Abstract
IL-17–producing CD4+ T helper (Th17) cells have recently been defined as a unique subset of proinflammatory helper cells whose development depends on signaling initiated by IL-6 and TGF-β, autocrine activity of IL-21, activation of STAT3, and induction of the orphan nuclear receptor RORγt. The maintenance, expansion, and further differentiation of the committed Th17 cells depend on IL-1β and IL-23. IL-17 was originally found produced by circulating human CD45RO+ memory T cells. A recent study found that human Th17 memory cells selectively express high levels of CCR6. In this study, we report that human peripheral blood and lymphoid tissue contain a significant number of CD4+FOXP3+ T cells that express CCR6 and have the capacity to produce IL-17 upon activation. These cells coexpress FOXP3 and RORγt transcription factors. The CD4+FOXP3+CCR6+ IL-17–producing cells strongly inhibit the proliferation of CD4+ responder T cells. CD4+CD25high-derived T-cell clones express FOXP3, RORγt, and IL-17 and maintain their suppressive function via a cell-cell contact mechanism. We further show that human CD4+FOXP3+CCR6− regulatory T (Treg) cells differentiate into IL-17 producer cells upon T-cell receptor stimulation in the presence of IL-1β, IL-2, IL-21, IL-23, and human serum. This, together with the finding that human thymus does not contain IL-17–producing Treg cells, suggests that the IL-17+FOXP3+ Treg cells are generated in the periphery. IL-17–producing Treg cells may play critical roles in antimicrobial defense, while controlling autoimmunity and inflammation.
Keywords: FOXP3, ROR gamma t, Th17, Treg, inflammation
IL-17 (also known as IL-17A) was identified in 1995 as a cytokine produced by activated human CD45RO+ memory T cells (1, 2). IL-17F, a closely related member with 50% amino acid sequence homology to IL-17A, was later discovered and is also expressed in activated peripheral blood (PB) CD4+ T cells (3). IL-17 (A and F) induces production of a broad range of proinflammatory cytokines and chemokines, including IL-6, colony-stimulating factors, CXC chemokines, human β-defensin-2 and metalloproteinases (4), by a variety of cells. IL-17 regulates host defense against infectious organisms through promoting granulopoiesis and neutrophil trafficking (5–7). In humans, elevated levels of IL-17 have been associated with inflammatory diseases, including rheumatoid arthritis, scleritis, uveitis, asthma, systemic lupus erythematosus, and allograft rejection (8–11). In mice, IL-17 contributes to the development of experimental autoimmune encephalomyelitis (12, 13), collagen-induced arthritis (14, 15), and colitis (16). IL-22, a product of IL-17–producing cells, on the other hand, induces acanthosis and psoriasis (17, 18).
The IL-17–producing CD4+ T helper cells (Th17) cells that produce both IL-17A and IL-17F are now defined as a separate subset (Th17) distinct from the Th1, Th2, and regulatory T (Treg) cells, in terms of developmental regulation and function. Th17 cell differentiation is induced by a combination of IL-6 and TGF-β and is augmented by induction of IL-21, which acts in an autocrine manner (19–22). Signaling induced by these cytokines results in phosphorylation of STAT3 and expression of the orphan nuclear receptor RORγt, transcription factors that are required for induction of IL-17 expression (19, 20, 22). The maintenance, expansion, and further differentiation of the committed Th17 cells depend on IL-1β and IL-23 (19, 23–25). The differentiation of naive T cells to Th17 cells can be inhibited by IFN-γ, IL-4, IL-27, IL-2, and retinoic acid, molecules critical for the differentiation of naive CD4+ T cells into Th1, Th2, and Treg cell pathways (13, 19, 26–29).
Although human IL-17–producing T cells were originally found enriched in the CD4+ CD45RA−CD45RO+ memory CD4+ T-cell population, it has been unclear whether they overlap with other Th cell subsets, such as Th1, Th2 memory T cells, or Treg T cells. Recently, human Th17 cells were defined as a subpopulation of circulating CD4+CD45RO+ memory T cells that expressed high levels of the chemokine (C-C motif) receptor 6 (CCR6) (30). Here, we report that human PB and lymphoid tissue contain a subpopulation of CD4+FOXP3+ Treg cells that express CCR6 and have the capacity to produce IL-17 upon activation. These cells coexpress FOXP3 and RORγt transcription factors critical for Treg or Th17 cell development and function (31–33). The CD4+FOXP3+CCR6+IL-17–producing cells could strongly inhibit the proliferation of CD4+ responder T cells. We further show that isolated CD4+FOXP3+IL-17–producing T-cell clones express FOXP3 and RORγt and maintain their suppressive function. In addition, human CD4+FOXP3+CCR6− Treg cells could differentiate into IL-17 producer cells upon T-cell receptor (TCR) stimulation in the presence of IL-1β, IL-2, IL-21, and IL-23. This, together with the finding that only PB and lymphoid tissue, but not thymus, contain the IL-17–producing Treg cells, suggests that the IL-17+FOXP3+ Treg cells are generated in the periphery. IL-17–producing Treg cells may play critical roles in antimicrobial defense while controlling autoimmunity and inflammation.
Results
A Subpopulation of CD4+CD25+FOXP3+ Treg Cells Has the Capacity to Produce IL-17.
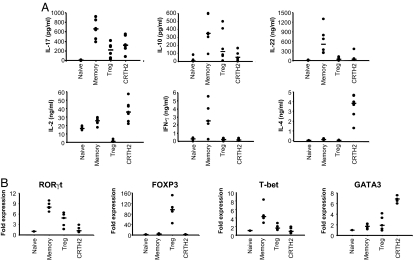
Although human IL-17–producing T cells were originally found enriched in the CD4+CD45RA− CD45RO+ memory CD4+ T-cell population (2), it is not known if they overlap with Th1 and Th2 memory T cells or Treg cells. To analyze the capacity of different CD4+ T cell subsets to secrete IL-17, we fractionated PB CD4+ T cells by flow cytometry into CD4+CD25high (Treg), CD4+CD25lowCD45RA+ (naive), CD4+CD25lowCD45RA− (memory), and CD4+CD25lowCD294+ (CRTH2) T cells and stimulated them with phorbol myristate acetate (PMA)/ionomycin for 24 h. We found that, in addition to memory CD4+ T cells, Treg and CRTH2 cells secreted a significant amount of IL-17 (Fig. 1A). The isolated T-cell subsets secreted the expected cytokine profiles upon stimulation: Treg cells secreted IL-10 but not IL-22, IL-2, IFN-γ, or IL-4; CRTH2− memory T cells, enriched for Th1 cells, secreted IL-10, IL-22, IL-2, and IFN-γ but not IL-4; and CRTH2+ Th2 memory cells secreted IL-4, IL-2, and IL-10 but not IFN-γ or IL-22. In addition, by real-time PCR analyses, we found that Treg cells and CRTH2− memory T cells expressed the RORγt transcription factor required for Th17 cell differentiation (Fig. 1B). These 3 T-cell subsets also expressed their lineage-specific transcription factors FOXP3, T-bet, and GATA3, respectively. These data suggest that all CD4+ memory T-cell subsets, including CRTH2 and Treg cells, may contain cells that have the capacity to produce IL-17.
Fig. 1.
Identification of T-cell subsets that secrete IL-17 and express key factors required for Th17 cell differentiation. (A) ELISA of cytokines in supernatants of T-cell subsets—CD4+CD25lowCD45RA+ (naive), CD4+CD25lowCD45RA− CRTH2− (memory), CD4+CD25high (Treg), and CD4+CRTH2+ (CRTH2)—sorted from PBMCs and stimulated with PMA/ionomycin for 24 h. The purity of each T-cell subset was >95%. (B) Real-time RT-PCR of RORγt, FOXP3, T-bet, and GATA-3 transcripts. Expression level was normalized to GADPH expression level and adjusted to corresponding expression levels in CD4+CD25−CD45RA+ naive T cells. Data are from 5–6 experiments taken from 5–6 different healthy donors. Horizontal bars indicate the median.
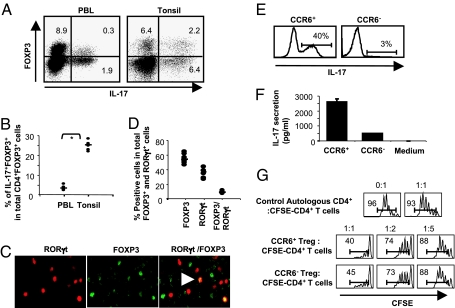
We next investigated the presence of FOXP3+IL-17+ T cells in PB and lymphoid tissue (tonsils) by flow cytometry. Human PB CD4+ T cells contained about 0.32 (±0.08) % IL-17+FOXP3+ and 2.63 (±1.22) % IL-17+FOXP3− T cells. Human tonsil CD4+ T cells contained about 2.4 (± 0.5) % IL-17+FOXP3+ and 4.68 (±1.08) % IL-17+FOXP3− T cells. The percentage of CD4+ T cells expressing FOXP3 for PB is about 8.6 (±1.7) %, of which 3.2 (±1.1) % expressed IL-17 (Fig. 2 A and B). Human tonsil CD4+ T cells contained about 11 (±2.5) % FOXP3+ T cells, of which 25 (±2.3) % expressed IL-17 (Fig. 2 A and B). The frequency of the FOXP3+IL-17+ T cells was 7 times higher in tonsils than in peripheral blood lymphocytes (PBLs). By immunostaining of human tonsil frozen sections, we found the presence of cells expressing FOXP3 only (green) and RORγt only (red) as well as both FOXP3 and RORγt (gold) (Fig. 2C). The percentage of FOXP3+RORγt+ double-staining cells in the total Foxp3+ and RORγt+ cells is in the range of 9 (±1) % (Fig. 2D), which is similar to that of FOXP3+IL17+ cells in the range of 13 (±1) % by FACS (Fig. S1). These results confirm the presence of FOXP3-expressing Treg cells that have the potential to make IL-17. Recently, Acosta-Rodriguez et al. (30) showed that human Th17 memory T cells selectively express high levels of CCR6 protein. We therefore fractionated the CD4+CD25high T cells into CCR6+ or CCR6− cells. Intracellular staining (Fig. 2E) and ELISA (Fig. 2F) showed that IL-17–producing cells are predominantly found among the CCR6+ T cells. Furthermore, both CCR6+CD4+CD25high and CCR6−CD4+CD25high T cells strongly inhibited the proliferation of CD4+ responder T cells (Fig. 2G), demonstrating that CD4+CD25highFOXP3+IL-17+ T cells are Treg cells.
Fig. 2.
Human tonsil contains a high percentage of FOXP3+IL-17+ CD4+ Treg cells. (A) Intracellular staining for FOXP3 and IL-17A proteins in PMA/ionomycin-stimulated CD4+ T cells isolated from PBL and tonsils. (B) Percentage of IL-17+FOXP3+ T cells among total CD4+FOXP3+ T cells. Arrowhead indicates double-positive for ROR gamma t and FOXP3 expression. A star indicates statistical significance (P < 0.05). (C and D) Immunohistochemistry of FOXP3 and RORγt proteins in human tonsil frozen sections. Horizontal bars indicate the median. (E) IL-17 producer cells are found in the CCR6+CD4+CD25high T-cell fraction. (F) Only CCR6+CD4+CD25high cells secrete abundant IL-17 when stimulated with anti-CD3 (2 μg/mL) and anti-CD28 (1 μg/mL). Representative experiments of 2 donors are shown. (G) CCR6+CD4+CD25high T cells suppress CD4+ responder T-cell proliferation. Data are representative of experiments with cells from 2 donors.
FOXP3 and IL-17 Double-Positive CD4+ T-Cell Clones Express RORγt and Display Suppressive Function.
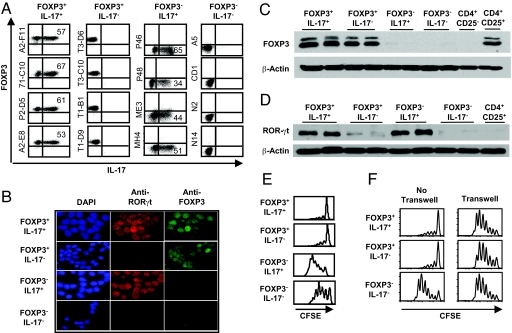
To characterize the CD4+CD25highIL-17+ Treg cells further, we generated T-cell clones from the PBL-derived CD4+CD25high Treg, CD4+CD25− memory, and naive CD4+ T cells, respectively, by limiting dilution methods. By intracellular staining, 4 representative clones derived from the CD4+CD25high Treg cells expressed both IL-17 and FOXP3 or only FOXP3 after 2 months in culture (Fig. 3A). By contrast, other T-cell clones derived from CD4+CD25low T cells were either IL-17+FOXP3− or IL-17−FOXP3−. By double-immunofluorescence staining, we further showed that FOXP3+IL-17+ T-cell clones coexpressed nuclear FOXP3 and RORγt, whereas FOXP3+IL-17− T cell clones expressed only FOXP3 (Fig. 3B). FOXP3−IL17+ T-cell clones expressed RORγt, and FOXP3−IL-17− clones expressed neither FOXP3 nor RORγt. The expression of FOXP3 and RORγt proteins in FOXP3+ or IL-17+ T cells was further confirmed by Western blot analysis showing that FOXP3+IL-17+ and FOXP3+IL-17− T-cell clones expressed the FOXP3 protein (Fig. 3C), whereas FOXP3+IL-17+ and FOXP3−IL-17+ T-cell clones expressed RORγt (Fig. 3D). In contrast, FOXP3− T cells did not express FOXP3, and IL-17− T cells expressed little RORγt. Upon TCR stimulation, all T-cell clones secreted a variable amount of IFN-γ, whereas some clones secreted both IL-17 and IL-10, IL-17 only, IL-10 only, or none (Table S1). We next investigated whether the IL-17+FOXP3+ T-cell clones derived from the CD4+CD25high Treg cells maintain suppression function. Fig. 3E shows that both IL-17+FOXP3+ and the IL-17−FOXP3+ T-cell clones potently suppressed the proliferation of CD4+CD25− T cells induced by anti-CD3 and anti-CD28, whereas FOXP3−IL-17+ or FOXP3−IL-17− T-cell clones did not exhibit suppressive activity. To investigate the suppressive mechanisms of these Treg cells, we tested a panel of neutralizing antibodies to IL-10; IL-10Rα; anti-TGF-β1,2,3; CTLA-4; PD-1; or TGF-β inhibitor and found that none of these blocked suppression (data not shown). We next performed transwell experiments and found that the suppressive function of the IL-17+ FOXP3+ Treg clones and IL-17−FOXP3+ Treg clones was absent in such conditions, indicating that suppression requires cell-cell contact (Fig. 3F).
Fig. 3.
Characterization of PBL-derived FOXP3+IL-17+CD4+ T-cell clones. (A) Intracellular staining for FOXP3 and IL-17A proteins in representative CD4+ T-cell clones derived from CD4+CD25high (FOXP3+IL-17+ and FOXP3+IL-17− lines) or CD4+CD25low T cells (FOXP3−IL-17+ and FOXP3−IL-17− lines). (B) Immunofluorescence microscopy of CD4+ T-cell clones fixed and stained with antibodies specific for human FOXP3 (green) and RORγt (red). DAPI (blue) was used to counterstain the nuclei. (Original magnification: ×400.) (C and D) Western blot analysis for FOXP3 and RORγt expression in T-cell clones as in A. Freshly sorted CD4+CD25lowCD45RA+ naive and CD4+CD24high T cells serve as a negative or positive control for FOXP3, respectively. IL-17− T cells serve as a negative control for RORγt. The β-actin protein serves as a protein loading control. (E) FOXP3+CD4+ T-cell clones suppressed proliferation of conventional CD4+CD25low responder T cells. Carboxyfluorescein succinimidyl ester-labeled CD4+CD25lowCD127+ responder cells were cultured with Treg cells at a ratio of Treg/responder cells (1:2). (F) T-cell suppression requires cell-cell contact. Equal numbers of Treg cells and CD4+ responder cells were used in the transwell experiments. The Treg cells were either cultured together with the responder cells as a positive control for suppression activity or cultured in inner wells. Results represent 1 of 2 independent experiments.
IL-1β, IL-2, IL-6, IL-21, and IL-23 Act Cooperatively to Induce IL-17 Production by the CCR6−CD4+CD25high Treg Cells.
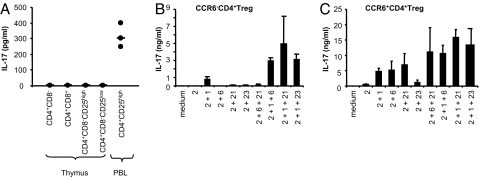
An important question is whether a subpopulation of CD4+CD25high Treg cells acquires the ability to produce IL-17 in the thymus during Treg development or in the periphery during inflammatory responses. We therefore isolated T-cell subsets from human thymus and assessed their capacity to produce IL-17 following activation, using flow cytometry and ELISA analyses. We found that CD4+CD8−CD25high, CD4+CD8−CD25low, CD4+CD8+, or CD4−CD8+ T cells isolated from thymus failed to secrete detectable amounts of IL-17 upon stimulation with PMA/ionomycin for 24 h (Fig. 4A). However, a significant amount of IL-17 was released from the CD4+CD25high fraction isolated from PB, suggesting that the FOXP3+ Treg cells may acquire the ability to produce IL-17 in the periphery. To determine whether the peripheral CD4+CD25high Treg cells can be induced to differentiate into IL-17 producer cells, we isolated CCR6− or CCR6+CD4+CD127−CD25high Treg cells from the PB and cultured these cells with anti-CD3, anti-CD28, a low concentration of IL-2, and 10% (vol/vol) human serum (which contains human TGF-β) in the presence of IL-1β, IL-6, IL-21, or IL-23 for 15–18 days. We found that IL-2 alone did not induce any Th17 cell differentiation from CCR6−CD4+CD25high Treg cells (Fig. 4B), although IL-2 alone or in combination with other cytokines significantly enhanced expansion of Th17 cells from CCR6+CD4+CD25high Treg cells (Fig. 4C). However, in the presence of IL-1β, CCR6−CD4+CD25high Treg cells could produce a low level of IL-17, and their IL-17 production was further enhanced with the addition of IL-6, IL-21, or IL-23 (Fig. 4B). Flow cytometry analysis of these cells shows that the majority of the IL-17 producer cells are FOXP3− (data not shown). These data suggest that IL-1β and IL-6 together could induce CCR6−CD4+CD25high Treg to differentiate into IL-17 producer cells in the presence of human serum.
Fig. 4.
Th17 cells are absent in thymus but can be derived from CCR6−CD4+ CD25high Treg cells by culturing with IL-1β. (A) T-cell subsets from thymus do not secrete IL-17 upon stimulation with PMA/ionomycin for 24 h. Data are from 3 experiments with 3 donors. (B) IL-1β promotes the differentiation of Th17 cells from CCR6−CD4+CD25high Treg cells. CCR6−CD4+CD25high Treg cells were activated with plate-bound anti-CD3 plus soluble anti-CD28 and then cultured for 15–18 days in the presence of indicated cytokines. Cells were then reactivated with anti-CD3 and anti-CD28 in the absence of cytokine for ELISA assays. Error bars represent SD. (C) IL-2 alone or in combination with IL-6, IL-21, or IL-23 further enhances expansion of Th17 cells from CCR6+CD4+CD25high Treg cells. Data are representative of 3 experiments with different donors.
Discussion
In this study, we have performed a thorough analysis of the ability of CD4+ T-cell subsets from human PB, tonsils, and thymus to produce IL-17. Unexpectedly, we found that up to 3% of FOXP3+ Treg cells in PB and 25% of FOXP3+ Treg in tonsils have the capacity to produce IL-17 upon activation. We further showed that the IL-17–producing Treg cells preferentially express CCR6, coexpress FOXP3 and RORγt, and strongly suppress responder CD4+ T-cell proliferation. Interestingly, the level of FOXP3 expression in the FOXP3+IL-17+ cells appears to be lower than that of the FOXP3+IL-17− cells in CD4+ T cells isolated from PB, suggesting that high FOXP3 expression might contribute to inhibition of Th17 differentiation. Although we do not rule out the contribution of FOXP3+IL-17− cells to the CCR6+CD4+CD25high T-cell suppression activity, our data suggest that both FOXP3+IL-17+ and FOXP3+IL-17− T cells are equally suppressive, because the strength of suppression of CCR6+ or CCR6−CD4+CD25high T cells on effector T-cell proliferation is similar in 3 of the Treg/effector ratios tested. To demonstrate the presence of IL-17–producing Treg cells at the clonal level further, we established a number of T-cell clones, including IL-17+FOXP3+, IL-17−FOXP3+, IL-17+FOXP3−, and IL-17−FOXP3− clones. We demonstrated that the IL-17+FOXP3+ T-cell clones after 2 months of culture coexpressed FOXP3 and RORγt proteins as well as the ability to produce IL-17 and to suppress CD4+ T-cell proliferation. Although our transwell assay indicates that the inhibition is cell-contact dependent, we do not rule out the possibility that Treg cells might also adsorb IL-2 to their IL-2 receptor and block proliferation of responder CD4+ T cells. We conclude from these data that IL-17–producing Treg cells may represent a significant subset of human CD4+ Th cells that display the functional features of both Treg and Th17 cells (e.g., IL-17 production, CCR6 expression, suppressive activity).
Although FOXP3+ Treg cells are critical for control of autoimmunity and inflammation (34), Th17 cells have been implicated in mediating inflammation and autoimmune diseases (4). The biological significance of T cells that display the function of Treg and the opposing function of Th17 is unclear. One of the key functions of IL-17 is to promote neutrophil differentiation from hematopoietic progenitor cells and neutrophil trafficking, critical mechanisms for innate immune defense against bacterial and fungal infection. Our finding that some FOXP3+ Treg cells acquire the ability to produce IL-17 suggests that Treg can potentially contribute to the antimicrobial innate immune defense while controlling inflammation and autoimmunity at the same time, particularly at mucosal sites. IFN-γ, a major product of Th1 cells, is critical for the initiation of cell-mediated immunity against intracellular pathogens and the induction of some autoimmune diseases (35, 36). Interestingly, many IFN-γ–producing Th1 cells were found to produce IL-10, an anti-inflammatory cytokine, in antimicrobial immune responses (37). This phenomenon has been called self-control of Th1 cells, which is a critical mechanism for effective antimicrobial immune responses while limiting self-tissue damage (37). Indeed, it has been shown that IL-10–deficient mice acutely infected with Toxoplasma gondii induced a lethal Th1 immune response accompanied by overproduction of IL-12, IFN-γ, and TNF-α (38).
In the mouse, the nuclear receptor RORγt is expressed in CD4+CD8+ thymocytes but not in single-positive CD4 or CD8 thymocytes (39). Accordingly, we did not detect any IL-17 produced by the single-positive thymic T-cell populations tested, including the FOXP3+ Treg thymocytes. There was also no IL-17 produced by human double-positive thymocytes, suggesting that the expression of RORγt is insufficient for T-lineage cells to acquire the ability to produce IL-17 in thymus. Our data suggest that peripheral CCR6−CD4+CD25high Treg cells stimulated in the presence of IL-1β and IL-6 differentiated into IL-17 producer cells in the presence of 10% (vol/vol) human serum, which contains TGF-β critical for human Th17 differentiation (40). This, together with the finding that a significant number of Treg cells in PB and particularly in tonsils produce IL-17, suggests that the IL-17+FOXP3+ Treg cells are generated at mucosal sites during inflammation. Indeed, a recent study in mice by Zhou et al. (41) has demonstrated the presence of FOXP3+RORγt+ T cells that have the ability to produce IL-17 in the lamina propria of the small intestine. The identification of IL-17–producing FOXP3+ Treg cells in both mice and humans suggests that Th17 and FOXP3+ Treg lineages are related in ontogeny. Both lineages appear to depend on TGF-β for their differentiation and/or maintenance, and additional cytokines may determine whether they become Th17, Treg, or dual-function effector T cells (41). FOXP3+ Treg cells may thus actively contribute to antimicrobial innate immunity by producing IL-17, while they control inflammation and autoimmunity at the same time.
Materials and Methods
Purification of CD4+ T-Cell Subsets.
Adult blood buffy coats from healthy donors were obtained from the Gulf Coast Regional Blood Center in Texas. CD4+ T cells were enriched using a CD4 T-cell isolation kit (Miltenyi Biotec) according to manufacturer's procedures. We isolated CRTH2 T cells from enriched CD4+ T cells by staining with biotin-CRTH2 antibody, followed by biotin-microbeads. Flow-through cells from LS column (Miltenyi Biotec) were stained with streptavidin-PE, APC-Cy7-CD4 antibody, and FITC-labeled lineage mixture antibodies against CD14, CD16, CD19, CD56, CD11c, and γδ-TCR and were sorted on a FACSAria (BD Bioscience) into a single fraction of CD4+CRTH2+. Cells retained in the LS column were eluted and stained with same FITC-labeled lineage mixture antibodies plus APC-Cy7-CD4, PE-Cy7-CD25, PE-CD127, and biotin-CD45RA and were then washed and stained with streptavidin-perCP-Cy5.5. Stained cells were sorted into 3 fractions of CD127−CD4+CD25high (top 2–3%, average of 90% FOXP3+) Treg cells, CD4+CD25lowCD45RA+ naive T cells, and CD4+CD25lowCD45RA−CRTH2− memory T cells.
Treg Cell Culture.
CCR6+ or CCR6− CD4+CD25high Treg cells were cultured for 5–6 days in 96-well flat-bottomed plates (Falcon) at a cell density of 5 × 104 cells per well in RPMI 1640 medium containing 10% (vol/vol) human AB serum (GemCell), 40 IU/mL IL-2, neutralizing anti-IFN-γ (5 μg/mL; 25718; R&D Systems), and anti-IL-4 (5 μg/mL; R&D Systems), along with plate-bound anti-CD3 (2 μg/mL) and soluble anti-CD28 (1 μg/mL). Where indicated, IL-1β (10 ng/mL), IL-6 (20 ng/mL), IL-21 (50 ng/mL), or IL-23 (20 ng/mL) was added to the cultures. Fresh culture medium containing the indicated cytokines was added every 5–6 days. On days 15–18, 5 × 104 cells were stimulated with plate-bound anti-CD3 (2 μg/mL) and anti-CD28 (1 μg/mL) and analyzed for IL-17 cytokine release.
Generation of Human PBL-Derived CD4+ Treg Cell Clones.
CD4+ T-cell clones were generated from flow cytometry-sorted CD4+CD25high (top 2%) cells by limiting dilution methods as described (42) using 0.5 T cell per well and 5 × 104 cells per well of irradiated allogeneic peripheral blood mononuclear cells (PBMCs; 7,000 rad) as feeder cells in lymphocyte stimulation medium containing RPMI 1640 (Invitrogen) supplemented with 2 mmol/L L-glutamine, 0.05 mmol/L β-mercaptoethanol, 10% human male AB serum (GemCell), 300 IU/mL IL-2, 75 ng/mL anti-CD3, 15 ng/mL anti-CD28, and 100 ng/mL anti-inducible T cell costimulator (ICOS). On day 14, one-fifth of the cells from each well with cell outgrowth were washed and restimulated with 3 μg/mL plate-bound anti-CD3 and 1 μg/mL soluble anti-CD28 in T-cell assay medium containing RPMI 1640 plus 4% (vol/vol) human AB serum and 2 mmol/L L-glutamine. IL-17 release from T cells was measured by ELISA. High IL-17 producer cells were then expanded, as previously described (42), using the same lymphocyte stimulation medium as above but supplemented with irradiated allogeneic 1.6 million/mL PBMCs (irradiation with 7,000 rad) and 0.3 million/mL EBV B-cell lines LCL111 and 112 (23,000 rad). Th17 T-cell clones derived from CD4+CD25low cells were generated as above, except that 5 ng/mL IL-1β and 10 ng/mL IL-23 were added in the expansion medium. The clonality of T-cell clones was determined with RT-PCR or monoclonal antibodies against the Vβ regions. All T cells were confirmed to be free of mycoplasma by a PlasmoTest kit (InvivoGen).
For more information, see SI Matierals and Methods.
Statistical Analysis.
A standard two-tailed t test was used for statistical analysis with P values of 0.05 or less considered significant.
Supplementary Material
Acknowledgments.
We thank Karen Ramirez and Zhiwei He for cell sorting and support. We thank Brenna Kelly and Xing Gong for screening and preparation of anti-RORγ antibodies. We thank Dr. Cliona M. Rooney for the gift of LCL lines. This work was supported by KECK Foundation 01, PP-4 and National Institutes of Health Grant AI091130 (to Y.-J.L.) and Howard Hughes Medical Institute (to D.R.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900408106/DCSupplemental.
References
- 1.Fossiez F, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao Z, et al. Human IL-17: A novel cytokine derived from T cells. J Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 3.Kawaguchi M, et al. Identification of a novel cytokine, ML-1, and its expression in subjects with asthma. J Immunol. 2001;167:4430–4435. doi: 10.4049/jimmunol.167.8.4430. [DOI] [PubMed] [Google Scholar]
- 4.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 5.Linden A, Laan M, Anderson GP. Neutrophils, interleukin-17A and lung disease. Eur Respir J. 2005;25:159–172. doi: 10.1183/09031936.04.00032904. [DOI] [PubMed] [Google Scholar]
- 6.Linden A, Adachi M. Neutrophilic airway inflammation and IL-17. Allergy. 2002;57:769–775. doi: 10.1034/j.1398-9995.2002.02164.x. [DOI] [PubMed] [Google Scholar]
- 7.Forlow SB, et al. Increased granulopoiesis through interleukin-17 and granulocyte colony-stimulating factor in leukocyte adhesion molecule-deficient mice. Blood. 2001;98:3309–3314. doi: 10.1182/blood.v98.12.3309. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal S, Gurney AL. IL-17: Prototype member of an emerging cytokine family. J Leukocyte Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- 9.Amadi-Obi A, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 10.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 12.Komiyama Y, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 13.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lubberts E, et al. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J Immunol. 2001;167:1004–1013. doi: 10.4049/jimmunol.167.2.1004. [DOI] [PubMed] [Google Scholar]
- 15.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Y, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 18.Ma HL, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 21.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 23.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson NJ, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 25.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 26.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 27.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 28.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 29.Stumhofer JS, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 30.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 31.Fontenot JD, Gavin MA, Rudensky AY. FOXP3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 32.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi S. Naturally arising FOXP3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 34.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 35.Haskins K, McDuffie M. Acceleration of diabetes in young NOD mice with a CD4+ islet-specific T cell clone. Science. 1990;249:1433–1436. doi: 10.1126/science.2205920. [DOI] [PubMed] [Google Scholar]
- 36.Sadick MD, Heinzel FP, Shigekane VM, Fisher WL, Locksley RM. Cellular and humoral immunity to Leishmania major in genetically susceptible mice after in vivo depletion of L3T4+ T cells. J Immunol. 1987;139:1303–1309. [PubMed] [Google Scholar]
- 37.Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med. 2007;204:239–243. doi: 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gazzinelli RT, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 39.Sun Z, et al. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 40.Manel N, Unutmaz D, Littman DR. Human TH-17 cell differentiation requires transforming growth factor-β and induction of the nuclear receptor RORγT. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou L, et al. TGF-β-induced FOXP3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang RF, Appella E, Kawakami Y, Kang X, Rosenberg SA. Identification of TRP-2 as a human tumor antigen recognized by cytotoxic T lymphocytes. J Exp Med. 1996;184:2207–2216. doi: 10.1084/jem.184.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.