Abstract
Living cartilaginous fishes, or chondrichthyans, include numerous elasmobranch (sharks and rays) species but only few chimaeroid (ratfish) species. The early history of chimaeroids, or holocephalans, and the modalities of their divergence from elasmobranchs are much debated. During Carboniferous times, 358–300 million years (Myr) ago, they underwent a remarkable evolutionary radiation, with some odd and poorly understood forms, including the enigmatic iniopterygians that were known until now from poorly informative flattened impressions. Here, we report iniopterygian skulls found preserved in 3 dimensions in ≈300-Myr-old concretions from Oklahoma and Kansas. The study was performed by using conventional X-ray microtomography (μCT), as well as absorption-based synchrotron microtomography (SR-μCT) [Tafforeau P, et al. (2006) Applications of X-ray synchrotron microtomography for non-destructive 3D studies of paleontological specimens. Appl Phys A 83:95–202] and a new holotomographic approach [Guigay P, Langer M, Boistel R, Cloetens P (2007) Mixed transfer function and transport of intensity approach for phase retrieval in the Fresnel region. Opt Lett 32:1617–1619], which revealed their peculiar anatomy. Iniopterygians also share unique characters with living chimaeroids, suggesting that the key chimaeroid skull features were already established 300 Myr ago. Moreover, SR-μCT of an articulated skull revealed a strikingly brain-shaped structure inside the endocranial cavity, which seems to be an exceptional case of soft-tissue mineralization of the brain, presumably as a result of microbially induced postmortem phosphatization. This was imaged with exceptional accuracy by using holotomography, which demonstrates its great potential to image preserved soft parts in dense fossils.
Keywords: Carboniferous, chondrichthyans, vertebrate, X-ray phase imaging
Iniopterygians have been described on the basis of partly articulated specimens preserved as impressions in the 310-Myr-old Carboniferous (Late Pennsylvanian) black shale of the northern U.S. (1). These fossils show 2 main chondrichthyan characteristics: a fragile layer of prismatic calcified cartilage lining the endoskeletal elements and pelvic claspers (special copulation organs). Iniopterygians display a very odd morphology, such as dorsolaterally inserted pectoral fins and complex tooth setting (1–4) (Fig. 1A). All iniopterygians described until now were flattened specimens that do not allow 3D reconstructions, although studies based on stereographic radiographs have suggested the presence of some chimaeroid-like characteristics (1–4), but the detailed anatomy of the group has remained largely unknown. However, among the numerous 300 Myr-old fish-bearing concretions from the Pennsylvanian sites of Oklahoma and Kansas (see Materials and Methods), most of which yield braincases of palaeonisciforms (ray-finned fishes) (5), some have turned out to contain chondrichthyan remains. Some 3D preserved skulls and associated postcranial elements have been attributed to iniopterygians, as evidenced by their jaw shape, characteristic tooth whorls, and star- or horn-shaped scutes covering their head (Fig. 1 B–E). Mechanical preparation coupled with conventional X-ray microtomography (μCT) [see supporting information (SI) Text], absorption-based synchrotron microtomography (SR-μCT) and SR holotomography (6–9) (see Materials and Methods) of the best-preserved specimens now provides complete reconstructions of the skull of these long-enigmatic chondrichthyans and supports previous insights that, despite their very peculiar characters, they are close relative of living chimaeroids (Figs. 1 and 2; and see Movie S1 and Movie S2).
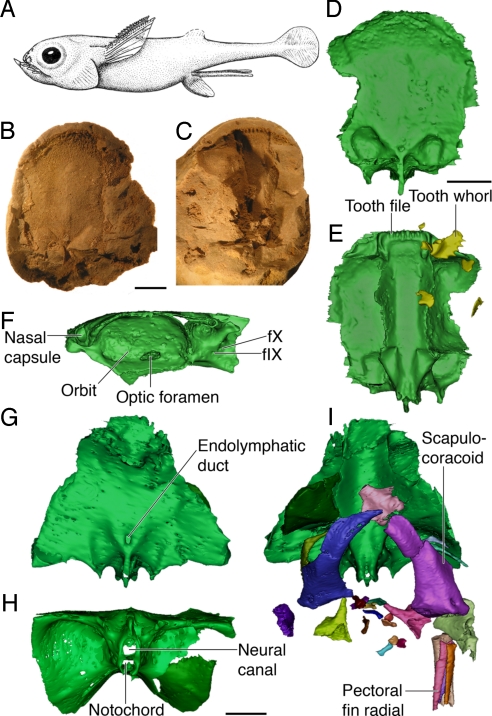
Fig. 1.
The anatomy of iniopterygians. (A) Reconstruction of Sibyrhynchus denisoni (based on ref. 5, not to scale). (B and C) Part (B) and counterpart (C) of a phosphatic nodule from the Pennsylvanian of Oklahoma (AMNH OKM38) containing the braincase and shoulder girdle of Sibyrhynchus sp. (D–F) Three-dimensional reconstruction of the same specimen, obtained from conventional X-ray μCT images, showing the braincase in dorsal (D), ventral (E), and lateral (F) view, with associated teeth. (G–I) Three-dimensional reconstruction of the braincase, shoulder girdle, and pectoral fin elements of a sibyrhynchid iniopterygian from the Pennsylvanian of Kansas (KUNHM 21894), based on SR-μCT images. Braincase in dorsal (G), posterior (H), and ventral views, with articulated shoulder girdles and pectoral fin radials (I). (Scale bar, 5 mm; f.IX and f.X, foramina for glossopharyngeus and vagus nerves).
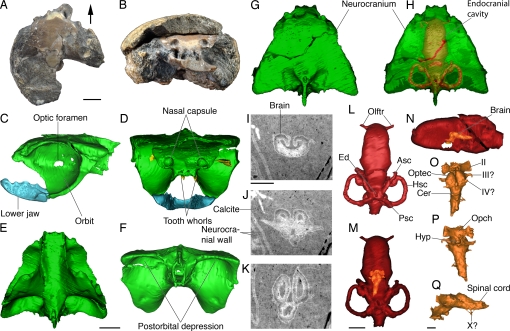
Fig. 2.
Braincase anatomy and exceptional brain preservation in a sibyrhynchid iniopterygian from the Pennsylvanian of Kansas. (A and B) Articulated skull preserved in a nodule (KUNHM 22060) (see also Fig. S1) in dorsal (A) and anterior (B) view (arrow points forward). (C–Q), Three-dimensional reconstructions and putative preserved brain structures of the same specimen, obtained from SR-μCT images (and holotomography for brain details). (C–H), Braincase, teeth, and lower jaw in lateral (C), anterior (D), ventral (E), posterior (F), and dorsal (G) view, showing by transparency the outline of the endocranial cavity and labyrinth (H). (I–K), Selected transverse (I and J), and horizontal (K) SR-μCT (holotomography) slices through the calcite-filled endocranial cavity, showing the probably phosphatized brain at the level of the rhombencephalon (I), hypophysis (J), and roof of the optic tectum and cerebellum (K). (L–N) Reconstruction of the endocranial cavity and otic capsule in dorsal (L and M) and lateral (N) view, showing the putative brain by transparency (M and N). (O–Q), reconstruction of the putative phosphatized brain in dorsal (O), ventral (P), and lateral (Q) view. (Scale bar, 5 mm for A–N and 1 mm for I—K and O–Q. Asc, anterior semicircular canal; Cer, cerebellum; Ed, endolymphatic duct; Hsc, horizontal semicircular canal; Hyp, hypophysis; Olftr, canals for olfactory tracts; Opch, optic chiasm; Optec, optic tectum; Psc, posterior semicircular canal; II, optic nerve; III?, oculomotorius nerve?; IV?, trochlear nerve?; X?, roots of vagus nerve?).
Results
The iniopterygian skulls from Oklahoma and Kansas are very similar to that of Sibyrhynchus denisoni (1), from the Indiana black shale, in the shape of the jaw and scutes, and in the outline of the braincase in dorsal view (Figs. 1 A and E and 2 C and D). They have very large orbits, bordered posteriorly and ventrally by an expanded postorbital wall and a suborbital shelf, but the braincase is significantly deeper than previously supposed (1–3). Anterior to the orbits is a pair of small, cup-shaped nasal capsules connected to the endocranial cavity by narrow canals for the olfactory tracts (Figs. 1F and 2 H and L). In front of the olfactory capsules, the braincase is prolonged by a rectangular cartilage plate bearing a transverse series of ridges that supported tooth families or tooth whorls (Figs. 1E and 2D). Large serrated teeth are also attached directly to the braincase floor, medially to the suborbital shelf (Fig. 2D). Posterior to the postorbital wall, the ventral part of the braincase is surprisingly narrow (Figs. 1H and 2F). It shows a deep median ridge containing canals for the spinal cord and the notochord and is flanked by very deep postorbital depressions that accommodated either jaw musculature or gills (Fig. 2F). Dorsal to these depressions, the otic capsules are extremely shallow and have small utricular cavities. The vertical and horizontal semicircular canals are almost in the same plane (Fig. 2 L and N), thereby recalling the condition otherwise found only in the strongly depressed braincase of certain placoderms (armored stem gnathostomes) (10, 11). The endocranial cavity is straight and relatively narrow but extends all along the braincase floor (Fig. 2H). The skull is thus platybasic, as in modern chimaeroids (12, 13), despite the very large size of the orbits. Some of the canals for cranial nerves can be identified (Figs. 1F and 2C), notably for the glossopharyngeus and vagus nerves. These exit from the braincase in much the same way as in modern chimaeroids; that is, there is no underlying hypotic lamina, in contrast to elasmobranchs (12–14).
The lower jaw is massive, duck bill-shaped, with a fused symphysis that bears the same ridges (and presumably the same kind of tooth families) as the anterior plate of the braincase. It articulates with the braincase at the level of the posteroventral corner of the orbital margin (Fig. 2 C and D), and there is thus no evidence of an independent palatoquadrate, as in extant adult chimaeroids (15). Behind the postorbital wall of the braincase are a number of elements belonging to the gill skeleton, the shoulder girdle, and pectoral fin (Fig. 1I).
In one of the articulated iniopterygian skulls from Kansas, preserved in an unweathered concretion, absorption SR-μCT revealed inside the endocranial cavity a peculiar structure that is denser than the surrounding infill of crystalline calcite (Fig. 2 I–K). We used a holotomographic approach adapted to absorbing objects (9) to resolve this structure in detail. It turned out to be a paired, symmetrical and elongated object that projects toward the optic foramina and the more posterior foramina, probably for the oculomotorius nerve (Fig. 2 J, M, and N). It also shows 2 hollow dorsal lobes and a large median ventral swelling (Fig. 2 J, P, Q), and it is continued posteriorly by an axial prolongation that fades away just before reaching a break through the specimen. The 3D reconstruction of this object is strikingly suggestive of part of an actual fish brain, showing the optic tectum of the midbrain, the cerebellum, hypophysial region, medulla oblongata, spinal cord, optic tracts, and the oculomotorius nerve (Fig. 2 O–Q; see SI Text and Movie S1 and Movie S2). Yet there seems to be no anterior continuation suggestive of a forebrain, apart from a vague anterior blade-shaped prolongation on one side only. However, unlikely as it may seem, the resemblance of this entirely mineral structure to a primitive gnathostome brain is remarkable. Because the specimen is unique, and this brain-like structure remains largely inaccessible, we have only a few hints about its nature. Microprobe analyses in areas where the brain-like structure reaches the surface of the specimen were performed and revealed a high concentration of calcium phosphate, whereas the surrounding calcite is almost pure calcium carbonate (see SI Text). Its shape, symmetry, and relations to the nerve foramina strongly suggest that it is an exceptionally preserved trace of the actual brain rather than a fortuitous artifact. This peculiar case of mineralization may be explained by the fact that the brain underwent microbially induced phosphatization shortly before decay (16, 17). This could have been favored by locally anoxic conditions inside the braincase and an environment probably saturated with calcium phosphate (hence the concretions). Such anoxic conditions, along with an increase of CO2 and the presence of volatile fatty acids in the brain, may have generated a fall in pH that could have shifted the equilibrium of precipitation in favor of calcium phosphate, rather than calcium carbonate (16–19). Then the phosphatized brain, in absence of bioturbation, could have been rapidly surrounded by diagenetic calcite, which preserved it in its almost natural position. However, holotomographic slices of this brain-like structure clearly show a fabric of thin radiating crystals that indicate recrystallization of the calcium phosphate (Fig. 2 I–K), leaving no hope of finding any structure at the histological or cellular level. Assuming that this object is a mineral replica of the brain and some cranial nerves, it shows an important size discrepancy relative to the endocranial cavity. The question of the size and proportions of the brain, relative to the endocranial cavity has been much debated by early vertebrate paleontologists, but anatomical studies of extant vertebrates show that the brain generally fills the endocranial cavity and that size discrepancy between the brain and endocranial cavity invoked for some taxa is in fact a consequence of inadequate preservation techniques (10, 20, 21). The size discrepancy observed in Sibyrhynchus could be explained by shrinking of the brain tissues that might have occurred just before phosphatization, hence the position of the cerebellum far anterior to the otic capsule (18). Yet the optic tract reaches its foramen in a normal position, as do the other putative cranial nerves, thereby suggesting that the shrinking of the brain was minor.
Indications of the shape of the actual brain was hitherto unrecorded in Palaeozoic vertebrates, apart from an ambiguous case in a Carboniferous actinopterygian fish (22). Moreover, possible phosphatized nerve fibers have been recorded in a Devonian placoderm (19). Although our discovery may seem anecdotal (a chondrichthyan, be it Carboniferous in age, must have possessed a brain), it suggests that actual gross neuroanatomical characters are potentially available under particular taphonomic conditions, thanks to new microtomographic techniques, and could throw some light on brain evolution during major evolutionary transitions. At any rate, and considering that iniopterygians share skeletal synapomorphies with chimaeroids, the position of their midbrain relative to the olfactory capsules suggests the presence of a very elongate telencephalon medium, as in chimaeroids, despite the lack of an interorbital septum (12, 23–26).
Discussion
Iniopterygians were first classified among the Subterbranchialia, corresponding to all chondrichthyans (including living chimaeroids), whose gill arches are situated beneath the braincase instead of extending behind it, as in sharks (2). However, this condition is also observed in osteichthyans and placoderms and is thus likely to be a primitive condition for jawed vertebrates (27). Therefore, this character alone cannot support the chimaeroid affinity of iniopterygians. In current chondrichthyan phylogenies including fossils, iniopterygians are either overlooked or turn up in unresolved positions, although generally as stem chimaeroids (3, 4, 30).
The earliest undisputed chimaeroids are early Triassic (250 Myr) in age (3), but in addition to iniopterygians a number of Palaeozoic taxa, notably the “bradyodonts” and echinochimaerids are also considered as stem chimaeroids because they share with the latter at least some derived characters (e.g., tubular dentine, prepelvic claspers) (3, 4, 28, 29). By contrast, previously described iniopterygian material did not show such characters. The material described here now demonstrates that, despite numerous specializations, iniopterygian skull anatomy is basically chimaeroid-like. Beside holostylic jaw suspension, sibyrhynchid iniopterygians display several characters that were known only in chimaeroid, namely the lack of foramina for internal carotid arteries (aborted carotids), which is compensated by a blood supply to the brain via the efferent pseudobranchial arteries that enter the suborbital shelf and reach the brain through the orbit (12, 13, 30–31). Iniopterygians, like chimaeroids, also lack a precerebral fontanelle, lagenar chamber, hyomandibular articulation, and their hyomandibular and palatine rami of the facial nerve pass through jugular and orbital canals, respectively (4, 15, 29).
Apart from the poorly preserved Carboniferous “bradyodont” Helodus simplex (28), no 3-dimensionally preserved skull of any fossil chimaeroid (or a supposedly chimaeroid-related taxon) was known to date, and all data used for reconstructing basal chimaeroid relationships were inferred from more or less flattened specimens or from tooth histology (3, 4, 28, 29). In contrast, some early elasmobranch and possible stem chondrichthyan skulls are now known in detail, notably thanks to CT-based studies (13, 21, 31). However, we are a long way to a robust phylogeny of chondrichthyan, extant and fossil. Detailed 3D anatomical information about other Paleozoic presumed chimaeroid relatives, such as eugeneodontids, petalodontids, and the diverse “bradyodont” clades (2), are badly needed. The iniopterygian skulls described here now provide means for a comparative study of skull anatomy in Palaeozoic representatives of the main 2 chondrichthyan clades, elasmobranchs and chimaeroids, and hints at an early appearance of chimaeroid specializations, possibly as early as the Devonian.
The possible indication of a fossilized vertebrate brain revealed by holotomography in an iniopterygian allows a tentative paleoneuroanatomical study of a fossil vertebrate based on the actual brain and not merely the endocranial cavity. It also points to similar findings in other vertebrates preserved under comparable conditions. This application of holotomography confirms the rapidly growing possibilities of X-ray synchrotron phase imaging techniques in palaeontology (6, 32–34), especially when dealing with the exceptional soft-tissue preservations. It imposes synchrotron radiation as a powerful tool for nondestructive imaging of fossils.
Materials and Methods
Origin of the Material.
The material described comes from the Upper Carboniferous (Pennsylvanian) of Kansas and Oklahoma.
Material from Kansas.
The Pennsylvanian fish-bearing concretions from Kansas and the palaeoniscoid braincases they contain have been known since the early twentieth century and have been extensively studied (5). They occur at the limit between the Haskell Limestone Member and the overlying Robbins Shale of the Stranger Formation (dated as Late Virgilian; 305–299 Myr) and crop out between the towns of Lawrence and Baldwin, KS. The specimens belong to the collection of the University of Kansas Natural History Museum, Lawrence (KUNHM 22060 and 21894).
Material from Oklahoma.
The specimen from Oklahoma (OKM38) was collected by Royal Mapes (Geology Department, Ohio University, Athens, OH) from the Tackett Shale, Coffeyville Formation (dated as Pennsylvanian, Missourian, ca. 307 Myr), at a roadcut in Tulsa County, OK (Center of NW sec. 2., T. 18 N, R. 12 E, Sapulpa North 7 1/2′ Quadrangle). Numerous paleoniscoid braincases were also recovered from small nodules at this site.
Methods of SR-μCT and Holotomography.
Samples were imaged primarily by using absorption-based X-ray synchrotron SR-μCT on beamline ID19 of the European Synchrotron Radiation Facility (ESRF). This technique has previously been demonstrated to be a powerful tool for nondestructive imaging of large fossils when conventional X-ray microtomography does not give good enough data quality (6). A monochromatic X-ray beam of 60-keV energy was used. The detector was a FReLoN (fast readout low noise) (35) CCD camera coupled with an optical magnification system, yielding an isotropic pixel size of 30.3 μm. For each tomography, covering a height of 5.6 mm, we used 1,200 projections on 180° with 0.4 s of exposure time. Several scans were performed after vertical displacement of the sample to image the whole specimens. Data were reconstructed by using the filtered back-projection algorithm (PyHST software, ESRF). Reconstructed slices were converted from 32 bits to 8 bits to reduce the data size for 3D processing. Successive scans of each sample were then set together by removing the common slices.
Observation of the original absorption scan of the KUNHM 22060 sample revealed a structure that may correspond to a fossilized brain, but the absorption contrast was not good enough to allow satisfactory segmentation of the structure. Another experiment was performed to resolve the structure with more details and better contrast. To increase the contrast, we decided to use quantitative phase tomography, also called holotomography (7, 8). The technique was originally designed to image pure phase objects (9) but was successfully used to image small fossil samples embedded in a mineral matrix (6). It is based on the acquisition of several propagation phase-contrast (36) scans that are then combined to retrieve a phase map of the sample for each angle of the tomographic acquisition. In the case of strongly absorbing objects, this phase retrieval process fails to bring accurate results because of the strong absorption contrast superimposed on the phase contrast and to the too-strong phase shift between the sample and the air that creates false low frequencies in the phase retrieval.
Here, an approach (9) for strongly absorbing samples is further refined to obtain a robust reconstruction. It involves a first scan in absorption mode, which is available in the contact plane of the sample and the detector. Like all differential phase-contrast methods, this technique is sensitive to noise in the low spatial frequency range if the propagation distance is relatively small and the X-ray energy is relatively high. This phenomenon was alleviated by introducing the assumption that the imaged object is roughly homogeneous. If the chemical composition of the sample is roughly known, an estimate of the ratio between absorption and phase shift can be calculated. This combined with the absorption scan is then used to regularize the low-frequency content of the phase shift. This allows robust and accurate phase reconstruction on complex, absorbing, and roughly homogeneous samples such as fossils.
For the holotomography, we used a monochromatic beam at an energy of 60 keV and a detector giving an isotropic pixel size of 14.92 μm. A tomographic scan with 1,500 projections, each with an exposure time of 0.3 s, was taken >180° for each of the 3 propagation distances. Two holotomographic acquisitions were necessary to cover the whole structure. The propagation distances were 50 mm (absorption), 400 mm, and 950 mm, respectively. After phase retrieval, the slices were reconstructed by using the filtered back-projection algorithm, then converted into 8 bits. Finally, the 2 holotomographic scans were combined to 1 volume where the common slices were removed. This volume constitutes a quantitative map of the electron density, hence approximately the mass density, through the sample. Because of the high contrast and good resolution provided by the holotomographic approach, it was possible to segment the putative brain in 3D with a good accuracy. The algorithmic approach opens possibilities for high-quality imaging of dense and complex fossils and can yield impressive results in cases of absorbing, roughly homogeneous samples with small internal variations, such as soft body parts preservation.
Despite a longer acquisition time than single distance phase retrieval protocols (32, 37–40), accuracy and sensitivity of this approach to image fossils are clearly higher. Because it does not require the object to be homogeneous and weakly absorbing, it can be applied on a much broader range of samples.
Supplementary Material
Acknowledgments.
We thank L. Martin (University of Kansas, Natural History Museum, Lawrence, KS) for the loan of the Kansas specimens and the permission to include them in the present article and R. Mapes (Ohio University, Athens, OH) for donating the Oklahoma specimen to the American Museum of Natural History, New York.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807047106/DCSupplemental.
References
- 1.Zangerl R, Case G. Iniopterygia a new order of chondrichthyan fishes from Pennsylvanian of North America. Fieldiana Geol Memoir. 1973;6:1–66. [Google Scholar]
- 2.Zangerl R. In: Handbook of Paleoichthyology. Schultze H-P, editor. Vol 3. Stuttgart: Gustav Fischer; 1981. pp. 1–138. [Google Scholar]
- 3.Stahl BJ. In: Handbook of Paleoichthyology. Schultze H-P, editor. Vol 4. Munich: Pfeil; 1999. pp. 1–163. [Google Scholar]
- 4.Grogan ED, Lund R. In: The Biology of Sharks and their Relatives. Carrier J, Musick JA, Heithaus M, editors. Boca Raton, FL: CRC; 2004. pp. 3–31. [Google Scholar]
- 5.Poplin C. Study of Some Paleoniscids from the Pennsylvanian of Kansas (Translated from French) Paris: Cahiers de Paleontologie, Editions du Centre National de la Recherche Scientifique; 1974. [Google Scholar]
- 6.Tafforeau P, et al. Applications of X-ray synchrotron microtomography for non-destructive 3D studies of paleontological specimens. Appl Phys A. 2006;83:195–202. [Google Scholar]
- 7.Cloetens P, et al. Holotomography: Quantitative phase tomography with micrometer resolution using hard synchrotron radiation X-rays. Appl Phys Lett. 1999;75:2912–2914. [Google Scholar]
- 8.Cloetens P, Mache R, Schlenker M, Lerbs-Mache S. Quantitative phase tomography of Arabidopsis seeds reveals intercellular network. Proc Natl Acad Sci USA. 2006;103:14626–14630. doi: 10.1073/pnas.0603490103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guigay P, Langer M, Boistel R, Cloetens P. Mixed transfer function and transport of intensity approach for phase retrieval in the Fresnel region. Opt Lett. 2007;32:1617–1619. doi: 10.1364/ol.32.001617. [DOI] [PubMed] [Google Scholar]
- 10.Stensiö E. In: Treatise of Paleontology (Translated from French) Piveteau J, editor. Vol 2. Paris: Masson; 1969. pp. 71–692. T 4. [Google Scholar]
- 11.Goujet D. The Placoderm Fishes from Spitsbergen (Translated from French) Paris: Cahiers de Paleontologie, Editions du Centre National de la Recherche Scientifique; 1974. [Google Scholar]
- 12.Holmgren N. Studies on the head in fishes. Part III. The phylogeny of elasmobranch fishes. Acta Zool. 1942;23:129–261. [Google Scholar]
- 13.Maisey JG. The braincase in Paleozoic symmoriiform and cladoselachian sharks. Bull Am Mus Nat Hist. 2007;541:1–122. [Google Scholar]
- 14.De Beer G. The Development of the Vertebrate Skull. Oxford: Clarendon; 1937. [Google Scholar]
- 15.Grogan ED, Lund R, Didier D. Description of the chimaerid jaw and its phylogenetic origin. J Morphol. 1999;239:45–59. doi: 10.1002/(SICI)1097-4687(199901)239:1<45::AID-JMOR3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 16.Briggs DEG. The role of decay and mineralization in the preservation of soft-bodied fossils. Ann Rev Earth Planet Sci. 2003;31:275–301. [Google Scholar]
- 17.Raff EC, et al. Embryo fossilization is a biological process mediated by microbial bioflims. Proc Natl Acad Sci USA. 2008;49:19360–19365. doi: 10.1073/pnas.0810106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briggs DEG, Kear AJ. Fossilization of soft tissue in the laboratory. Science. 1993;259:1439–1442. doi: 10.1126/science.259.5100.1439. [DOI] [PubMed] [Google Scholar]
- 19.Trinajstic K, Marshall C, Long J, Bifield K. Exceptional preservation of nerve and muscle tissues in Late Devonian placoderm fish and their evolutionary implications. Biol Lett. 2007;3:197–200. doi: 10.1098/rsbl.2006.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Northcutt RG. Brain variation and phylogenetic trends in elasmobranch fishes. J Exp Zool Suppl 1989. 1989;2:83–100. doi: 10.1002/jez.1402520410. [DOI] [PubMed] [Google Scholar]
- 21.Maisey JG. In: Recent Advances in the Origin and Early Radiation of Vertebrates. Arratia G, Wilson MVH, Cloutier R, editors. Munich: Verlag Dr. Friedrich Pfeil; 2004. pp. 439–454. [Google Scholar]
- 22.Coates MI. Endocranial preservation of a Carboniferous actinopterygian from Lancashire, U.K., and the interrelationship of primitive actinopterygians. Phil Trans R Soc London Ser B. 1999;354:435–462. [Google Scholar]
- 23.Yopak KE, Montgomery JC. Brain organization and specialization in deep-sea chondrichthyans. Brain Behav Evol. 2008;71:287–304. doi: 10.1159/000127048. [DOI] [PubMed] [Google Scholar]
- 24.Yopak KE, Lisney TJ, Collin SP, Montgomery JC. Variation in brain organization and cerebellar foliation in chondrichthyans: Sharks and holocephalans. Brain Behav Evol. 2007;69:280–300. doi: 10.1159/000100037. [DOI] [PubMed] [Google Scholar]
- 25.Kuhlenbeck H, Niimi K. Further observations on the morphology of the brain in the holocephalan elasmobranch Chimaera and. Callorhynchus. J Hirnfosch. 1969;11:267–314. [PubMed] [Google Scholar]
- 26.Northcutt RG. In: Sensory Biology of Sharks, Skates and Rays. Hodgson ES, Mathewson RF, editors. Arlington, VA: Office of Naval Research, Department of the Navy; 1978. pp. 117–193. [Google Scholar]
- 27.Janvier P. Early Vertebrates. Oxford: Oxford Univ Press; 1996. [Google Scholar]
- 28.Patterson C. The phylogeny of the chimaeroids. Phil Trans R Soc London Ser B. 1965;249:101–219. [Google Scholar]
- 29.Maisey JG. Chondrichthyan phylogeny: A look at the evidence. J Vert Paleontol. 1984;4:359–371. [Google Scholar]
- 30.Maisey JG. In: Major Events in Early Vertebrate Evolution. Ahlberg PE, editor. London: Taylor and Francis; 2001. pp. 263–288. [Google Scholar]
- 31.Maisey JG. Braincase of the Upper Devonian shark Cladodoides wildungensis (Chondrichthyes, Elasmobranchii), with observations on the braincase in early chondrichthyans. Bull Am Mus Nat Hist. 2005;228:1–103. [Google Scholar]
- 32.Friis EM, et al. Phase-contrast X-ray microtomography links Cretaceous seeds with Gnetales and Bennettitales. Nature. 2007;450:549–552. doi: 10.1038/nature06278. [DOI] [PubMed] [Google Scholar]
- 33.Feist M, Liu J, Tafforeau P. New insights into Paleozoic charophyte morphology and phylogeny. Am J Bot. 2005;92:1152–1160. doi: 10.3732/ajb.92.7.1152. [DOI] [PubMed] [Google Scholar]
- 34.Smith TM, et al. Earliest evidence of modern human life history in North African early Homo sapiens. Proc Natl Acad Sci USA. 2007;104:6128–6133. doi: 10.1073/pnas.0700747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labiche J-C, et al. The fast readout low noise camera as a versatile X-ray detector for time resolved dispersive extended X-ray absorption fine structure and diffraction studies of dynamic problems in materials science, chemistry, and catalysis. Rev Sci Instrum. 2007;78 doi: 10.1063/1.2783112. 091301. [DOI] [PubMed] [Google Scholar]
- 36.Snigirev A, Snigireva I, Kohn V, Kuznetsov S, Schelokov I. On the possibilities of X-ray phase contrast microimaging by coherent high-energy synchrotron radiation. Rev Sci Instrum. 1995;66:5486. [Google Scholar]
- 37.Paganin D, Mayo SC, Gureyev TE, Miller PR, Wilkins SW. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J Microsc. 2002;206:33–40. doi: 10.1046/j.1365-2818.2002.01010.x. [DOI] [PubMed] [Google Scholar]
- 38.Zabler S, Cloetens P, Guigay J-P, Baruchel J, Schlenker M. Optimization of phase contrast imaging using hard X-rays. Rev Sci Instrum. 2005;76 073705. [Google Scholar]
- 39.Gureyev TE, Paganin DM, Myers GR, Nesterets YI, Wilkins SW. Phase-and-amplitude computer tomography. Appl Phys Lett. 2006;89 034102–1-034102–3. [Google Scholar]
- 40.Tafforeau P, Bentaleb I, Jaeger J-J, Martin C. Nature of laminations and mineralization in rhinoceros enamel using histology and X-ray synchrotron microtomography: Potential implications for palaeoenvironmental isotopic studies. Palaeogeogr Palaeoclimat Palaeoecol. 2007;246:206–227. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.