Abstract
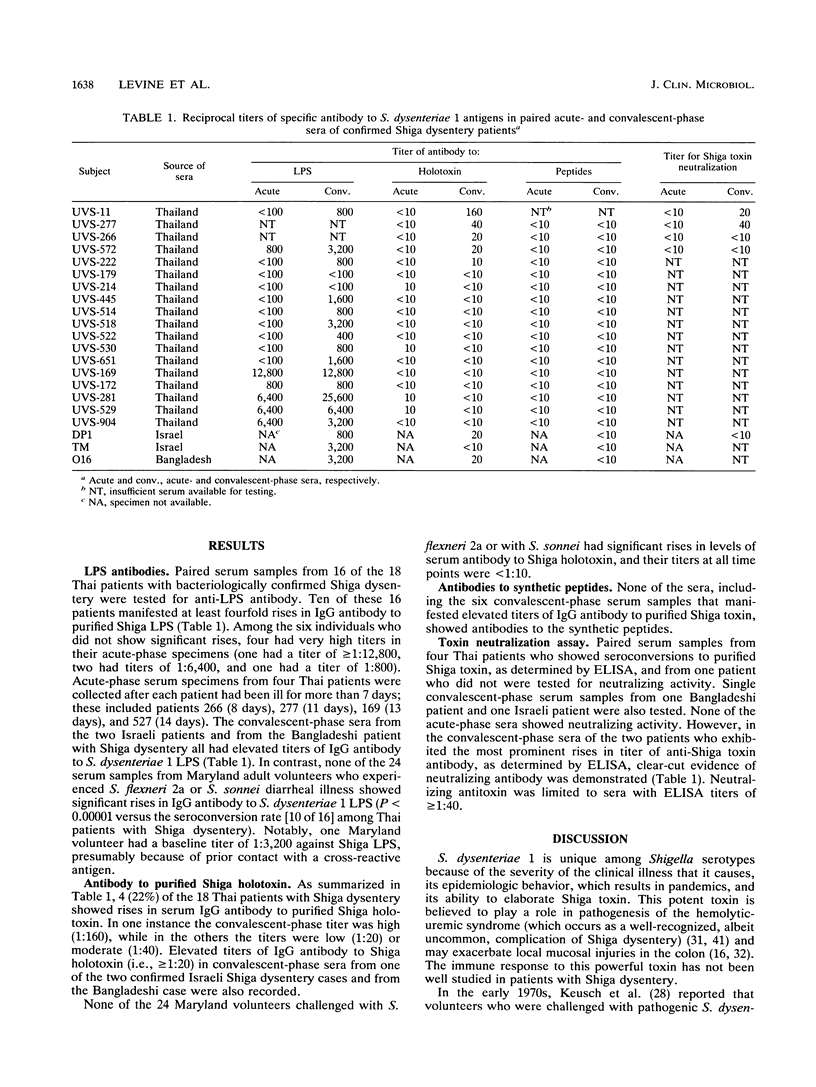
Acute- and convalescent-phase sera from 18 Thai patients and convalescent-phase sera from two Israeli patients and one Bangladeshi patient with Shigella dysenteriae 1 (Shiga) dysentery were tested by enzyme-linked immunosorbent assay to detect antibodies that bind S. dysenteriae lipopolysaccharide (LPS), Shiga holotoxin, or two synthetic peptides representing epitopes from the B subunit of Shiga toxin. Paired sera from 24 Maryland adults with Shigella flexneri 2a or Shigella sonnei diarrhea served as negative controls. Of the 16 paired Thai serum samples tested for immunoglobulin G LPS antibody, 10 had greater than or equal to 4-fold rises (the two subjects with the highest convalescent-phase titers exhibited toxin-neutralizing activity); acute-phase specimens from four of the remaining six individuals already had elevated Shiga LPS titers in their acute specimens ranging from 1:800 to 1:12,800. Similarly, convalescent-phase sera from the two Israeli patients and the Bangladeshi patient revealed LPS titers of 1:800 to 1:3,200. In contrast, none of the Maryland volunteers with S. flexneri or S. sonnei diarrhea manifested rises in Shiga anti-LPS (P less than 0.00001 versus 10 of 16 Thai patients). Only 4 of the 18 Thai patients had significant rise in antibody to purified Shiga toxin, while one of the two Israeli patients and the one Bangladeshi patient had elevated convalescent-phase titers. None of the sera that reacted with Shiga holotoxin had antibody that bound to the peptides. This report, which describes a search for serum antibodies that bind Shiga toxin in patients with Shiga dysentery, demonstrates such antibodies in only a minority of patients with bacteriologically confirmed disease. During Shiga dysentery, Shiga toxin may be elaborated in such small quantities in vivo that it fails to elicit an immune response in most patients even though it may exert biological effects. In this behavior Shiga toxin resembles tetanus toxin, another potent exotoxin that fails to elicit antitoxic responses in people who recover from clinical tetanus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya I. L., Lowe C. U., Thapa R., Gurubacharya V. L., Shrestha M. B., Cadoz M., Schulz D., Armand J., Bryla D. A., Trollfors B. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi. A preliminary report. N Engl J Med. 1987 Oct 29;317(18):1101–1104. doi: 10.1056/NEJM198710293171801. [DOI] [PubMed] [Google Scholar]
- Ashkenazi S., Cleary K. R., Pickering L. K., Murray B. E., Cleary T. G. The association of Shiga toxin and other cytotoxins with the neurologic manifestations of shigellosis. J Infect Dis. 1990 May;161(5):961–965. doi: 10.1093/infdis/161.5.961. [DOI] [PubMed] [Google Scholar]
- Bartlett A. V., 3rd, Prado D., Cleary T. G., Pickering L. K. Production of Shiga toxin and other cytotoxins by serogroups of Shigella. J Infect Dis. 1986 Dec;154(6):996–1002. doi: 10.1093/infdis/154.6.996. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Rothman S. W., Doctor B. P. Inhibition of protein synthesis in intact HeLa cells by Shigella dysenteriae 1 toxin. Infect Immun. 1980 Jul;29(1):98–107. doi: 10.1128/iai.29.1.98-107.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chart H., Scotland S. M., Rowe B. Serum antibodies to Escherichia coli serotype O157:H7 in patients with hemolytic uremic syndrome. J Clin Microbiol. 1989 Feb;27(2):285–290. doi: 10.1128/jcm.27.2.285-290.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Madrid-Marina V., Estrov Z., Freedman M. H., Lingwood C. A., Dosch H. M. Expression of glycolipid receptors to Shiga-like toxin on human B lymphocytes: a mechanism for the failure of long-lived antibody response to dysenteric disease. Int Immunol. 1990;2(1):1–8. doi: 10.1093/intimm/2.1.1. [DOI] [PubMed] [Google Scholar]
- Cáceres A., Mata L. J. Serologic response of patients with shiga dysentery. J Infect Dis. 1974 Apr;129(4):439–443. doi: 10.1093/infdis/129.4.439. [DOI] [PubMed] [Google Scholar]
- Donohue-Rolfe A., Acheson D. W., Kane A. V., Keusch G. T. Purification of Shiga toxin and Shiga-like toxins I and II by receptor analog affinity chromatography with immobilized P1 glycoprotein and production of cross-reactive monoclonal antibodies. Infect Immun. 1989 Dec;57(12):3888–3893. doi: 10.1128/iai.57.12.3888-3893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont H. L., Hornick R. B., Dawkins A. T., Snyder M. J., Formal S. B. The response of man to virulent Shigella flexneri 2a. J Infect Dis. 1969 Mar;119(3):296–299. doi: 10.1093/infdis/119.3.296. [DOI] [PubMed] [Google Scholar]
- Echeverria P., Hanchalay S., Taylor D. N. Serological response to plasmid-encoded antigens in children and adults with shigellosis. Diagn Microbiol Infect Dis. 1988 Jun;10(2):75–80. doi: 10.1016/0732-8893(88)90043-0. [DOI] [PubMed] [Google Scholar]
- Eiklid K., Olsnes S. Animal toxicity of Shigella dysenteriae cytotoxin: evidence that the neurotoxic, enterotoxic, and cytotoxic activities are due to one toxin. J Immunol. 1983 Jan;130(1):380–384. [PubMed] [Google Scholar]
- Eldridge J. H., Gilley R. M., Staas J. K., Moldoveanu Z., Meulbroek J. A., Tice T. R. Biodegradable microspheres: vaccine delivery system for oral immunization. Curr Top Microbiol Immunol. 1989;146:59–66. doi: 10.1007/978-3-642-74529-4_6. [DOI] [PubMed] [Google Scholar]
- Fontaine A., Arondel J., Sansonetti P. J. Role of Shiga toxin in the pathogenesis of bacillary dysentery, studied by using a Tox- mutant of Shigella dysenteriae 1. Infect Immun. 1988 Dec;56(12):3099–3109. doi: 10.1128/iai.56.12.3099-3109.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Baron L. S., Kopecko D. J., Washington O., Powell C., Life C. A. Construction of a potential bivalent vaccine strain: introduction of Shigella sonnei form I antigen genes into the galE Salmonella typhi Ty21a typhoid vaccine strain. Infect Immun. 1981 Dec;34(3):746–750. doi: 10.1128/iai.34.3.746-750.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry M. K., Dalrymple J. M. Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol. 1980 Sep;12(3):361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J. A priori delineation of a peptide which mimics a discontinuous antigenic determinant. Mol Immunol. 1986 Jul;23(7):709–715. doi: 10.1016/0161-5890(86)90081-7. [DOI] [PubMed] [Google Scholar]
- Gill D. M. Bacterial toxins: a table of lethal amounts. Microbiol Rev. 1982 Mar;46(1):86–94. doi: 10.1128/mr.46.1.86-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari I., Arnon R. Carboxy-terminal peptides from the B subunit of Shiga toxin induce a local and parenteral protective effect. Mol Immunol. 1990 Jul;27(7):613–621. doi: 10.1016/0161-5890(90)90003-i. [DOI] [PubMed] [Google Scholar]
- Harari I., Donohue-Rolfe A., Keusch G., Arnon R. Synthetic peptides of Shiga toxin B subunit induce antibodies which neutralize its biological activity. Infect Immun. 1988 Jun;56(6):1618–1624. doi: 10.1128/iai.56.6.1618-1624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington D. A., Van de Verg L., Formal S. B., Hale T. L., Tall B. D., Cryz S. J., Tramont E. C., Levine M. M. Studies in volunteers to evaluate candidate Shigella vaccines: further experience with a bivalent Salmonella typhi-Shigella sonnei vaccine and protection conferred by previous Shigella sonnei disease. Vaccine. 1990 Aug;8(4):353–357. doi: 10.1016/0264-410x(90)90094-3. [DOI] [PubMed] [Google Scholar]
- Jackson M. P. Structure-function analyses of Shiga toxin and the Shiga-like toxins. Microb Pathog. 1990 Apr;8(4):235–242. doi: 10.1016/0882-4010(90)90050-z. [DOI] [PubMed] [Google Scholar]
- Karmali M. A., Petric M., Lim C., Fleming P. C., Arbus G. S., Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985 May;151(5):775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- Keusch G. T., Grady G. F., Mata L. J., McIver J. The pathogenesis of Shigella diarrhea. I. Enterotoxin production by Shigella dysenteriae I. J Clin Invest. 1972 May;51(5):1212–1218. doi: 10.1172/JCI106915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T., Jacewicz M., Levine M. M., Hornick R. B., Kochwa S. Pathogenesis of shigella diarrhea. Serum anticytotoxin antibody response produced by toxigenic and nontoxigenic Shigella dysenteriae 1. J Clin Invest. 1976 Jan;57(1):194–202. doi: 10.1172/JCI108259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T., Jacewicz M., Mobassaleh M., Donohue-Rolfe A. Shiga toxin: intestinal cell receptors and pathophysiology of enterotoxic effects. Rev Infect Dis. 1991 Mar-Apr;13 (Suppl 4):S304–S310. doi: 10.1093/clinids/13.supplement_4.s304. [DOI] [PubMed] [Google Scholar]
- Keusch G. T., Jacewicz M. Serum enterotoxin-neutralizing antibody in human shigellosis. Nat New Biol. 1973 Jan 3;241(105):31–32. doi: 10.1038/newbio241031a0. [DOI] [PubMed] [Google Scholar]
- Keusch G. T., Jacewicz M. The pathogenesis of Shigella diarrhea. VI. Toxin and antitoxin in Shigella flexneri and Shigella sonnei infections in humans. J Infect Dis. 1977 Apr;135(4):552–556. doi: 10.1093/infdis/135.4.552. [DOI] [PubMed] [Google Scholar]
- Koster F., Levin J., Walker L., Tung K. S., Gilman R. H., Rahaman M. M., Majid M. A., Islam S., Williams R. C., Jr Hemolytic-uremic syndrome after shigellosis. Relation to endotoxemia and circulating immune complexes. N Engl J Med. 1978 Apr 27;298(17):927–933. doi: 10.1056/NEJM197804272981702. [DOI] [PubMed] [Google Scholar]
- Kotloff K. L., Herrington D. A., Hale T. L., Newland J. W., Van De Verg L., Cogan J. P., Snoy P. J., Sadoff J. C., Formal S. B., Levine M. M. Safety, immunogenicity, and efficacy in monkeys and humans of invasive Escherichia coli K-12 hybrid vaccine candidates expressing Shigella flexneri 2a somatic antigen. Infect Immun. 1992 Jun;60(6):2218–2224. doi: 10.1128/iai.60.6.2218-2224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., DuPont H. L., Formal S. B., Hornick R. B., Takeuchi A., Gangarosa E. J., Snyder M. J., Libonati J. P. Pathogenesis of Shigella dysenteriae 1 (Shiga) dysentery. J Infect Dis. 1973 Mar;127(3):261–270. doi: 10.1093/infdis/127.3.261. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Herrington D., Murphy J. R., Morris J. G., Losonsky G., Tall B., Lindberg A. A., Svenson S., Baqar S., Edwards M. F. Safety, infectivity, immunogenicity, and in vivo stability of two attenuated auxotrophic mutant strains of Salmonella typhi, 541Ty and 543Ty, as live oral vaccines in humans. J Clin Invest. 1987 Mar;79(3):888–902. doi: 10.1172/JCI112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Young C. R., Black R. E., Takeda Y., Finkelstein R. A. Enzyme-linked immunosorbent assay to measure antibodies to purified heat-labile enterotoxins from human and porcine strains of Escherichia coli and to cholera toxin: application in serodiagnosis and seroepidemiology. J Clin Microbiol. 1985 Feb;21(2):174–179. doi: 10.1128/jcm.21.2.174-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonsky G. A., Ferreccio C., Kotloff K. L., Kaintuck S., Robbins J. B., Levine M. M. Development and evaluation of an enzyme-linked immunosorbent assay for serum Vi antibodies for detection of chronic Salmonella typhi carriers. J Clin Microbiol. 1987 Dec;25(12):2266–2269. doi: 10.1128/jcm.25.12.2266-2269.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver J., Grady G. F., Formal S. B. Immunization with Shigella dysenteriae type 1: evaluation of antitoxic immunity in prevention of experimental disease in rhesus monkeys (Macaca mulatta). J Infect Dis. 1977 Sep;136(3):416–421. doi: 10.1093/infdis/136.3.416. [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., Holmes R. K. Shiga and Shiga-like toxins. Microbiol Rev. 1987 Jun;51(2):206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks E. V., Hale T. L., Formal S. B. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun. 1986 Jul;53(1):57–63. doi: 10.1128/iai.53.1.57-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado D., Cleary T. G., Pickering L. K., Ericsson C. D., Bartlett A. V., 3rd, DuPont H. L., Johnson P. C. The relation between production of cytotoxin and clinical features in shigellosis. J Infect Dis. 1986 Jul;154(1):149–155. doi: 10.1093/infdis/154.1.149. [DOI] [PubMed] [Google Scholar]
- Raghupathy P., Date A., Shastry J. C., Sudarsanam A., Jadhav M. Haemolytic-uraemic syndrome complicating shigella dystentery in south Indian children. Br Med J. 1978 Jun 10;1(6126):1518–1521. doi: 10.1136/bmj.1.6126.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland S. M., Smith H. R., Rowe B. Two distinct toxins active on Vero cells from Escherichia coli O157. Lancet. 1985 Oct 19;2(8460):885–886. doi: 10.1016/s0140-6736(85)90146-1. [DOI] [PubMed] [Google Scholar]
- Strockbine N. A., Marques L. R., Newland J. W., Smith H. W., Holmes R. K., O'Brien A. D. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect Immun. 1986 Jul;53(1):135–140. doi: 10.1128/iai.53.1.135-140.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. N., Bodhidatta L., Brown J. E., Echeverria P., Kunanusont C., Naigowit P., Hanchalay S., Chatkaeomorakot A., Lindberg A. A. Introduction and spread of multi-resistant Shigella dysenteriae I in Thailand. Am J Trop Med Hyg. 1989 Jan;40(1):77–85. doi: 10.4269/ajtmh.1989.40.77. [DOI] [PubMed] [Google Scholar]
- VICARI G., OLITZKI A. L., OLITZKI Z. The action of the thermolabile toxin of Shigella dysenteriae on cells cultivated in vitro. Br J Exp Pathol. 1960 Apr;41:179–189. [PMC free article] [PubMed] [Google Scholar]
- Verma N. K., Lindberg A. A. Construction of aromatic dependent Shigella flexneri 2a live vaccine candidate strains: deletion mutations in the aroA and the aroD genes. Vaccine. 1991 Jan;9(1):6–9. doi: 10.1016/0264-410x(91)90308-s. [DOI] [PubMed] [Google Scholar]



