Abstract
Atmospheric aerosol deposition is an important source of nutrients and trace metals to the open ocean that can enhance ocean productivity and carbon sequestration and thus influence atmospheric carbon dioxide concentrations and climate. Using aerosol samples from different back trajectories in incubation experiments with natural communities, we demonstrate that the response of phytoplankton growth to aerosol additions depends on specific components in aerosols and differs across phytoplankton species. Aerosol additions enhanced growth by releasing nitrogen and phosphorus, but not all aerosols stimulated growth. Toxic effects were observed with some aerosols, where the toxicity affected picoeukaryotes and Synechococcus but not Prochlorococcus. We suggest that the toxicity could be due to high copper concentrations in these aerosols and support this by laboratory copper toxicity tests preformed with Synechococcus cultures. However, it is possible that other elements present in the aerosols or unknown synergistic effects between these elements could have also contributed to the toxic effect. Anthropogenic emissions are increasing atmospheric copper deposition sharply, and based on coupled atmosphere–ocean calculations, we show that this deposition can potentially alter patterns of marine primary production and community structure in high aerosol, low chlorophyll areas, particularly in the Bay of Bengal and downwind of South and East Asia.
Laboratory experiments, field observations, and numerical simulations all link atmospheric deposition events to increases in ocean chlorophyll concentrations and phytoplankton biomass (1–3), suggesting that atmospheric deposition of nutrients and trace metals can stimulate phytoplankton growth. Indeed, enrichment experiments with iron (a required nutrient scarce in seawater and enriched in dust) show that in high-nutrient low-chlorophyll areas (representing 20–40% of the ocean), iron addition can increase primary production, export production, and carbon sequestration (4–7). In areas where phosphorus and nitrogen concentrations are low, aerosol deposition can supply both iron and phosphate, nutrients that stimulate nitrogen fixation (8–9). It has been suggested that increases in dust deposition during glacial periods have been responsible for lowering atmospheric carbon dioxide concentrations thus impacting climate (10–12).
Aerosol particles consist of many natural and anthropogenic components, including mineral dust, soot, organic molecules, sea salt crystals, spores, bacteria, and other microscopic particles (13), and can supply many elements and compounds to seawater (14–16). Little research has been done to elucidate what specific component(s) in aerosols affect phytoplankton at the level of community or individual species or how certain taxa within the community respond to distinct aerosol deposition events and to aerosols of different composition.
Results and Discussion
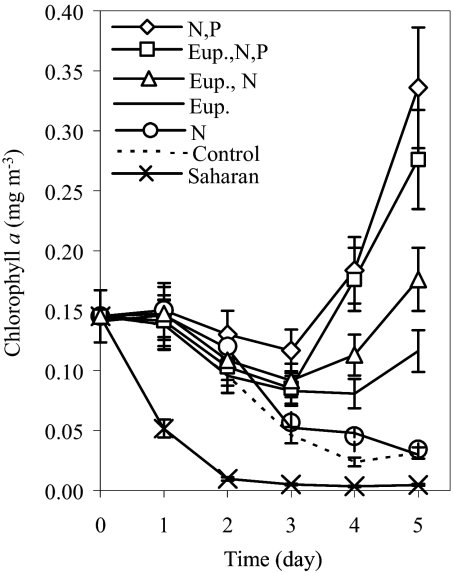
To assess the short-term response of phytoplankton communities to aerosol deposition, we performed bioassay experiments on northern Red Sea surface seawater (17) using locally collected dry deposition aerosol samples that represent the bulk of the deposition in this arid area [see detailed methods in supporting information (SI) Text]. We found that the phytoplankton were under colimitation of nitrogen (N) and phosphorus (P), because N (combined nitrate and ammonium) and P (phosphate) additions on their own did not increase the amount of chlorophyll a (Chl a), whereas there were significant increases in Chl a relative to untreated controls when N and P were added together (P < 0.001). Iron (Fe) concentrations are elevated in these surface waters (18, 19), thus the effect of Fe was not considered. Additions of 6 mg of locally collected aerosols (dry deposition collected on a filter by using a high-volume total-suspended-particle sampler) from the most prevalent (European) air mass source in this area, based on air mass back trajectories (19) along with N and P resulted in increases similar to those with combined N and P but without aerosol (Fig. 1). Treatments with European aerosols without inorganic N and P or with single-nutrient amendment resulted in doubling of Chl a relative to the control (significant increase P = 0.04, Fig. 1). The amount of aerosol added in these incubations (6 mg in 8 L) corresponds to the expected dust input (mg L−1 of surface seawater) from cumulative deposition (mg m−2) over 2 weeks and a mixed layer of 10–20 m (typical dust storms in this region last days to weeks). The response of the phytoplankton to aerosol additions indicates that aerosols were able to supply bioavailable N and P (although at concentrations less than those supplied in the nutrient additions we used) and reverse nutrient limitation of the phytoplankton community (Fig. 1). The N/P ratio in the aerosols was very high (on average N/P = 170) compared with the Redfield ratio typically required by marine phytoplankton (N/P = 16), emphasizing the importance and potential impact of atmospheric deposition as a nitrogen source to the ocean (3, 12, 20).
Fig. 1.
The response of phytoplankton from the Gulf of Aqaba to major-nutrients and aerosol addition monitored over 5 days in bioassay experiments. A 1-way ANOVA indicated that mean Chl a levels differed significantly across treatments, F (6, 17) = 15.4, P < 0.001. Shown are Chl a concentrations relative to control (no additions), combined nitrogen and phosphorus (N, P) addition, European aerosol together with N and P additions (Eup., N, P), European aerosol additions alone (Eup.) or with N addition (Eup., N), singular amendment with only nitrogen (N), and addition of African aerosol (Sahara). Addition of European aerosol combined with phosphate was similar to that of the aerosol with N addition, whereas addition of P alone yielded results similar to the control or addition of N alone. Note that only the addition of African aerosols resulted in lower Chl a compared with the control (in all 3 replicate incubations). Error bars show standard error of the mean of 3 replicate samples.
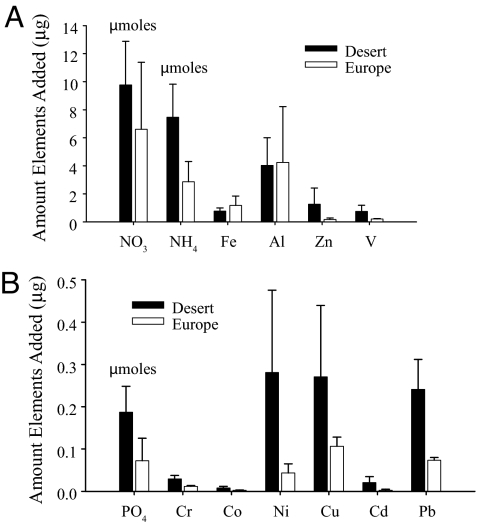
We also compared aerosols of African (Sahara Desert) trajectory to the more common European aerosol (Fig. 1). We use the same amount of aerosol as for the European aerosol additions that stimulated growth (6 mg in 8 L). The concentrations of soluble nutrients and trace metals added from these aerosols to the bioassay incubations were determined by measuring the total amount (micrograms or micromoles) of each component released to seawater from the aerosol filters after adding the filter to the incubation vessel. Fig. 2 shows the elements and compounds released from aerosols that were significantly different between the European and African aerosols. Although the African aerosol released more soluble nitrate, ammonium, and phosphate than aerosols from European origin (Fig. 2), we nevertheless observed sharp declines in Chl a in all treatments (3 replicates) receiving African aerosols. We concluded that the lack of growth after African aerosol addition may have been a result of toxic effects of one or more of the trace metals added to solution from the aerosol. Because of the high concentrations of copper (Cu) added from the African aerosols compared with the European aerosols (Fig. 2) and the well-documented negative effects of Cu on phytoplankton (21), we tested whether Cu caused the observed toxicity in our bioassay samples. Our results support this hypothesis (see below); however, the possibility that other elements present in the aerosols or unknown synergistic effects between these elements could have also contributed to the toxic effect cannot be ruled out.
Fig. 2.
Nutrient and trace metal contribution from aerosols of different origin. Enrichment of trace element and nutrient concentrations from the addition of African (Sahara Desert) aerosols (black bars) and European aerosols (white bars) collected locally at the Gulf of Aqaba. These amounts (micrograms or micromoles) were released to the incubation bottles from the addition of 6 mg of African aerosols (AD), or 6 mg of European aerosols (ED). Note that each incubation received a distinct aerosol filter collected simultaneously (triplicate treatments were not homogenized), resulting in some variability between triplicates. The top of the wide bar for each component represent the replicate with the lowest measured concentration, and the top of the thin line represents the highest concentration measured (e.g., the full range is represented not errors). Concentrations were measured as described in ref. 19. We use the lower value of Cu in the African dust (top of the broad black bar) for calculating the threshold concentration that corresponds to the chlorophyll levels in the Gulf. The lowest Cu concentration in the African aerosols is ≈3 times higher than the highest in the European aerosols. The amount of Cu added from the aerosol resulted in a 2-fold increase compared with the ambient Cu concentrations in Gulf of Aqaba surface water (which are similar to Atlantic surface water concentrations).
To determine the threshold Cu toxicity level and compare it to that calculated from our bioassays, we preformed serial Cu addition (Cu standard prepared from high-purity metal Cu dissolved in 2% nitric acid—1,000 mg/L; Sigma–Aldrich) experiments in the laboratory using Synechococcus WH8102 (see SI Text). It is important to note that we measured total Cu in all of our experiments (Fig. 2, Fig. S1) and used total Cu in the model results (Fig. 4 C and D and Fig. S2), yet toxicity does not depend on the total Cu added but rather on free (noncomplexed) Cu concentrations (22). However, in the open ocean, free Cu levels are controlled primarily by Cu-binding organic ligands released by organisms (22, 23). Accordingly, to account for variable levels of organic ligands in solution (and thus the free non-ligand-bound Cu), we report (Fig. S1 and Fig. 4 C and D) total Cu concentrations normalized to Chl a (a proxy for biomass and proportional to organic ligand levels); such normalization also allows for global extrapolation of our results, because data on free Cu concentration in surface seawater is sparse. The observed threshold toxicity in our laboratory experiments was between 0.2 and 2 μg of Cu per microgram of Chl a for this organism. The toxicity response was rapid, depressing Fv/Fm measurements (a measure of photosynthetic capacity) within 1 h and impairing cell growth within 24 h of Cu addition (see SI Text). These results are consistent with our field observations (toxicity at 0.4 μg of Cu per μg Chl a apparent within the first day of incubation) and with published results from the Sargasso Sea and other culture experiments (24, 25). Similar experiments with nickel (Ni) or lead (Pb) (which were also higher in the African aerosols) did not result in similar toxicity responses as were seen with the field incubations.
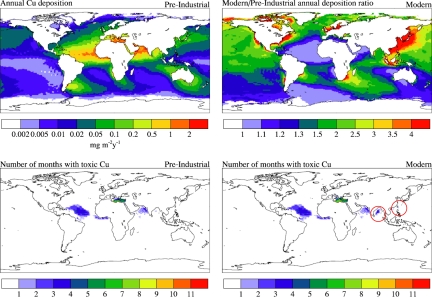
Fig. 4.
Global spatial patterns of copper deposition and potential toxicity impacts on phytoplankton. (Upper) Annual preindustrial aerosol Cu deposition fields (Left) and modern to preindustrial annual Cu deposition ratios (Right) throughout the world oceans. (Lower) Regions with potential high (toxic) Cu levels relative to chlorophyll identified by the number of months with Cu/Chl above the toxicity threshold during preindustrial (Left) and modern (Right) times. Red circles show the areas of increased toxicity in the modern ocean compared with the preindustrial case.
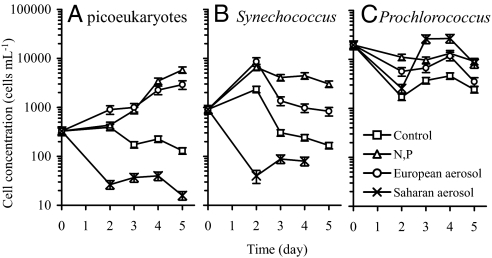
Flow cytometry measurements (Fig. 3) show that picoeukaryote algae (Fig. 3A) and cyanobacterial populations of Synechococcus (Fig. 3B) and Prochlorococcus (Fig. 3C) did not respond uniformly to aerosol treatments in the Red Sea incubation experiment. Picoeukaryotes showed a large increase in cell numbers when enriched with 6 mg of European aerosol, or N and P together. Enrichment with 6 mg of African aerosol caused a marked decrease in picoeukaryote cell numbers despite having provided fertilizing levels of N and P (Fig. 2). Synechococcus abundances were likewise greater than in the control in treatments receiving European aerosol or N and P together. Like the picoeukaryotes, African dust led to a sharp decline in Synechococcus cell number. Prochlorococcus showed no large changes in cell number with the different treatments, regardless of the aerosol's origin. Our results suggest that genetic and physiological properties of individual taxa within the community result in markedly divergent responses to aerosol additions. In contrast to strains from the Atlantic (24), the ecotype of Prochlorococcus present in the Gulf at the time of our sampling appears to be less sensitive to metal toxicity, and this could contribute to the overall ubiquitous global distribution of Prochlorococcus worldwide (26).
Fig. 3.
Variable response of local phytoplankton taxa to nutrient and aerosol additions. The response of picoeukaryotes (A), Synechoccocus (B), and Prochlorococcus (C) from surface seawater in the Gulf to aerosol additions. Note that log scales are used in these plots. Error bars denote 90% confidence intervals. Data for day 5 for Synechococcus is not shown in the figure because the levels crashed to zero (i.e., all cells were dead) by day 5, and it is not possible to show a value of zero on the log scale we have used here.
Collectively our results indicated that: (i) Aerosol deposition can contribute substantial amounts of N and P to the ocean and may enhance phytoplankton growth (Fig. 1); (ii) aerosols arriving to the northern Red Sea along different trajectories differ in their chemical characteristics (Fig. 2); (iii) aerosols from different sources induce considerably different responses of phytoplankton biomass including fertilization as well as adverse toxic effects (Fig. 1); and (iv) different phytoplankton taxa respond differently to aerosol additions; thus, phytoplankton community structure may be affected by aerosol additions (Fig. 3).
To investigate the global implications of atmospheric Cu deposition, we estimated preindustrial and contemporary aerosol Cu deposition fields by using available observations and a 3-dimensional atmospheric tracer transport model (Fig. 4A) (see SI Text). This calculation is based on an extensive dataset for Cu concentrations in aerosols (not just our data), global aerosol distributions, and aerosol deposition rates. We note that the model results represent a minimum Cu deposition value because they take into account only dry deposition. This is a first attempt to estimate global Cu deposition, and thus our deposition fluxes must be considered tentative. However, the estimated dust concentrations as well as the fraction of Cu from combustion match available atmospheric observations across a wide range of environments and concentration values. Consistent with our Red Sea observations, most of the estimated Cu deposition comes from desert dust (65%), but anthropogenic sources of Cu account for ≈30%. Because much of the anthropogenic Cu is emitted by combustion (also see Fig. S3 and Table S1), especially in industrial processes, we estimate a large increase in Cu deposition to oceans far from desert regions in the current era relative to the preindustrial (Fig. 4B). Using this Cu deposition model, we identify regions with potential high (toxic) Cu levels relative to chlorophyll by comparing the monthly modeled atmospheric Cu deposition fields with monthly climatological satellite-derived surface chlorophyll fields from the SeaWiFS (Sea-viewing Wide Field-of-view Sensor) satellite ocean-color instrument (27). The calculation results do not depend directly in any way on the amount of aerosol used in our incubation experiments but, rather, takes into account atmospheric aerosol loads, aerosol Cu data, aerosol Cu solubility, and the toxicity threshold for Cu (identified in this study and consistent with previous data reported by others). We use aerosol Cu solubility of 40% [this is typical for aerosols from many locations and lower than the average Cu solubility for the aerosols we collected (28)], estimate surface ocean Cu concentrations by using a seasonally varying mixed layer depth, and assume a range of published Cu or other metal removal time scales ranging from 1 to 5 years (29–31). Under preindustrial conditions, potential Cu toxicity effects are concentrated in the northern hemisphere downwind of natural dust sources in low-chlorophyll subtropics in the Atlantic, Mediterranean and Indian Ocean basins (Fig. 4C; using an intermediate time scale of 3 years). Anthropogenic emissions expand the potential toxicity zones into the Bay of Bengal and small areas in the west Pacific downwind of Asian industrial regions (Fig. 4D). We note that if natural phytoplankton communities in the world's ocean are more sensitive to Cu toxicity than the Gulf of Aqaba populations, our model results may underestimate the actual area that may be impacted by Cu toxicity.
Conclusions
The unique response of different phytoplankton to aerosols of different origin and chemical composition and our model results collectively illustrate the variable and globally significant impacts of aerosols on marine phytoplankton. Specifically, we report here a negative effect of aerosols in the open ocean in contrast to multiple examples of negative effects on land. Many climate models, however, assume that aerosol deposition is equivalent to Fe and/or P enrichment, which, in turn, uniformly stimulates phytoplankton growth across all taxonomic groups. Our work demonstrated that this is an incorrect oversimplification of the effects of aerosols, and more detailed and specific aerosol composition should be considered, as is done here. Moreover, the selective response of different taxa to aerosol additions demonstrates that aerosol deposition results in changes in phytoplankton community composition. On a local scale, these phytoplankton community shifts may affect grazing by higher trophic levels, thereby potentially impacting marine fisheries in coastal communities. Such changes may also directly affect the amount of export production, because species-dependent cell size, density, and aggregation potentially affect sinking rates. Predicted changes in dust deposition globally, from the present to the end of the century, range from a 300% increase (32) to a 60% decrease (33, 34). The complex mutual interactions between phytoplankton, atmospheric chemistry, and climate are important in view of predicted changes in aerosols deposition rates and distribution and the possible increase in future anthropogenic copper emissions. Accordingly, to predict the impacts of expected future changes in aerosol deposition, global climate change models should incorporate the variable effects of aerosol on the marine ecosystem (including negative toxic effects) and the complex interactions between aerosols and marine phytoplankton of different taxa (2, 35).
Methods
Bioassay Incubation Experiments.
Incubation experiments with natural phytoplankton assemblages took place in the Gulf of Aqaba in the northern Red Sea, an oligo- to mesotrophic marine ecosystem with significant aerosol deposition rates (17, 19). The nutrient concentrations in these waters during the stratified season when our experiment was conducted were very low—nitrate ≈0.2 μM and soluble reactive phosphate (SRP) ≈0.02 μM. Trace metals in the surface layer are high compared with open ocean conditions (Cu, 1.7 nM; Fe, 2.1 nM; Zn, 1.1 nM; Pb, 0.05 nM). However, because all of the treatment started with the same seawater composition, and we are comparing differences relative to control, these concentrations are not very important. Incubations were done in clear (acid and sample washed) polyethylene 10-L cubitainers (Fold-A-Carrier; Reliance). Screening material was used to attenuate the sunlight intensity reaching the containers; 50% light attenuation yielded maximum midday irradiance of ≈1,000 μmol m−2 s−1 and was equivalent to the upper 10 m of the euphotic zone of the Gulf during summer months. We used concentrations of 7 μM N (combined nitrate and ammonium) and 0.6 μM P (as phosphate) that are representative of the deep-water concentrations in the Gulf. In each experiment, a similar amendment of N and/or P was used across all treatments. Where no nutrient additions were made, the nutrients were just from the seawater (control) or from the aerosol sources dissolving into the seawater. Concentrations released from aerosols were typically lower than our additions (inducing less growth) (see Fig. 2). We added ≈6 mg of locally collected aerosol that is equivalent to ≈2 weeks of deposition during dust storms in this area in a mixed layer of 10–20 m. Similar amounts of aerosol particles (≈6 mg) were added to each experiment from the aerosol collection filters regardless of aerosol source (based on back trajectories). Additions were made into 8 L of 100-μm filtered surface seawater. Incubations were placed in a pond with surface seawater flowing through under ambient light and temperature conditions and were over a 5-day period. We monitored the response of bulk phytoplankton using Chl a concentrations and assessed the impact on specific phytoplankton taxa using flow cytometry as described in ref. 17. For more detail see SI Text.
Toxicity Bioassays.
Serial copper (Cu standard prepared from high-purity metal Cu dissolved in 2% nitric acid—1,000 mg/L; Sigma–Aldrich) addition incubation experiments were done in the laboratory under controlled trace metal clean conditions by using Synechococcus WH8102 to determine the threshold toxicity. Control treatments with 2% nitric acid (no Cu) were included. Cells were grown (in triplicate) in F/2 medium at 20 μmol quanta m−2 s−1. We added 0.003–300 μg of total Cu L−1 (using 10-fold concentration increments) to log-phase Synechococcus cultures. Measurements of Chl a, OD750, and Fv/Fm were taken before Cu additions and at 1, 25, 68, and 93 h after Cu additions. Chl a was also measured 48 h and 5 days after Cu additions (see SI Text). Note that we observe a threshold response. Once the Cu concentration exceeds the chelating capacity and the free-Cu concentration is above the toxic level, the cells die; thus, additional Cu does not result in additional negative response. Similar experiments with Ni or Pb did not cause toxic effects at concentrations similar to those seen in the aerosol field bioassays (higher concentrations and/or longer exposure times were needed). For more detail see SI Text.
Copper Deposition.
Cu-deposition fields are based on Cu concentrations derived from atmospheric aerosol composition datasets and compared with available observations over land and ocean regions (see SI Text). Based on observational relationships between Cu and other elements, we model fossil fuel, biofuel, biomass burning, dust, and primary biogenic particle contributions to atmospheric Cu. These estimates suggest that Cu in mineral dust dominates the global budget of Cu deposited to the oceans (65%) but that anthropogenic sources of Cu are important away from the desert regions. Assuming that 90% of biomass burning and 100% of fossil fuel and biofuel comes from anthropogenic sources, we estimate a large increase in Cu deposition to oceans away from desert regions in the current climate relative to the preindustrial (SI Text). Here, we ignore potentially large, but poorly constrained, changes in desert dust itself in response to humans (31). Although these results are preliminary, they represent the best state of our knowledge about Cu deposition globally. For more detail see SI Text.
Supplementary Material
Acknowledgments.
We thank our colleagues at the Interuniversity Institute for Marine Science in Eilat, Israel, for assisting in data collection and providing laboratory space and equipment during the study; P. Artaxo, G. Bergametti, D. Cohen, N. Kubilay, W. Maenhut, and J. Hand of the IMPROVE network for contributing data for the Cu deposition estimates; and T. Bond and C. Luo for contributing atmospheric deposition model results. This work was supported by National Aeronautics and Space Agency (NASA) New Investigator Program Grant NAG5-1266 (to A.P.) and North Atlantic Treaty Organization Science for Peace Grant SfP 982161 (to A.P. and A.F.P.). K.R.M.M. was supported through the National Science Foundation (NSF) Graduate Research Fellowship Program and the U.S. Department of Energy Global Change Education Program. S.C.D., I.D.L., and N.M. were supported in part by NASA Grant NNG06G127G and the NSF-sponsored Center for Microbial Oceanography Research and Education (C-MORE; NSF CCF-424599), and N.M. was supported in part by NSF Grant ATM-0758369.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811486106/DCSupplemental.
References
- 1.Fung IY, et al. Iron supply and demand in the upper ocean. Global Biogeochem Cycles. 2000;14:281–295. [Google Scholar]
- 2.Jickells TD, et al. Global iron connections between desert dust, ocean biogeochemistry, and climate. Science. 2005;308:67–71. doi: 10.1126/science.1105959. [DOI] [PubMed] [Google Scholar]
- 3.Doney SC, et al. Impact of anthropogenic atmospheric nitrogen and sulfur deposition on ocean acidification and the inorganic carbon system. Proc Natl Acad Sci USA. 2007;104:14580–14585. doi: 10.1073/pnas.0702218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd PW, et al. Mesoscale iron enrichment experiments 1993–2005: Synthesis and future directions. Science. 2007;315:612–617. doi: 10.1126/science.1131669. [DOI] [PubMed] [Google Scholar]
- 5.Bishop JKB, Davis RE, Sherman JT. Robotic observations of enhanced carbon biomass and export at 55°S during SOFEX. Science. 2002;298:817–821. doi: 10.1126/science.1087717. [DOI] [PubMed] [Google Scholar]
- 6.Erickson DJ, III, et al. Atmospheric iron delivery and surface ocean biological activity in the Southern Ocean and Patagonian region. Geophys Res Lett. 2003;30:1609–1613. [Google Scholar]
- 7.Buesseler KO, Andrews JE, Pike SM, Charette MA. The effects of iron fertilization on carbon sequestration in the Southern Ocean. Science. 2004;304:414–417. doi: 10.1126/science.1086895. [DOI] [PubMed] [Google Scholar]
- 8.Mills MM, Ridame C, Davey M, La Roche J, Geider RJ. Iron and phosphorus co-limit nitrogen fixation in the eastern tropical North Atlantic. Nature. 2004;429:292–294. doi: 10.1038/nature02550. [DOI] [PubMed] [Google Scholar]
- 9.Gruber N, Sarmiento JL. Global patterns of marine nitrogen fixation and denitrification. Global Biogeochem Cycles. 1997;11:235–266. [Google Scholar]
- 10.Martin JH. Glacial-interglacial CO2 change: The iron hypothesis. Paleoceanography. 1990;5:1–13. [Google Scholar]
- 11.Kohfeld KE, Le Quéré C, Harrison S P, Anderson RF. Role of marine biology for glacial–interglacial CO2 cycles. Science. 2005;308:74–78. doi: 10.1126/science.1105375. [DOI] [PubMed] [Google Scholar]
- 12.Moore JK, Doney SC, Lindsay K, Mahowald N, Michaels AF. Nitrogen fixation amplifies the ocean biogeochemical response to decadal timescale variations in mineral dust deposition. Tellus. 2006;58B:560–572. [Google Scholar]
- 13.Duce RA. Aerosols. In: Oliver JE, editor. Encyclopedia of World Climates. Dordrecht, The Netherlands: Kluwer; 2005. pp. 4–6. [Google Scholar]
- 14.Baker AR, Jickells TD, Witt M, Linge KL. Trends in the solubility of iron, aluminium, manganese and phosphorus in aerosol collected over the Atlantic Ocean. Mar Chem. 2006;98:43–58. [Google Scholar]
- 15.Sedwick PN, Sholkovitz ER, Church TM. Impact of anthropogenic combustion emissions on the fractional solubility of aerosol iron: Evidence from the Sargasso Sea. Geochem Geophys Geosyst. 2007;8:Q10Q06. doi: 10.1029/2007GC001586. [DOI] [Google Scholar]
- 16.Baker AR, et al. Dry and wet deposition of nutrients from the tropical Atlantic atmosphere: Links to primary productivity and nitrogen fixation. Deep Sea Res (Part I) 2007;54:1704–1720. [Google Scholar]
- 17.Mackey KRM, et al. Phosphorus availability, phytoplankton community dynamics, and taxon specific phosphorus status in the Gulf of Aqaba, Red Sea. Limnol Oceanogr. 2007;52:873–885. [Google Scholar]
- 18.Chase Z, Paytan A, Johnson KS, Street J, Chen Y. Input and cycling of iron in the Gulf of Aqaba, Red Sea. Global Biogeochem Cycles. 2006;20:GB3017. [Google Scholar]
- 19.Chen Y, et al. Sources and fluxes of atmospheric trace elements to the Gulf of Aqaba, Red Sea. J Geophys Res. 2008;113:D05306. [Google Scholar]
- 20.Krishnamurthy A., Moore JK, Zender CS, Luo C. Effects of atmospheric inorganic nitrogen deposition on ocean biogeochemistry. J Geophys Res. 2007;112:G02019. doi: 10.1029/2006JG000334. [DOI] [Google Scholar]
- 21.Nayar S, Goh BPL, Chou LM. Environmental impact of heavy metals from dredged and re-suspended sediments on phytoplankton and bacteria assessed in in-situ mesocosms. Ecotoxicol Environ Saf. 2004;59:349–369. doi: 10.1016/j.ecoenv.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Sunda WG, Huntsman SA. Processes regulating cellular metal accumulation and physiological effects: Phytoplankton as model systems. Sci Total Environ. 1998;219:165–181. [Google Scholar]
- 23.Morel MMF, Price NM. The biogeochemical cycles of trace metals in the ocean. Science. 2003;300:944–947. doi: 10.1126/science.1083545. [DOI] [PubMed] [Google Scholar]
- 24.Mann EL, Ahlgren N, Moffett JW, Chisholm SW. Copper toxicity and cyanobacteria ecology in the Sargasso Sea. Limnol Oceanogr. 2002;47:976–988. [Google Scholar]
- 25.Moffett JW, Brand LE. Production of strong, extracellular Cu chelators by marine cyanobacteria in response to Cu stress. Limnol Oceanogr. 1996;41:873–885. [Google Scholar]
- 26.Moffett JW, Brand LE, Croot PL, Barbeau K. Cu speciation and cyanobacterial distribution in harbors subject to anthropogenic Cu inputs. Limnol Oceanogr. 1997;42:789–799. [Google Scholar]
- 27.McClain CR, Feldman GC, Hooker SB. An overview of the SeaWiFS project and strategies for producing a climate research quality global ocean bio-optical time series. Deep Sea Res II. 2004;51:5–42. [Google Scholar]
- 28.Chester RK, et al. Factors controlling the solubilities of trace metals from non-remote aerosols to the sea surface by the ‘dry’ deposition mode. Mar Chem. 1993;42:107–126. [Google Scholar]
- 29.Han Q, Moore JK, Zender C, Measures C, Hydes D. Constraining oceanic dust deposition using surface ocean dissolved Al. Global Biogeochem Cycles. 2008;22:GB2003. doi: 10.1029/2007GB002975. [DOI] [Google Scholar]
- 30.Measures CI, Vink S. On the use of dissolved aluminium in surface waters to estimate dust deposition to the ocean. Global Biogeochem Cycles. 2000;14:317–327. [Google Scholar]
- 31.Moore JK, Braucher O. Sedimentary and mineral dust sources of dissolved iron to the world ocean. Biogeosciences. 2008;5:631–656. [Google Scholar]
- 32.Woodward S, Roberts D, Betts R. A simulation of the effect of climate change-induced desertification on mineral dust aerosol. Geophys Res Lett. 2005;32:GL023482. [Google Scholar]
- 33.Mahowald N, Luo C. A less dusty future? Geophys Res Lett. 2003;30:GL017880. [Google Scholar]
- 34.Tegen M, Werner S, Harrison P, Kohfeld KE. Relative importance of climate and land use in determining present and future global soil dust emission. Geophys Res Lett. 2004;31:L05105. [Google Scholar]
- 35.Malin G. Oceans—New pieces for the marine sulfur cycle jigsaw. Science. 2006;314:607–608. doi: 10.1126/science.1133279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.