effect on overall survival is still controversial [17-22,31,33-35].
In this study, we tried to clarify the role of BRCA mutations on the outcome of breast cancer patients from North Sardinia, where an official cancer registry is available [26]. In particular, we evaluated the 5-year survival rates among women who had received the diagnosis of breast cancer from 1997 to 2002 and gave their consent to undergo a BRCA genetic testing. Among the 508 analyzed patients, we assessed the breast cancer-specific survival rates for women with (44 cases; 9%) or without (464 cases; 91%) a BRCA2 germline mutation.
Using the Kaplan-Meier method, the survival rate of patients with a positive BRCA2 genetic test was lower than that of patients with negative genetic tests within the first two years after diagnosis in our series. However, the two survival curves tended to merge at the end of five years from diagnosis (see Figure 1). This trend may indeed account for the absence of significance of the Cox regression for BRCA2 status, due to the failure of proportionality of hazards (a situation in which calculation of a total hazard ratio with the Cox model is unsuitable). The relative survival of the entire Sardinian series at five years from diagnosis is slightly below the average survival rate observed in breast cancer cases from the other Italian regions [17]; moreover, it always remains lower in the subset of BRCA2 mutation carriers than in that of BRCA2 mutation-negative patients, regardless the adjustments according to the different prognostic parameters in multivariate analysis (see Figures 2 and 3). Indeed, the relative survival seems to be worse in cases with positive BRCA2 genetic tests, even after adjustment for age of onset (which represents one of the factors with the greatest influence on cancer prognosis, according to the studies in other populations [34-36]). Although survival is also deeply influenced by disease stage at the time of diagnosis, we observed no significant difference between the two BRCA2 subsets after stratification by stage.
Although our study has a number of limitations mainly due to the fact that we identified only a limited fraction (44/508; 9%) of mutation carriers and, thus, the subgroup analyses relied on a small number of subjects, we can conclude that a prolonged follow-up observation seems to minimize the effect of the presence of BRCA2 germline mutations on prognosis among breast cancer patients from Sardinian population.
Our findings are consistent with data recently reported in Israeli women of Ashkenazi Jewish ancestry (who present a high prevalence of hereditary breast cancer and BRCA founder mutations) [22] as well as in a Dutch series of BRCA1-associated breast carcinoma patients [37]. In both studies, the breast cancer-specific survivals for carriers and noncarriers of BRCA mutations were similar, even considering a longer period of observation (ten years) from diagnosis [22,37]. On the basis of these results, one could speculate that additional factors may influence the prognosis in such patients.
Search for prognostic factors in breast cancer patients is still a challenge; several lifestyle and environmental risk factors for breast cancer are being investigated. For example, evidence have been recently found that an increase in body mass index is associated with a poorer prognosis in women receiving diagnosis of breast cancer [36]. Probably, further studies toward the comprehension of the underlying interactions between all genetic and environmental factors could really improve the classification of the different subsets of patients who would be expected to have better or worse prognosis as well as to be more or less likely to respond to specific therapeutic interventions.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MB participated to the design of the study and performed statistical analyses. RC participated to analysis and interpretation of data. VC participated to statistical analysis. OS participated to analysis and interpretation of data. DP performed the data management. AC participated to patients' collection and performed some screening analyses. FT participated to patients' collection. MP participated to mutation analysis. GrP performed mutation screening. GiP conceived of the study and drafted the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Mario Budroni, Email: mariobudroni@tiscali.it.
Rosaria Cesaraccio, Email: rosariacesaraccio@tiscali.it.
Vincenzo Coviello, Email: enzo.coviello@alice.it.
Ornelia Sechi, Email: orneliasechi@tiscali.it.
Daniela Pirino, Email: danielapirino@tiscali.it.
Antonio Cossu, Email: cossu@uniss.it.
Francesco Tanda, Email: tandaf@uniss.it.
Marina Pisano, Email: marina.pisano@icb.cnr.it.
Grazia Palomba, Email: graziap68@yahoo.it.
Giuseppe Palmieri, Email: gpalmieri@yahoo.com.
Acknowledgements
Authors are grateful to patients and families for their important contribution to this study. Work was funded by Regione Autonoma della Sardegna, Ricerca Finalizzata Ministero della Salute, Associazione Italiana Ricerca sul Cancro (AIRC), and Associazione UMANA Onlus.
References
- Lønning PE. Breast cancer prognostication and prediction: are we making progress? Ann Oncol. 2007;18(Suppl 8):viii3–7. doi: 10.1093/annonc/mdm260. [DOI] [PubMed] [Google Scholar]
- van't Veer LJ, Dai H, Vijver MJ van de, He YD, Hart AA, Mao M, Peterse HL, Kooy K van der, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- Jarzabek K, Koda M, Kozlowski L, Mittre H, Sulkowski S, Kottler ML, Wolczynski S. Distinct mRNA, protein expression patterns and distribution of oestrogen receptors alpha and beta in human primary breast cancer: correlation with proliferation marker Ki-67 and clinicopathological factors. Eur J Cancer. 2005;41:2924–34. doi: 10.1016/j.ejca.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Ford D, Easton DF, Bishop DT, Narod SA, Godgar DE. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994;343:692–695. doi: 10.1016/S0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- Easton DF, Ford D, Bishop DT. Breast Cancer Linkage Consortium. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Am J Hum Genet. 1995;56:265–271. [PMC free article] [PubMed] [Google Scholar]
- Easton DF, Steele L, Fields P, Ormiston W, Averill D, Daly PA, McManus R, Neuhausen SL, Ford D, Wooster R, Cannon-Albright LA, Stratton MR, Goldgar DE. Cancer risks in two large breast cancer families linked to BRCA2 on chromosome 13q12-13. Am J Hum Genet. 1997;61:120–128. doi: 10.1086/513891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, Bishop DT, Weber B, Lenoir G, Chang-Claude J, Sobol H, Teare MD, Struewing J, Arason A, Scherneck S, Peto J, Rebbeck TR, Tonin P, Neuhausen S, Barkardottir R, Eyfjord J, Lynch H, Ponder BA, Gayther SA, Zelada-Hedman M. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirova YM, Stoppa-Lyonnet D, Savignoni A, Sigal-Zafrani B, Fabre N, Fourquet A. Institut Curie Breast Cancer Study Group. Risk of breast cancer recurrence and contralateral breast cancer in relation to BRCA1 and BRCA2 mutation status following breast-conserving surgery and radiotherapy. Eur J Cancer. 2005;41:2304–11. doi: 10.1016/j.ejca.2005.02.037. [DOI] [PubMed] [Google Scholar]
- Dent R, Warner E. Screening for hereditary breast cancer. Semin Oncol. 2007;34:392–400. doi: 10.1053/j.seminoncol.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Metcalfe KA, Lubinski J, Ghadirian P, Lynch H, Kim-Sing C, Friedman E, Foulkes WD, Domchek S, Ainsworth P, Isaacs C, Tung N, Gronwald J, Cummings S, Wagner T, Manoukian S, Møller P, Weitzel J, Sun P, Narod SA. Hereditary Breast Cancer Clinical Study Group. Predictors of contralateral prophylactic mastectomy in women with a BRCA1 or BRCA2 mutation: the Hereditary Breast Cancer Clinical Study Group. J Clin Oncol. 2008;26:1093–7. doi: 10.1200/JCO.2007.12.6078. [DOI] [PubMed] [Google Scholar]
- Adem C, Reynolds C, Soderberg CL, Slezak JM, McDonnell SK, Sebo TJ, Schaid DJ, Myers JL, Sellers TA, Hartmann LC, Jenkins RB. Pathologic characteristics of breast parenchyma in patients with hereditary breast carcinoma, including BRCA1 and BRCA2 mutation carriers. Cancer. 2003;97:1–11. doi: 10.1002/cncr.11048. [DOI] [PubMed] [Google Scholar]
- Chappuis PO, Nethercot V, Foules WD. Clinico-pathological characteristics of BRCA1 and BRCA2-related breast cancer. Semin Surg Oncol. 2000;18:287–295. doi: 10.1002/(SICI)1098-2388(200006)18:4<287::AID-SSU3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Honrado E, Benítez J, Palacios J. Histopathology of BRCA1- and BRCA2-associated breast cancer. Crit Rev Oncol Hematol. 2006;59:27–39. doi: 10.1016/j.critrevonc.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Palacios J, Honrado E, Osorio A, Cazorla A, Sarrió D, Barroso A, Rodríguez S, Cigudosa JC, Diez O, Alonso C, Lerma E, Sánchez L, Rivas C, Benítez J. Immunohistochemical characteristics defined by tissue microarray of hereditary breast cancer not attributable to BRCA1 or BRCA2 mutations: differences from breast carcinomas arising in BRCA1 and BRCA2 mutation carriers. Clin Cancer Res. 2003;9:3606–3614. [PubMed] [Google Scholar]
- Lakhani SR, Jacquemier J, Sloane JP, Gusterson BA, Anderson TJ, Vijver MJ van de, Farid LM, Venter D, Antoniou A, Storfer-Isser A, Smyth E, Steel CM, Haites N, Scott RJ, Goldgar D, Neuhausen S, Daly PA, Ormiston W, McManus R, Scherneck S, Ponder BA, Ford D, Peto J, Stoppa-Lyonnet D, Bignon YJ, Struewing JP, Spurr NK, Bishop DT, Klijn JG, Devilee P, Cornelisse CJ, Lasset C, Lenoir G, Barkardottir RB, Egilsson V, Hamann U, Chang-Claude J, Sobol H, Weber B, Stratton MR, Easton DF. Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst. 1998;90:1138–1145. doi: 10.1093/jnci/90.15.1138. [DOI] [PubMed] [Google Scholar]
- The Breast Cancer Linkage Consortium. Pathology of familial breast cancer: differences between breast cancers in carriers of BRCA1 or BRCA2 mutations and sporadic cases. Lancet. 1997;349:1505–1510. doi: 10.1016/S0140-6736(96)10109-4. [DOI] [PubMed] [Google Scholar]
- Foulkes WD, Chappuis PO, Wong N, Brunet JS, Vesprini D, Rozen F, Yuan ZQ, Pollak MN, Kuperstein G, Narod SA, Begin LR. Primary node negative breast cancer in BRCA1 mutation carriers has a poor outcome. Ann Oncol. 2000;11:307–13. doi: 10.1023/A:1008340723974. [DOI] [PubMed] [Google Scholar]
- Robson ME, Chappuis PO, Satagopan J, Wong N, Boyd J, Goffin JR, Hudis C, Roberge D, Norton L, Begin LR, Offit K, Foulkes WD. A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res. 2004;6 doi: 10.1186/bcr658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppa-Lyonnet D, Ansquer Y, Dreyfus H, Gautier C, Gauthier-Villars M, Bourstyn E, Clough KB, Magdelenat H, Pouillart P, Vincent-Salomon A, Fourquet A, Asselain B. Familial invasive breast cancers: worse outcome related to BRCA1 mutations. J Clin Oncol. 2000;18:4053–4059. doi: 10.1200/JCO.2000.18.24.4053. [DOI] [PubMed] [Google Scholar]
- Johannsson OT, Ranstam J, Borg A, Olsson H. Survival of BRCA1 breast and ovarian cancer patients: a population-based study from southern Sweden. J Clin Oncol. 1998;16:397–404. doi: 10.1200/JCO.1998.16.2.397. [DOI] [PubMed] [Google Scholar]
- Brekelmans CTM, Seynaeve C, Menke-Pluymers M. Survival and prognostic factors in BRCA1-associated breast cancer. Ann Oncol. 2006;17:391–400. doi: 10.1093/annonc/mdj095. [DOI] [PubMed] [Google Scholar]
- Rennert G, Bisland-Naggan S, Barnett-Griness O. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 2007;357:115–123. doi: 10.1056/NEJMoa070608. [DOI] [PubMed] [Google Scholar]
- Palmieri G, Palomba G, Cossu A, Pisano M, Dedola MF, Sarobba M, Farris A, Olmeo N, Contu A, Pasca A, Satta M, Persico I, Carboni A, Cossu-Rocca P, Contini M, Mangion J, Stratton MR, Tanda F. BRCA1 and BRCA2 germline mutations in Sardinian breast cancer families and their implications for genetic counseling. Ann Oncol. 2002;13:1899–1907. doi: 10.1093/annonc/mdf326. [DOI] [PubMed] [Google Scholar]
- Palomba G, Pisano M, Cossu A, Budroni M, Dedola MF, Farris A, Contu A, Baldinu P, Tanda F, Palmieri G. Spectrum and prevalence of BRCA1 and BRCA2 germline mutations in Sardinian breast cancer patients through a hospital-based screening. Cancer. 2005;104:1172–1179. doi: 10.1002/cncr.21298. [DOI] [PubMed] [Google Scholar]
- Palomba G, Cossu A, Friedman E, Budroni M, Farris A, Contu A, Pisano M, Baldinu P, Sini MC, Tanda F, Palmieri G. Origin and distribution of the BRCA2-8765delAG mutation in breast cancer. BMC Cancer. 2007;7:132. doi: 10.1186/1471-2407-7-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budroni M, Cesaraccio R, Pirino D, Sechi O, Oggiano M, Piras D, Sechi A, Cossu A, Palmieri G, Tanda F. In: Cancer Incidence in Five Continents, International Agency for Research on Cancer (IARC) Scientific Publications, No. 160. Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, Boyle P, editor. IX. Lyon, IARC; 2007. Cancer incidence in Sassari Province (1998–2002) [Google Scholar]
- Hakulinen T. Cancer survival corrected for heterogeneity in patient withdrawal. Biometrics. 1982;38:933–942. doi: 10.2307/2529873. [DOI] [PubMed] [Google Scholar]
- Verdecchia A, Capocaccia R, Hakulinen T. In: Survival of cancer patients in Europe. The EUROCARE study. IARC scientific publication 95. Berrino F, SAnt M, Verdecchia A, Capocaccia R, Hakulinen T, Esteve J, editor. Lyon International Agency for Research on Cancer; 1995. Methods of data analysis. [Google Scholar]
- Brenner H, Hakulinen T. Age adjustment of cancer survival rates: methods, point estimates and standard errors. Br J Cancer. 2005;93:372–5. doi: 10.1038/sj.bjc.6602704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mote PA, Leary JA, Avery KA, Sandelin K, Chenevix-Trench G, Kirk JA, Clarke CL. Germ-line mutations in BRCA1 or BRCA2 in the normal breast are associated with altered expression of estrogen-responsive proteins and the predominance of progesterone receptor. Genes Chromosomes Cancer. 2004;39:236–48. doi: 10.1002/gcc.10321. [DOI] [PubMed] [Google Scholar]
- Veronesi A, de Giacomi C, Magri MD, Lombardi D, Zanetti M, Scuderi C, Dolcetti R, Viel A, Crivellari D, Bidoli E, Boiocchi M. Familial breast cancer: characteristics and outcome of BRCA 1–2 positive and negative cases. BMC Cancer. 2005;5:70. doi: 10.1186/1471-2407-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musolino A, Bella MA, Bortesi B, Michiara M, Naldi N, Zanelli P, Capelletti M, Pezzuolo D, Camisa R, Savi M, Neri TM, Ardizzoni A. BRCA mutations, molecular markers, and clinical variables in early-onset breast cancer: a population-based study. Breast. 2007;16:280–92. doi: 10.1016/j.breast.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Eerola H, Vahteristo P, Sarantaus L. Survival of breast cancer patients in BRCA1, BRCA2, and non-BRCA1/2 breast cancer families: a relative survival analysis from Finland. Int J Cancer. 2001;93:368–72. doi: 10.1002/ijc.1341. [DOI] [PubMed] [Google Scholar]
- El-Tamer M, Russo D, Troxel A. Survival and recurrence after breast cancer in BRCA1/2 mutation carriers. Ann Surg Oncol. 2004;11:157–64. doi: 10.1245/ASO.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Moller P, Evans DG, Reis MM, Gregory H, Anderson E, Maehle L, Lalloo F, Howell A, Apold J, Clark N, Lucassen A, Steel CM. Surveillance for familial breast cancer: Differences in outcome according to BRCA mutation status. Int J Cancer. 2007;121:1017–1020. doi: 10.1002/ijc.22789. [DOI] [PubMed] [Google Scholar]
- Barnett GC, Shah M, Redman K, Easton DF, Ponder BAJ, Pharoah PDP. Risk factors for the incidence of breast cancer: do they affect survival from the disease? J Clin Oncol. 2008;26:3310–3316. doi: 10.1200/JCO.2006.10.3168. [DOI] [PubMed] [Google Scholar]
- Brekelmans CT, Seynaeve C, Menke-Pluymers M, Brüggenwirth HT, Tilanus-Linthorst MM, Bartels CC, Kriege M, van Geel AN, Crepin CM, Blom JC, Meijers-Heijboer H, Klijn JG. Survival and prognostic factors in BRCA1-associated breast cancer. Ann Oncol. 2006;17:391–400. doi: 10.1093/annonc/mdj095. [DOI] [PubMed] [Google Scholar]


