Abstract
The overenrichment (eutrophication) of aquatic ecosystems with nutrients leading to algal blooms and anoxic conditions has been a persistent and widespread environmental problem. Although there are many studies on the ecological impact of elevated phosphorus (P) levels (e.g., decrease in biodiversity and water quality), little is known about the evolutionary consequences for animal species. We reconstructed the genetic architecture of a Daphnia species complex in 2 European lakes using diapausing eggs that were isolated from sediment layers covering the past 100 years. Changes in total P were clearly associated with a shift in species composition and the population structure of evolutionary lineages. Although environmental conditions were largely re-established after peak eutrophication during the 1970s and 1980s, original species composition and the genetic architecture of species were not restored but evolved along new evolutionary trajectories. Our data demonstrate that anthropogenically induced temporal alterations of habitats are associated with long-lasting changes in communities and species via interspecific hybridization and introgression.
Keywords: biological archive, eutrophication, hybridization, introgression, invasiveness
During the past century, most European lakes went through a phase of eutrophication (i.e., overenrichment with nutrients) and many recovered their original trophic state as a result of pollution control (1). This process was accompanied by a shift in species composition and diversity of both pelagic and littoral communities, reduction of water quality, and even occasional fish kills (2). In northern temperate lakes, total phosphorus (P) concentration is regarded as the key factor of eutrophication (3). Human-made increased levels of P (urban and industrial sewage, erosional runoff, and leaching from agricultural areas) caused algal blooms, which subsequently affected species of higher trophic levels such as zooplankton and fish. Here, we use P concentration as a surrogate to characterize a major human-caused environmental change.
Among the most important planktonic grazers in pelagic foodwebs are species of the genus Daphnia (Crustacea: Anomopoda; water fleas). Daphnia species serve as food for fish and invertebrates, and they feed on algae and bacteria. Daphnia produce diapausing stages, which are deposited in the sediments of lakes (Fig. 1A). Because subfossil resting eggs are often viable for up to 100 years (4) and provide sufficient quality and quantities of DNA for genetic analyses (5, 6), resting egg banks represent a unique biological archive to unravel ecological and evolutionary changes (7, 8). Currently, most European lakes are inhabited by 3 genetically divergent species of the Daphnia longispina complex (D. galeata, D. cucullata, and D. hyalina) as well as their interspecific hybrids (9). These species, and most other Daphnia species, reproduce via cyclic parthenogenesis. The induction of sexual females and males is determined by environmental factors such as the change in food level, crowding (high densities of conspecifics), and photoperiod (10). Therefore, abundances of resting eggs over time do not necessarily represent the short-term success or fitness of taxa or clonal lineages at a given time period (11, 12). Instead, relative abundances of taxa or genotypes derived from resting egg banks reveal information about lineages that successfully contributed genes to the next generation (13). Thus, analyses of resting egg banks allow one to trace the long-term evolutionary fate of lineages (7, 8, 13). Although sediment remains have been used to assess the species compositional changes in association with eutrophication (14, 15), hardly anything is known about changes in the molecular genetic composition of populations during a period of rapid anthropogenic changes and subsequent recovery. Furthermore, rapid environmental changes allow opportunities for species invasions and potential gene exchange between species, which may fundamentally alter ecological interactions and influence rates of replacement.
Fig. 1.
Reconstruction of the Daphnia taxon composition and ecological changes in Lake Constance and Greifensee over time. (A) Sediment core covering the years between 1900 and 2004 in Lake Constance was used for the isolation of resting eggs. (B) P concentration in Lake Constance (light green and upper x axis) and Greifensee (green and lower x axis) over time. (C and D) Temporal variation in relative abundances of Daphnia taxa in Lake Constance and Greifensee, respectively (light blue = D. hyalina, red = D. galeata × hyalina, gray = D. galeata). Species and hybrid identification is based on ITS-RFLP, mtDNA, and microsatellite analyses. (E) Temporal pattern of nuclear and mitochondrial DNA introgression of D. galeata in Greifensee. Relative abundance of all those D. galeata individuals, as classified by microsatellite analyses, which exhibit either nuclear (ITS) or mitochondrial (16S) DNA of D. hyalina. Blue triangles represent ITS alleles and blue dots represent mitochondrial haplotypes of D. hyalina found in D. galeata resting eggs.
To understand the role of human-made changes on the evolutionary history of species, we reconstructed the taxon composition and patterns of genetic variation using Daphnia diapausing (ephippial) eggs from 2 perialpine lakes, Lake Constance (Austria, Germany, and Switzerland) and Greifensee (Switzerland). As in the majority of European lakes, both lakes were subjected to increased levels of P, mostly because of the intensive application of fertilizers and P-containing detergents (1). However, after the reduction of phosphorus inflow, as a result of the installation of sewage treatment plants, P-levels decreased to lower levels comparable to those before eutrophication. This setting allowed us to compare populations before and during habitat alterations as well as after the recovery. Previous studies suggested that the phase of disturbance also involved an invasion (16), thus allowing for a change in lineage diversification (via introgression) rather than the more typical loss in genetic diversity. There were 2 main goals of our study: First, we tested for a potential association of inter- and intraspecific genetic variation in Daphnia with changes in P-levels. Second, we aimed to assess the evolutionary consequences of rapid ecological changes on the genetic makeup of natural populations.
Results
Changes in Daphnia species and hybrid composition are associated with rapid environmental shifts in Lake Constance and Greifensee (Fig. 1 B, C, and D). In the first half of the 20th century, both lakes were inhabited by D. hyalina, which has been shown to be indigenous in Lake Constance (Obersee; ref. 17). In addition, our data confirm previous observations that D. galeata invaded Lake Constance in the mid-1950s (16). Similarly, Greifensee was successfully invaded by the same species in the mid-1940s. Species invasions in both lakes were followed by interspecific hybridization and a subsequent decrease of D. hyalina ephippia in the resting egg bank. This reduction, and the virtual absence of D. hyalina resting eggs during recent decades (Fig. 1 C and D), is probably attributable to the reduction of sexual activity, because planktonic D. hyalina individuals have been reported in recent studies of Lake Constance (12, 18).
In both lakes, population genetic analyses suggest that the occurrence of interspecific hybrids was associated with the relative abundance of parental species, because we found a significant correlation of observed interspecific hybrids and expected number of hybrids (based on parental frequencies). In addition, most of the samples showed no significant deviation from Hardy-Weinberg expectations (see Table S1 and Fig. S1).
Comparative analyses of nuclear and mitochondrial DNA markers over time indicate that population genetic changes are mediated by introgression since the onset of interspecific hybridization (Fig. 1E). In general, we obtained very similar patterns of hybridization and introgression irrespective of the applied statistical techniques [e.g., STRUCTURE (19), NEW- HYBRIDS (20)]. The directionality and level of mitochondrial introgression varied between lakes (Fig. 2). In Lake Constance, we found a significant number of D. hyalina (defined by microsatellite analysis) exhibiting mitochondrial DNA of D. galeata, whereas in Greifensee, mainly the invasive species D. galeata exhibited nuclear and mitochondrial genes of D. hyalina. Overall, we observed a general shift in the genotypic architecture of species complexes in both lakes over time (Fig. 3).
Fig. 2.
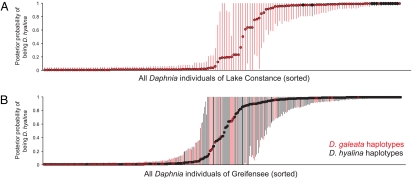
Level of mitochondrial DNA introgression in Daphnia species. Genotypic structure of Daphnia populations in Lake Constance (A) and Greifensee (B) was based on the posterior probability of belonging to D. hyalina [as implemented in STRUCTURE (19)]. Each dot represents a single individual sorted according to probabilities of belonging to D. hyalina (e.g., F1 hybrids are found around the value 0.5, individuals close to 0 or 1 represent later generation back crosses). Species-specific mitochondrial DNA haplotypes (16S rDNA RFLP analyses) are labeled with different colors (black = D. hyalina, red = D. galeata). Error bars represent 95% probability intervals (black lines = D. hyalina, red lines = D. galeata).
Fig. 3.
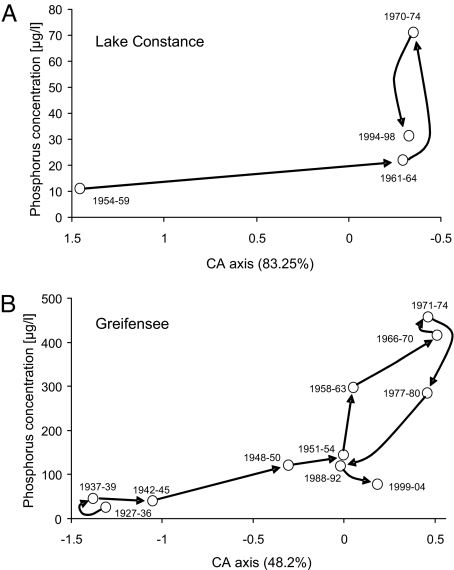
Association of genotypic shifts in the Daphnia species complex and environmental change over time. Genetic architecture of the Daphnia hyalina-galeata species complex in Lake Constance (A) and Greifensee (B) based on a canonical correspondence analysis (see SI Text) of microsatellite data (60). Each dot represents the genetic composition of a sample of Daphnia resting eggs (including species and hybrids) isolated from sediment cores of a given time period. The y axis represents the average P concentration (μg/L) for each sample, and arrows indicate the sequence of temporal changes.
Discussion
The occurrence of eutrophication processes are well known to be associated with reduced biodiversity and water quality, but the evolutionary consequences for species are largely unexplored (13). Our study associates rapid ecological changes in lakes with the evolutionary fate and genetic consequences of hybridization and introgression covering a time period of nearly 100 years. Paleogenetic analyses of Daphnia resting eggs showed that the large variation in trophic status of Greifensee and Lake Constance facilitated the establishment of D. galeata and subsequent interspecific hybridization with D. hyalina. D. galeata × hyalina resting eggs were detected during times of rapid changes in P-levels (e.g., Greifensee: 18–525 μg of P/L). These data suggest either that hybrids are produced because of the increased mating probabilities (co-occurrence of parental species) or that hybrids exhibit a higher level of fitness than parental species under “intermediate” or rapidly changing environmental conditions (21). Although we have no direct evidence for increased hybrid fitness, the existence of back crosses and mitochondrial DNA introgression suggests that D. galeata × hyalina individuals successfully reproduced and contributed to future generations (Fig. 1E). On the other hand, population genetic analyses indicate that D. galeata and D. hyalina abundances are associated with the relative abundances of interspecific hybrids (across all time periods in both lakes; Fig. S1). In addition, parental species seem to mate nearly randomly in most cases (Table S1), with apparently low levels of pre- or postzygotic isolation (22). Despite the fact that we cannot differentiate between the 2 potential scenarios, either hybrid superiority during intermediate trophic conditions or random mating among parental species, both scenarios imply that the anthropogenetically induced changes are responsible for the origin of interspecific hybrids. Alternative explanations for the association of taxon composition and the elevated P-levels are (i) a general invasion of D. galeata in this region during the 1950s and 1960s or (ii) reduced sexual reproduction of D. galeata in Lake Greifensee during the first part of the past century. A simultaneous invasion of D. galeata into both lakes seems unlikely, because D. galeata was not able to invade lakes that maintained their oligotrophic status throughout the past century (e.g., Lake Brienz) (23). Although these lakes are located in the vicinity of eutrophic systems, they only exhibit populations of D. hyalina or D. longispina (a synonym of D. hyalina; ref. 24). Furthermore, many studies have shown that D. hyalina is predominantly found in oligotrophic lakes, whereas D. galeata represents a generalist that also occurs in strongly polluted habitats (25, 26). Further, large genetic differences (3 fixed loci) among the initial D. galeata invaders of both lakes reject the hypothesis of a simultaneous regional invasion. Because D. galeata was present in the region before the 1940s (e.g., in lower Lake Constance, a more eutrophic and shallow basin of Lake Constance) (27), its invasion of upper Lake Constance is most likely attributable to environmental changes (i.e., eutrophication in upper Lake Constance during the 1950s). It is, however, not clear yet why we found no D. galeata ephippia in the Greifensee sediment before the 1950s (as predicted by P-levels). In contrast to upper Lake Constance, no detailed historic observations exist for this lake to support the occurrence of D. galeata in Greifensee. Alternatively to the “simultaneous invasion” hypothesis, D. galeata might have been present in the lake for a long time already but ceased or at least strongly reduced ephippial production during the first part of the past century. A change in the frequency of sexual reproduction with eutrophication (17), as well as differential hatching success of parental and hybrid taxa (28), has also been observed in Lake Constance. This hypothesis is supported by the presence of a few interspecific hybrids in Greifensee sediment layers of the 1920s, which suggests that D. galeata was present in Greifensee during this time but did not reproduce sexually (i.e., make ephippia). Thus, our data support the assumption that the occurrence and successful sexual reproduction of Daphnia hybrids are associated with ecological parameters. Both resting egg banks show that an increase of D. galeata and a decrease of D. hyalina over time are linked to human-made changes in P-levels, supporting the hypothesis of an association between eutrophication and species composition (13, 23, 29).
Although Lake Constance and Greifensee went through a very similar history of changes in P, they differ in the magnitude of P concentrations. Lake Constance P-levels rose from less than 10 μg/L (winter mixis) in the 1940s to a maximum of 87 μg/L in 1979, and Greifensee peaked in 1971 with 525 μg/L after an average value of about 40 μg/L in the 1930s (25). This observation suggests that other (environmental) factors limited an earlier establishment of D. galeata in Greifensee. One of the most important factors, other than P, which alters Daphnia communities, is fish predation (26). Because predation has different impacts in shallow and deep lakes (e.g., predator control is higher in shallow lakes than in deep lakes) (30), Lake Constance and Greifensee are expected to respond differently. The interaction of multiple factors such as predation and food quality might have determined the differential establishment success of D. galeata in both lakes (31–34).
One potential consequence of interspecific hybridization is introgression (i.e., the spread of genetic material of one species into the gene pool of the other), which facilitates the origin of new recombinant genotypes that may establish new evolutionary lineages. This process is expected to be irreversible, because it is highly unlikely that assortative mating, genetic drift, or selection may re-establish the original genotypic architecture. Lake Constance Daphnia populations have rapidly evolved tolerance to increased cyanobacterial densities but hardly any reverse evolution after reoligotrophication (8, 35). This pattern supports our expectation that no amount of environmental remediation can restore pre-eutrophication species composition and genetic architecture. Recently, a number of empirical studies and a simulation study reported on gene flow mainly from the local to the invasive species, suggesting that one need not invoke selection, unequal mating behavior, or differences in relative abundances to explain substantial unidirectional introgression (36). This process is largely explained by the successive dilution of the invasive gene pool by a few interbreeding events during the first wave of hybridization and the logistic growth occurring in newly founded populations. Our empirical data on the Greifensee population support this hypothesis (i.e., higher level of introgression in D. galeata); however, the Lake Constance population shows a different pattern. Here, we found a high level of mitochondrial DNA introgression from D. galeata, the invasive species, to D. hyalina, the indigenous species. The reason for this discrepancy is not clear; it might be caused by variation in the mating system or natural selection. Previous studies on the directionality of hybridization among North American Daphnia species suggest that females of the common species and males of the rare species form interspecific hybrids (37). However, studies of European species did show a different pattern, indicating that allochronic differentiation of parental species and asymmetric interspecific mate choice are very important (31). Our preliminary experimental data show that certain cytonuclear recombinants (e.g., D. galeata carrying mtDNA of D. hyalina) exhibit, at least temporarily, greater fitness than other recombinants (32). Whether selection or other factors are responsible for high levels of introgression remains an open question; however, our data confirm a steady rise of interspecific gene flow in Daphnia since the onset of hybridization. Within only a relatively short time period (a few decades), genomes of both species and in both lakes have significantly altered their architecture. Thus, interspecific hybridization of invasive and local Daphnia species prohibited the recovery of the taxon composition before the period with elevated P-levels despite the fact that both lakes nearly returned to their initial trophic states.
Because resting egg banks allow the recruitment of “ancestral” genotypes, any shift from one species to another, or from one lineage to another, will incorporate time-dependent lags that might delay an immediate response to environmental shifts (33). Whether this phenomenon is responsible for a delayed transition to the original species and genotypic constitution in Lake Constance and Greifensee is an open question for future research. However, several lines of evidence suggest that even if a time lag occurs, hybridization and introgression will most likely prohibit the re-establishment of ancestral forms. Recent experimental studies have shown that Daphnia populations adapt rapidly to environmental changes (8, 34, 38); thus, ancestral “predisturbance” genotypes (i.e., D. hyalina) will be in competition with recent well-adapted recombinant genotypes. In addition, we expect that the likelihood of successful re-establishment of ancestral forms decreases with time, because the hatching success declines and the ecological differentiation between past and present habitats increases. Furthermore, life history experiments using Daphnia clones hatched from before, during, and after disturbance were subjected to different food qualities (high or low P-levels in algae) and temperatures. Fitness comparisons (e.g., based on somatic growth rate) revealed (i) higher differentiation in ecologically relevant traits (Qst) than in neutral markers (Fst) and (ii) differential fitness of cytonuclear genotypes (32).
Recently, empirical data of aquatic animals have challenged the view that populations of invasive species suffer from low genetic diversity and expected low evolutionary potential and high risk for extinction (39). In many cases of aquatic invasions, large propagule pools and multiple introductions seem to overrule founder effects (40). In Daphnia, like in plants, a second mechanism, interspecific hybridization, might explain the success of invading species. Some Daphnia species, such as D. galeata, seem to overcome the loss of genetic diversity because of their ability to hybridize with local species. In addition, sexual reproduction with local taxa and, subsequently, introgression will allow fast and efficient adaptation of new lineages. This phenomenon might explain why the species with the highest level of interspecific hybridization in the genus Daphnia (with 5 other species; ref. 41), D. galeata, represents the taxon with the widest distributional range and largest ecological tolerance in European freshwater lakes. Invasions of novel habitats and the subsequent capture of “local” genes may lead to successful establishment of lineages or fast invasions across large areas. However, this process may lead to genetic homogenization caused by increased genetic admixture, effectively reversing speciation (42).
In summary, our data are consistent with field and laboratory experiments that suggest a severe impact of changes in trophic status on community composition of aquatic habitats (23). For example, studies using zooplankton remains of several lakes across Europe covering a large part of the past century revealed that species composition over time was associated with trophic levels (43–45). In addition, field and laboratory studies showed that Daphnia species and hybrids vary in their habitat preference and fitness and respond to variation in the P-content of their food (23, 46–48). Changes in P-levels (as an indicator of human impact) seem to facilitate coexistence of species and, subsequently, interspecific hybridization, causing long-lasting changes in the genotypic architecture of Daphnia.
In addition to eutrophication, other environmental challenges such as climate change and biodiversity loss currently represent the largest challenges for aquatic systems. The rise of surface temperature and the invasion of alien species offer ample opportunities for interspecific hybridization, because previously geographically isolated organisms come into contact. Because our data show that species introductions not only alter local communities but genetic structures of indigenous species, human-mediated introductions pose direct and indirect effects on the diversity of local populations. The environmental problem of eutrophication as well as that of alien species invasions might be more serious than previously thought, because genetic consequences can be cryptic and persistent.
Materials and Methods
Sediment cores of Lake Constance, which is located at the border of Germany, Switzerland, and Austria, were collected in September 2004 from a depth of 180 m close to the Langenargener Bucht. Greifensee (Switzerland) cores were collected in November 2004 from the middle of the lake at a depth of 30 m. The greatest possible lake depth for sediment sampling (below the thermocline, anoxic conditions in summer and in the dark) was chosen to prevent sampling of biological archives that have been subjected to multiple hatching stimuli. Sediments from Lake Constance were dated by lamination counting (15, 49). In addition, cores of both lakes were dated using reference cores that have been subjected to 137Cs-dating (Lake Constance and Greifensee; refs. 49 and 50, respectively). Sediments from Greifensee were portioned into 1-cm slices. To obtain a sufficient number of resting eggs for both lakes, we pooled sediment layers covering, on average, 5.6 years (range: 3–18 years; Table S2). To prevent contamination of cores by movement of the Perspex tube through the sediment, we removed the outer sediment ring (ca. 1 cm) from subsequent treatments. Ephippia were isolated by washing the sediments through a metal sieve (220-μm mesh size). P concentrations, either based on direct measurements or on reconstructions using diatoms in sediments, were provided by the Landesanstalt für Umweltschutz Baden-Württemberg, Institut für Seenforschung, Langenargen, Germany (Lake Constance) and the Canton of Zürich (Greifensee; ref. 25).
Resting eggs were isolated from their ephippial shells, and DNA was prepared separately in 35 μL of H3 buffer [10 mM Tris-HCl (pH 8.3) at 25 °C, 0.05 M potassium chloride, 0.005% Tween-20, and 0.005% Nonidet P-40) and 1.2 μL of proteinase K (10 μg/mL; Sigma). After an incubation time of 12 h, proteinase K was deactivated by heating the sample 12 min to 95 °C. An internal transcribed spacer (ITS) fragment (a short piece of the ITS1 region, 5.8S rDNA, and a large part of ITS2 region) was amplified using a total reaction volume of 14 μL. Two microliters of template, 3 mM MgCl2, 1× PCR buffer, 0.2 mM dNTP, 0.3 μM each primer (ITS2–5.8S: 5′-GGA AGT AAA AGT CGT AAC AAG G-3′ and ITS1–18S: 5′-CGG TGG TCG ACG ACA CTT CGA CAC GC-3′), and 1 U/reaction Taq polymerase (Invitrogen) were amplified at 94 °C for 3 min; 5 cycles at 94 °C for 1 min, 52 °C for 1 min, and 72 °C for 1.5 min; and 35 cycles at 94 °C for 1 min, 50 °C for 30 sec, and 72 °C for 1 min, with a final synthesis step at 72 °C for 5 min. A restriction fragment length polymorphism (RFLP) analysis was used for taxon identification (51). Amplicons of the ITS region were digested with the restriction enzyme Mwo I for 2.5 h at 60 °C in a total reaction volume of 9.6 μL containing 8 μL of PCR product and 10× NEBuffer for Mwo I, 0.8 U of the restriction enzyme, and autoclaved dH2O.
To distinguish between D. galeata and D. hyalina mitochondrial haplotypes, a digestion of a 16S rDNA fragment with restriction enzymes was conducted (52). PCR amplifications were performed with 2 μL of template in a 14-μL reaction volume containing 3 mM MgCl2, 1× PCR buffer, 0.2 mM dNTP, 0.3 μM each primer (S1: 5′-CGG CCG CCT GTT TAT CAA AAA CAT-3′ and S2: 5′-GGA GCT CCG GTT TGA ACT CAG ATC-3′), and 1 U of TaqDNA polymerase (all chemicals and primers by Invitrogen). Cycling conditions started with 2 cycles of 93 °C for 2.5 min, 55 °C for 1 min, and 72 °C for 2 min, followed by 41 cycles of 1-min steps at 93 °C, 55 °C, and 72 °C. A final 5-min synthesis step at 72 °C completed the amplification. Four microliters of the amplicon was separately digested for 3 h with 3 different enzymes: Mnl I, Dde I, and Rsa I (all NEB). The combination of digestion banding patterns identified the mitochondrial haplotypes (Table S3).
Microsatellite analyses are based on either 6 (Lake Constance) or 8 (Greifensee) loci: DaB10/15, DaB17/17, DaB17/16, DaB10/14 (53), Dp512, Dp519 (54), Dgm101, and Dgm109 (55). All amplifications were performed in a 10-μL reaction volume containing 2.4 mM MgCl2, 1× PCR buffer, 0.25 mM dNTP, 0.2 μM each primer, and 0.5 U of TaqDNA polymerase (chemicals and primers by Invitrogen). Cycling conditions started with a 3-min denaturing step at 95 °C, followed by 35 cycles of 1-min steps at 95 °C, primer-specific annealing temperature (see below), and 72 °C. A final 7-min synthesis step at 72 °C completed the program. Annealing temperatures varied depending on primer pairs: DaB10/15, DaB17/17, DaB17/16, and DaB10/14 at 55 °C; Dp512 at 56 °C; Dp519 at 50 °C; Dgm101 at 54 °C; and Dgm109 at 60 °C. Amplicons were diluted and electrophoresed on an A.L.F. sequencer (Amersham) with self-designed size standards based on Lambda virus DNA.
Species and hybrid identification was based on 2 different approaches. First, we pooled all available information [ITS, microsatellites, and mtDNA (“total evidence”)]. Second, we identified species based on microsatellite analysis and inferred the relative frequency of a nuclear (ITS) and a mitochondrial (16S rDNA) species-specific marker over time (“congruence approach”). Microsatellite data were subjected to 2 different model-based Bayesian statistical techniques, STRUCTURE version 2.1 and NEWHYBRIDS version 1.1, which use the information of highly polymorphic molecular markers (19, 20). The following options were used for each STRUCTURE run: assignment without any prior information of population membership; 2-population model (K = 2); 106 replicates after a burn-in of 105; admixture model; α inferred with an initial value of 1, a maximum value of 10, a uniform prior, and the same value for all populations; different values of FST for different subpopulations; a prior mean FST of 0.01; a prior SD of 0.0; and constant λ with a value of 1. NEWHYBRIDS analyses are based on more than 106 Markov chain Monte Carlo simulation sweeps following a burn-in period of 104 sweeps, 6 genotype frequency classes, and no prior information. Data sets were analyzed several times with different starting values, lengths of burn-in period, and numbers of sweeps, as recommended by Anderson and Thompson (20).
One of the methods used for species and hybrid identification, the ITS RFLP analysis (51), has been verified using additional restriction sites, because recent findings indicate a polymorphic recognition site of Mwo I for D. galeata and D. cucullata (56). However, we did not detect any ambiguous genotypes in the populations of Lake Constance and Greifensee.
Gene flow among species was detected by comparisons of ITS, microsatellite, and mitochondrial DNA markers. Each comparison (microsatellites vs. ITS, microsatellites vs. mtDNA, ITS vs. mtDNA, or selected microsatellite loci vs. microsatellites) revealed evidence for recombinant genotypes and introgression. Our results, together with previous multiple findings of introgression among Daphnia species, provide strong evidence for gene flow between species as opposed to alternative explanations such as ancestral polymorphism or homogenization of rDNA intergenic spacers after hybridization (9, 37, 57–59).
Supplementary Material
Acknowledgments.
We thank Nelson Hairston, Michael L. Arnold, Erik Jeppesen, Derek Taylor, Luc De Meester, Barbara Keller, Michael Reid, and Adam Petrusek for their constructive inputs. In particular, we thank 3 anonymous reviewers for comments that substantially improved the manuscript. We are grateful to the undergraduate students of the Ecology and Evolution course 2005 and Gisela Schmiedeskamp for technical assistance and Martin Wessels and Alfred Sulger for sampling of sediment cores. Research was supported by grants from the German Research Foundation (Grant SCHW 830/7–1), the European Science Foundation (Grant 05_EDIV_FP102_BIOPOOL), and the Biodiversity and Climate Research Centre (to K.S.) and a doctoral scholarship from the Hessen State Ministry of Higher Education, Research, and the Arts (to N.B).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. N.G.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807187106/DCSupplemental.
References
- 1.Correll DL. The role of phosphorus in the eutrophication of receiving waters: A review. J Environ Qual. 1998;27:261–266. [Google Scholar]
- 2.Schindler DW. Recent advances in the understanding and management of eutrophication. Limnol Oceanogr. 2006;51:356–363. [Google Scholar]
- 3.Schindler DW. Factors regulating phytoplankton production and standing crop in world's freshwaters. Limnol Oceanogr. 1978;23:478–486. [Google Scholar]
- 4.Hairston NG, Vanbrunt RA, Kearns CM, Engstrom DR. Age and survivorship of diapausing eggs in a sediment egg bank. Ecology. 1995;76:1706–1711. [Google Scholar]
- 5.Schwenk K. Interspecific hybridization in Daphnia: Distinction and origin of hybrid matrilines. Mol Biol Evol. 1993;10:1289–1302. doi: 10.1093/oxfordjournals.molbev.a040076. [DOI] [PubMed] [Google Scholar]
- 6.Limburg PA, Weider LJ. ‘Ancient’ DNA in the resting egg bank of a microcrustacean can serve as a palaeolimnological database. Proc R Soc London Ser B. 2002;269:281–287. doi: 10.1098/rspb.2001.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brendonck L, De Meester L. Egg banks in freshwater zooplankton: Evolutionary and ecological archives in the sediment. Hydrobiologia. 2003;491:65–84. [Google Scholar]
- 8.Hairston NG, et al. Rapid evolution revealed by dormant eggs. Nature. 1999;401:446. [Google Scholar]
- 9.Schwenk K, Spaak P. Evolutionary and ecological consequences of interspecific hybridization in cladocerans. Experientia. 1995;51:465–481. [Google Scholar]
- 10.Hobæk A, Larsson P. Sex determination in Daphnia magna. Ecology. 1990;71:2255–2268. [Google Scholar]
- 11.Keller B, Spaak P. Non-random sexual reproduction and diapausing egg production in a Daphnia hybrid species complex. Limnol Oceanogr. 2004;49:1393–1400. [Google Scholar]
- 12.Jankowski T, Straile D. A comparison of egg-bank and long-term plankton dynamics of two Daphnia species, D hyalina and D galeata: Potentials and limits of reconstruction. Limnol Oceanogr. 2003;48:1948–1955. [Google Scholar]
- 13.Kerfoot WC, Weider LJ. Experimental paleoecology (resurrection ecology): Chasing Van Valen's Red Queen hypothesis. Limnol Oceanogr. 2004;49:1300–1316. [Google Scholar]
- 14.Jeppesen E, et al. Lake responses to reduced nutrient loading—An analysis of contemporary long-term data from 35 case studies. Freshwater Biol. 2005;50:1747–1771. [Google Scholar]
- 15.Weider LJ, Lampert W, Wessels M, Colbourne JK, Limburg P. Long-term genetic shifts in a microcrustacean egg bank associated with anthropogenic changes in the Lake Constance ecosystem. Proc R Soc London Ser B. 1997;264:1613–1618. [Google Scholar]
- 16.Muckle R, Dillmann-Vogel H. Die bisexuelle Fortpflanzung in der Daphnia longispina Gruppe des Bodensee-Obersees. Beiträge zur Naturkundlichen Forschung in Südwestdeutschland. 1976;35:81–94. [Google Scholar]
- 17.Elster HJ, Schwoerbel I. Beiträge zur Biologie und Populationsdynamik der Daphnien im Bodensee. Arch Hydrobiol Suppl Algol Stud. 1970;38:18–72. [Google Scholar]
- 18.Jankowski T, Straile D. Allochronic differentiation among Daphnia species, hybrids and backcrosses: The importance of sexual reproduction for population dynamics and genetic architecture. J Evol Biol. 2004;17:312–321. [PubMed] [Google Scholar]
- 19.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson EC, Thompson EA. A model-based method for identifying species hybrids using multilocus genetic data. Genetics. 2002;160:1217–1229. doi: 10.1093/genetics/160.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewontin RC, Birch LC. Hybridization as a source of variation for adaptation to new environments. Evolution. 1966;20:315–336. doi: 10.1111/j.1558-5646.1966.tb03369.x. [DOI] [PubMed] [Google Scholar]
- 22.Schwenk K, Bijl M, Menken SBJ. Experimental interspecific hybridization in Daphnia. Hydrobiologia. 2001;442:67–73. [Google Scholar]
- 23.Rellstab C. Zürich, Switzerland: ETH Zürich; 2008. Population dynamics and genetic structure of Daphnia in ultra-oligotrophic Lake Brienz. PhD thesis. [Google Scholar]
- 24.Petrusek A, et al. A taxonomic reappraisal of the European Daphnia longispina complex. Zool Scr. 2008:507–519. [Google Scholar]
- 25.Elber F, Hürlimann J, Niederberger K. Entwicklung des Gesamtphosphors im Greifensee anhand der im Sediment eingelagerten Kieselalgen. AWEL, Amt für Abfall, Wasser, Energie und Luft des Kantons Zürich. 2004 Available at http://www.labor.zh.ch/internet/bd/awel/gs/gq/de/doku/doku_seen.html.
- 26.Vakkilainen K, et al. Response of zooplankton to nutrient enrichment and fish in shallow lakes: A pan-European mesocosm experiment. Freshwater Biol. 2004;49:1619–1632. [Google Scholar]
- 27.Kiefer F. Naturkunde des Bodensees. Lindau–Konstanz, Germany: Jan Thorbecke; 1955. [Google Scholar]
- 28.Brede N, Straile D, Streit B, Schwenk K. The contribution of differential hatching success to the fitness of species and interspecific hybrids. Hydrobiologia. 2007;594:83–89. [Google Scholar]
- 29.Kerfoot WC, Robbins JA, Weider LJ. A new approach to historical reconstruction: Combining descriptive and experimental paleolimnology. Limnol Oceanogr. 1999;44:1232–1247. [Google Scholar]
- 30.Jeppesen E, et al. The impact of nutrient state and lake depth on top-down control in the pelagic zone of lakes: A study of 466 lakes from the temperate zone to the arctic. Ecosystems. 2003;6:313–325. [Google Scholar]
- 31.Schwenk K. Utrecht, The Netherlands: University of Utrecht; 1997. Evolutionary genetics of Daphnia species complexes— Hybridism in syntopy. PhD thesis. [Google Scholar]
- 32.Brede N. Frankfurt am Main, Germany: Goethe University Frankfurt am Main; 2008. The reconstruction of evolutionary patterns from Daphnia resting egg banks. PhD thesis. [Google Scholar]
- 33.Hairston NG, De Stasio BD. Rate of evolution slowed by a dormant propagule pool. Nature. 1988;336:239–242. [Google Scholar]
- 34.Decaestecker E, et al. Host-parasite ‘Red Queen’ dynamics archived in pond sediment. Nature. 2007;450:870–U816. doi: 10.1038/nature06291. [DOI] [PubMed] [Google Scholar]
- 35.Hairston NG, et al. Natural selection for grazer resistance to toxic cyanobacteria: Evolution of phenotypic plasticity? Evolution. 2001;55:2203–2214. doi: 10.1111/j.0014-3820.2001.tb00736.x. [DOI] [PubMed] [Google Scholar]
- 36.Currat M, Ruedi M, Petit RJ, Excoffier L. The hidden side of invasions: Massive introgression by local genes. Evolution. 2008;62:1908–1920. doi: 10.1111/j.1558-5646.2008.00413.x. [DOI] [PubMed] [Google Scholar]
- 37.Taylor DJ, Hebert PDN. Habitat-dependent hybrid parentage and differential introgression between neighboringly sympatric Daphnia species. Proc Natl Acad Sci USA. 1993;90:7079–7083. doi: 10.1073/pnas.90.15.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cousyn C, et al. Rapid, local adaptation of zooplankton behavior to changes in predation pressure in the absence of neutral genetic changes. Proc Natl Acad Sci USA. 2001;98:6256–6260. doi: 10.1073/pnas.111606798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roman J, Darling JA. Paradox lost: Genetic diversity and the success of aquatic invasions. Trends Ecol Evol. 2007;22:454–464. doi: 10.1016/j.tree.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 40.De Meester L, Gomez A, Okamura B, Schwenk K. The Monopolization Hypothesis and the dispersal-gene flow paradox in aquatic organisms. Acta Oecol. 2002;23:121–135. [Google Scholar]
- 41.Schwenk K, Posada D, Hebert PDN. Molecular systematics of European Hyalodaphnia: The role of contemporary hybridization in ancient species. Proc R Soc London Ser B. 2000;267:1833–1842. doi: 10.1098/rspb.2000.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seehausen O, Takimoto G, Roy D, Jokela J. Speciation reversal and biodiversity dynamics with hybridization in changing environments. Mol Ecol. 2008;17:30–44. doi: 10.1111/j.1365-294X.2007.03529.x. [DOI] [PubMed] [Google Scholar]
- 43.Guilizzoni P, Lami A, Manca M, Musazzi S, Marchetto A. Palaeoenvironmental changes inferred from biological remains in short lake sediment cores from the Central Alps and Dolomites. Hydrobiologia. 2006;562:167–191. [Google Scholar]
- 44.Manca M, Torretta B, Comoli P, Amsinck S, Jeppensen E. Major changes in trophic dynamics in large, deep sub-alpine Lake Maggiore from 1940s to 2002: A high resolution comparative palaeo-neolimnological study. Freshwater Biol. 2007;52:2256–2269. [Google Scholar]
- 45.Manca M, Armiraglio M. Zooplankton of 15 lakes in the Southern Central Alps: Comparison of recent and past (pre-ca 1850 AD) communities. J Limnol. 2002;61:225–231. [Google Scholar]
- 46.Seidendorf B, Boersma M, Schwenk K. Evolutionary stoichiometry: The role of food quality for clonal differentiation and hybrid maintenance in a Daphnia species complex. Limnol Oceanogr. 2007;52:385–394. [Google Scholar]
- 47.Hessen DO, Faafeng BA, Andersen T. Replacement of herbivore zooplankton species along gradients of ecosystem productivity and fish predation pressure. Can J Fish Aquat Sci. 1995;52:733–742. [Google Scholar]
- 48.Tessier AJ, Leibold MA, Tsao J. A fundamental trade-off in resource exploitation by Daphnia and consequences to plankton communities. Ecology. 2000;81:826–841. [Google Scholar]
- 49.Wessels M, Lenhard A, Giovanoli F, Bollhofer A. High resolution time series of lead and zinc in sediments of Lake Constance. Aquat Sci. 1995;57:291–304. [Google Scholar]
- 50.Lotter AF, Sturm M, Teranes JL, Wehrli B. Varve formation since 1885 and high-resolution varve analyses in hypertrophic Baldeggersee (Switzerland) Aquat Sci. 1997;59:304–325. [Google Scholar]
- 51.Billiones R, Brehm M, Klee J, Schwenk K. Genetic identification of Hyalodaphnia species and interspecific hybrids. Hydrobiologia. 2004;526:43–53. [Google Scholar]
- 52.Schwenk K, et al. Genetic markers, genealogies and biogeographic patterns in the Cladocera. Aquat Ecol. 1998;32:37–51. [Google Scholar]
- 53.Ender A, Schwenk K, Städler T, Streit B, Schierwater B. RAPD identification of microsatellites in Daphnia. Mol Ecol. 1996;5:437–441. [PubMed] [Google Scholar]
- 54.Colbourne JK, Robison B, Bogart K, Lynch M. Five hundred and twenty-eight microsatellite markers for ecological genomic investigations using Daphnia. Mol Ecol Notes. 2004;4:485–490. [Google Scholar]
- 55.Fox JA. New microsatellite primers for Daphnia galeata mendotae. Mol Ecol Notes. 2004;4:544–546. [Google Scholar]
- 56.Skage M, et al. Intra-specific rDNA-ITS restriction site variation and an improved protocol to distinguish species and hybrids in the Daphnia longispina complex. Hydrobiologia. 2007;594:19–32. [Google Scholar]
- 57.Taylor DJ, Sprenger HL, Ishida S. Geographic and phylogenetic evidence for dispersed nuclear introgression in a daphniid with sexual propagules. Mol Ecol. 2005;14:525–537. doi: 10.1111/j.1365-294X.2005.02415.x. [DOI] [PubMed] [Google Scholar]
- 58.Spaak P. Temporal changes in the genetic structure of the Daphnia species complex in Tjeukemeer, with evidence for backcrossing. Heredity. 1996;76:539–548. [Google Scholar]
- 59.Gießler S, Mader E, Schwenk K. Morphological evolution and genetic differentiation in Daphnia species complexes. J Evol Biol. 1999;12:710–723. [Google Scholar]
- 60.Belkhir K, Borsa P, Chikhi L, Raufaste N, Bonhomme F. Montpellier, France: Laboratoire Génome, Populations, Interactions, CNRS UMR 5000, Université de Montpellier II; 1996–2004. GENETIX 4.05, logiciel sous Windows TM pour la génétique des populations. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.