Abstract
Many species that engage in parental behavior exhibit infanticide under certain circumstances. The neural signals regulating the transition from infant care giver to infant killer and back remain unclear. Previously we demonstrated that progesterone (P) and its receptor (PR) have inhibitory effects on parental behavior and increase infant-directed aggression in male mice. In the present studies we sought to elucidate the mechanisms by which the effects of P are manifested. Because the onset of parental behavior in females is associated with the withdrawal of P at the end of pregnancy we tested the hypothesis that withdrawal of P would similarly enhance parental behavior in males. Virgin male mice were implanted with P or vehicle for 21 days, replicating the duration of pregnancy in females. Tests were run for parental and infanticidal behavior 5 days after removal of the capsules. P increased the proportion of nonparental males that attacked pups. However, neither the number of males exhibiting parental care nor the quality of care was affected by P treatment. Serum P and testosterone (T) levels were not different from controls at the time of behavioral testing indicating continued elevations in peripheral hormones are not required for the expression of infanticide. In conclusion, withdrawal of P does not trigger the onset of parental behavior in males. Rather, prior exposure to P induces persistent infanticidal behavior in adult male mice.
Keywords: Progesterone, Infanticide, Paternal behavior, Testosterone, Parental Behavior
Introduction
Male mammals display a wide range of complex social behaviors toward young that can range from aggression to indifference to parental care [10,16,37]. Parental care is rare among animals and is estimated to be present in approximately 10% of mammalian species [18] and only 6% of rodent species [9]. In addition to a lack of parental care, male mammals may also exhibit extreme aggression towards infants resulting in infanticide. Initially characterized as maladaptive, some studies have concluded that infanticide can increase a male’s reproductive success and is therefore an adaptive behavior [14,44]. For many species in which individual males engage in high levels of parental behavior at one time the same male will, at another stage of life, exhibit aggression towards young. The frequency of infanticide expressed by individuals of a species can be influenced by a number of factors including: prenatal hormone exposure, prenatal stress, age, sex, previous sexual experience, previous exposure to young and previous social experience [4,10,11,13,16,20,23–25,28,43]. For example, many individuals of the highly parental California mouse (Peromyscus californicus) exhibit infanticide when tested prior to mating, but exhibit parental behavior subsequent to mating and the birth of their own pups [12,13]. However, the neural and endocrine mechanisms responsible for these changes in behavior are unknown.
Early reports on the proximate causes of infanticide provide confusing and often conflicting results [19]. However, a consensus points to a role for both the organizational and activational effects of hormonal stimuli as proximate mechanisms regulating the probability a male will engage in infanticidal behavior [21,36,40,42,43]. Reproductive steroids, primarily estrogen and testosterone (T), have long been known to exert organizational effects during fetal and early postnatal life as well as activational effects in adulthood [29]. It has also been proposed that some of the sex differences in the brain enable the expression of similar behaviors, despite different hormonal environments [8].
Traditionally considered a hormone that controls female mating behavior and physiology, progesterone (P) inhibits parental behavior in female rodents [39]. In many mammals, the withdrawal of P at the end of pregnancy provides a permissive environment for the expression of maternal behavior [27]. Recent work has suggested that P may also influence male reproductive behaviors [36,45,47]. Male California mice that are fathers have lower P levels than do males that are not [40]. However, data from two species of dwarf hamster (Phodopus) suggest that the emergence of paternal behavior is not associated with a change in circulating levels of P [38].Thus there may be phylogenetic variation in the role of P in the mediation of these behaviors.
Using male progesterone receptor knockout mice (PRKO) we have demonstrated an important role for P and progesterone receptor (PR) in the expression of infanticide and the suppression of parental behavior [37]. Mated PRKO males exhibit no infanticidal behavior and virgins display little aggression towards young. Furthermore, in wild-type mice P treatment increases and blockade of PR decreases aggressive tendencies toward young [37]. P is also likely to be involved in the inhibition of parental care as PRKO males exhibit high levels of direct parental care [37].
In the current study we tested the hypothesis that withdrawal of P would reduce infanticidal behavior and induce parental behavior in male mice. We designed the current study to examine the effects of P exposure over a time frame that mimicked the duration of pregnancy in females and the emergence of parental behavior after mating in males.
Material and Methods
Animals
All surgical and behavioral experimental procedures were conducted with the approval of the Northwestern University Animal Care and Use Committee. Male offspring from established breeding pairs of C57BL/6 mice (Charles River Laboratories, Inc., Wilmington, MA) were weaned at three weeks and caged alone from that time throughout the duration of the experiment. All animals were 8–10 weeks old at the onset of testing. Animals were housed in a 14 h light:10 h dark (lights on 0500–1900h) light cycle. Food and water were available ad libitum.
Progesterone Administration
Virgin males were implanted with a Silastic capsule containing P (25 mg/ml) suspended in sesame oil vehicle (Sigma, St. Louis, MO) or control capsules containing vehicle. This concentration delivers a physiological dose of P in mice for at least 28 days [26]. Silastic medical-grade tubing (0.04” ID, 0.085” OD; American Scientific Products, McGaw Park, IL) was cut into segments and one end was sealed with Silicone type A medical adhesive (Dow Corning, Midland, MI). After the adhesive had dried the capsules were filled to a length of 1 cm with either P or vehicle and the remaining end sealed. Capsules were allowed to cure overnight before implantation. Males were anesthetized with isofluorane prior to insertion of the capsules under the skin.
The current study was designed to examine the behavior of males over a time frame that mimicked the duration of pregnancy, the onset of maternal behavior in females and is consistent with the onset of paternal behavior in males following mating. The capsules were left in place for 21 days and then removed under anesthesia. Behavioral tests were conducted on all P treated (n=19) or control (n=20) males 5 days after removal of the capsules. We chose to test behavior 5 days after hormone withdrawal to mimic the timing of the early post-partum period when both parents would be expected to exhibit parental behavior. Blood was collected immediately after testing and plasma frozen for later radioimmunoassay (RIA).
Aggression and Parental Behavior Tests
On the day before testing all males were given cotton and allowed to build a nest overnight. Mice were moved to the testing room the next day and allowed to acclimate at least 1 hour. A pup, aged 3–7 days, was placed in the farthest corner from the nest and behavior of the male towards the pup was observed for 10 minutes (600 seconds). If pups were attacked the test was stopped immediately and the pup removed, otherwise the latency to contact the pup, latency to pick up the pup (if applicable), and the time to retrieve the pup to the nest were recorded. If a male failed to exhibit a behavior within the test period, a latency of 600 sec was assigned. The presence or absence of the following behaviors was recorded during the observation period: contacted a pup, picked up a pup, retrieved the pup to the nest, and nurtured the pup in the nest (e.g. licking or crouching continuously over the pup for at least two minutes). To compare the present data with previous studies, a parental behavior index was calculated [37]. The index score was calculated by awarding points in the following manner: +1 for contacting pup, +1 for picking up pup, +4 for retrieving pup to nest, +1 for nurturing behaviors. A perfect score of +7 indicates the highest level of parental care; a score of 0 indicates no parental behavior [17]. Because contact and pick-up behavior may represent investigatory behavior rather than parental behavior and have been shown to persist in females in which maternal behavior had been disrupted by knocking-out the prolactin receptor, retrieval of a pup to the nest was given a heavier weighting than the other behaviors. During the observations it was noted that males that failed to retrieve pups (termed nonparental) exhibited two different types of behavior- some males attacked while others ignored the pups. All behavioral tests were scored by an observer blind to treatment group.
Hormone Measurement
Immediately following the behavior test mice were deeply anesthetized via isofluorane inhalation and a terminal blood sample collected by cardiac puncture. Blood was heparinized and centrifuged. Plasma was frozen at −20C for later measurements of hormones by RIA using the Immunchem Progesterone or Testosterone 125I kits (ICN Pharmaceuticals, Costa Mesa, CA). All samples for each hormone were measured in a single assay to eliminate interassay variability. Intraassay CV for T was 8.7% and 4% (high pool) and 8.0% (low pool) for P.
Statistical Analyses
The Chi2 was used to test for differences among groups in the proportions of males exhibiting behaviors that were recorded as categorical data (presence or absence of a specified behavior). Scores of the behavioral index or latencies were compared using student’s t-tests. Plasma hormone levels were compared using student’s t-test and one-way analysis of variance (ANOVA) as appropriate for each analysis. Statistical analyses were computed using GraphPad Prism (ver 3.02; GraphPad Software, San Diego, CA) or Number Cruncher Statistical System (NCSS 2004, Kaysville, UT).
Results
Effect of Progesterone Treatment on Parental Behavior
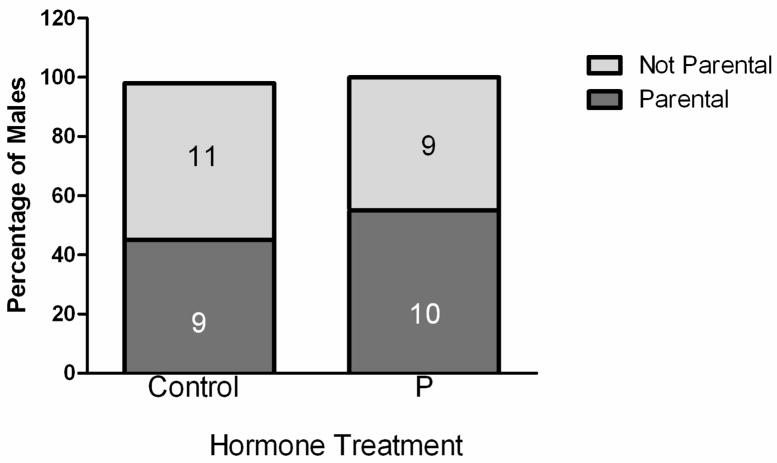
Five days after hormone withdrawal, approximately 50% of the males from both treatment groups exhibited parental behavior spontaneously with 9/20 control and 9/17 P-treated males retrieving the pup (Figure 1). The latencies to contact a pup, pick-up a pup, or retrieve the pup to the nest were calculated for all males; there were no differences among groups (data not shown). The quality of parental behavior as estimated by the Parental Behavior Index Score was determined for those males that exhibited parental behavior. Chronic P treatment had no effect on the quality of parental care as measured by the Parental Behavior Index Scores of males towards unfamiliar pups (3.6 ± 0.7 (C) vs 4 ± 0.7 (P)).
Figure 1.
Proportions of males exhibiting Parental or Nonparental behavior. Approximately fifty percent of adult, virgin, male C57Bl/6 mice exhibited parental behavior towards an unfamiliar infant. The propensity to express parental behavior was not altered by prior treatment with progesterone either 5 days ( χ2 test, p = 0.76) after removal of the hormone capsule. Data are presented as percentage of each group; numbers inside each bar are the numbers of individuals exhibiting each type of behavior.
Effect of Progesterone Treatment on Attack Behavior
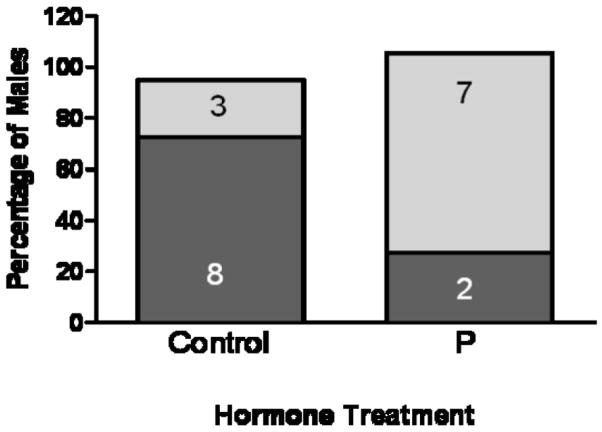
Nonparental males (those that did not retrieve pups) exhibited two distinct behaviors-some males ignored while others attacked the young; prior exposure to P significantly increased the propensity of males to attack. Males treated with P were more likely to attack young (7 of 9; 78%) than control males (3 of 8; 27.3%) (Fig. 2; F = 5.05, p < 0.02).
Figure 2.
Percentage of Nonparental males that ignored or attacked young. Prior treatment with progesterone significantly increases aggression towards young in these Nonparental males. Five days after capsule withdrawal the majority of the subgroup of Control males that were Nonparental ignored the pup (8 of 11); in contrast, the majority of P-treated males that were Nonparental (7 of 9) attacked pups (χ2 test, p<0.02). Data are presented as the percentage of males in each treatment group expressing each type of behavior. Numbers inside each bar are the numbers of individuals expressing each type of behavior.
Hormone-behavior Associations
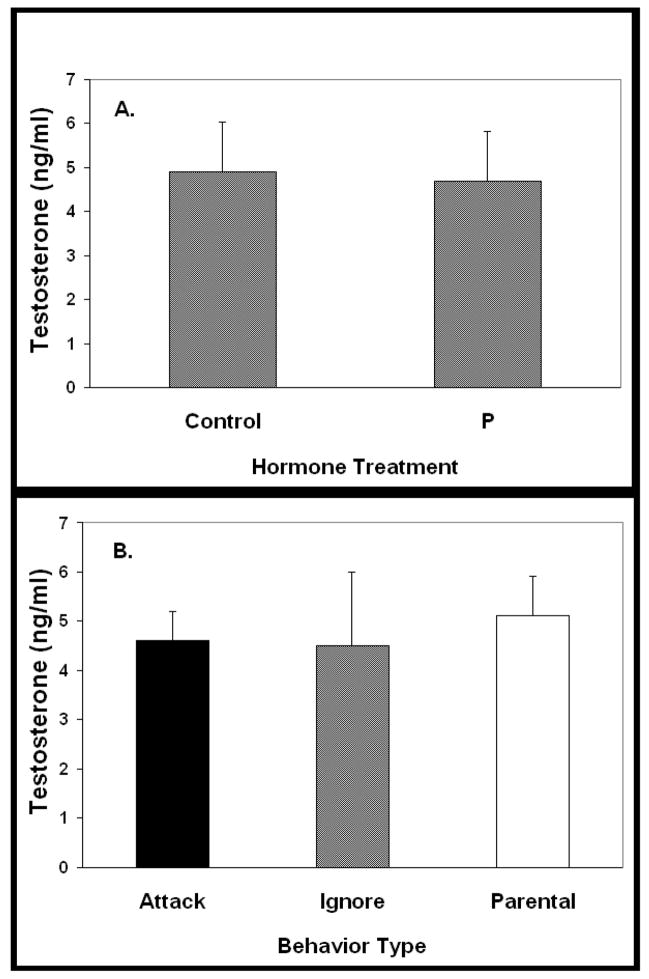
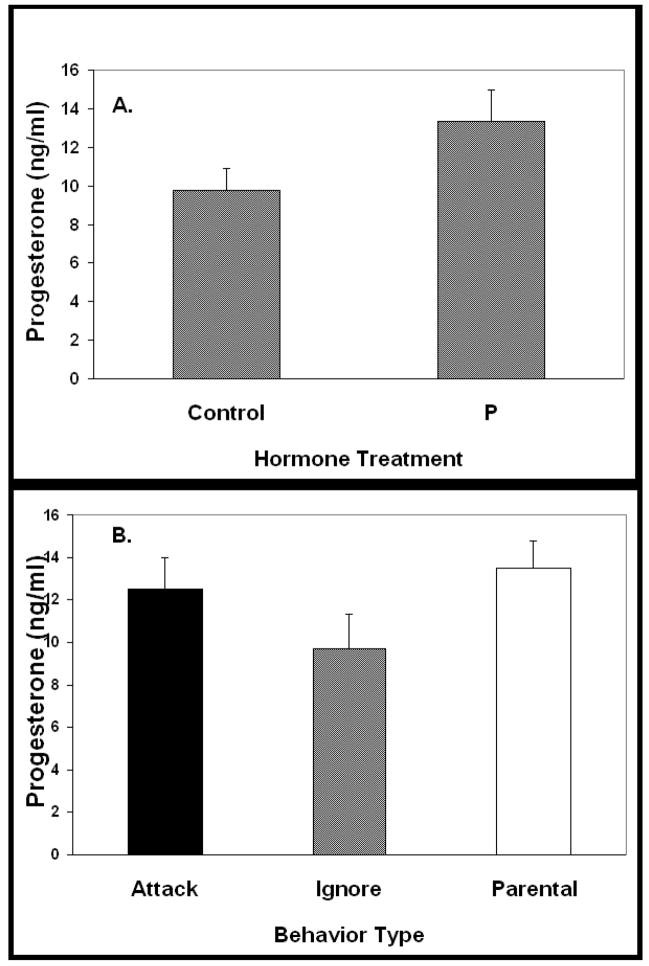
T levels (Figure 3A) were similar among control and P-treated animals (control 4.9±1.1 ng/ml, n=20 vs. P-treated 4.7 ±1.0 ng/ml, n=18). When data were configured to permit comparison of T concentrations as a function of the behavior exhibited by the males, no difference in T concentrations among groups was observed (Figure 3B, p > 0.05). As with the serum T concentrations, P levels (Figure 4A) were similar among control and P-treated mice (control 9.8±2.2 ng/ml, n=20 vs. P-treated 13.3±1.6 ng/ml, n=18, p>0.05). When data were configured to permit comparison of P concentrations as a function of the behavior exhibited, no difference in P concentrations among groups was observed (Figure 4B, p>0.05).
Figure 3.
Serum testosterone concentrations at the time of behavioral testing. A. Serum T concentrations of Control and P-treated males did not differ after capsule withdrawal (p>0.05). Serum samples were taken immediately upon the conclusion of the behavior test. B. Data from A were redrawn to permit comparison of serum T concentrations among groups exhibiting different types of behavior. Serum T concentrations did not differ as a function of the type of behavior exhibited by the males (p>0.05). Data presented as mean plus SEM.
Figure 4.
Serum progesterone concentrations at the time of behavioral testing. A. Serum P concentrations of P and control-treated males after capsule withdrawal did not differ (p>0.05). Serum samples were taken immediately upon the conclusion of the behavior test. B. Data from A were redrawn to permit comparison of serum P concentrations among groups exhibiting different types of behavior. Serum P concentrations did not differ as a function of the type of behavior exhibited by the males (p>0.05). Data presented as mean plus SEM.
Discussion
In contrast to the hypothesis, withdrawal of P from males did not increase parental behavior or reduce infanticidal behavior. Instead, chronic P treatment increased infant-directed aggression. These findings agree with previous studies which found that P increased infant-directed aggression [37,40] and that infant-directed aggression does not correlate with peripheral T levels [5,22,37,38,41,42]. The present study extends the previous results by suggesting that exposure of the adult brain to P may induce a sustained enhancement of infant-directed aggressive behavior..
These results are in contrast to our previous study in which PRKO and wild-type males treated with RU486 show enhanced levels of parental care. Differences between the current and previous results may be explained by considering the role of PR activation under different hormonal treatments. First, PRKO mice lack PRA and PRB both during development and adulthood, thus there may be developmental effects of the PR on parental care that are distinct from the effects of P and its receptor on infant-directed aggression in the adult brain. Second, treatment with RU486 prevents activation of PR by endogenous P. In the present study, although the hormone capsules were withdrawn, the males still possessed normal circulating levels of P which could activate PR. The current results suggest that stimulation of PR enhances infant directed aggression in mice.
Although P is primarily known for its role in maintaining pregnancy in females, increasing evidence demonstrates a significant role for P in males [36,37,45,47]. In male rats P secretion has recently been shown to be a component of the stress response and the extent of the response differs between prepubertal and adult rats [33]. Infanticide has been hypothesized to occur in response to social stress or overcrowding [6,7,19]. If this is the case, then P may provide a mechanistic link between social stress and the onset of infant-directed aggression.
Previous work in female rats has shown the effects of P in mediating maternal behavior are dependent upon the interval between withdrawal of the hormone and presentation of young. Maternal behavior increases dramatically 24–48 hours after P withdrawal in these animals [1,2]. We chose the five day time point to determine if withdrawal of P elicited changes in paternal behavior that persisted after removal of the hormone. However, the possibility exists that males tested 24–48 hours after withdrawal of hormone would be responsive to pups and the later time point used in this study represents a reemergence of aggression towards young.
Many studies have focused on the role of T in the onset and maintenance of parental behavior and/or the suppression of infanticidal behavior. In many studies, T levels have not been found to reliably predict male behavior patterns towards young [5,22,41,42]. And there are conflicting reports about the effects of castration and T-replacement on parental behavior in many species, including prairie voles [22,46], gerbils [5], hamsters [15] and rats [3,30–32,34,35]. An important consideration in these studies is that castration also significantly reduces P levels and it is possible that some of the subsequent behavioral changes could be a consequence of lowered P. For example, in the California mouse, virgin males exhibit higher P levels when they are infanticidal and decreased P levels after mating and onset of parental behavior [40]. A functional relationship between P and T-dependent behaviors is further suggested by the observation that the loss of PR expression is correlated with increased androgen receptor expression in the medial preoptic area and bed nucleus of the stria terminalis [36]. Additional studies are necessary to elucidate the roles of P and T in regulating both spontaneous and mating-induced parental behaviors by males.
Considerable variability exists in the behavior of males towards infants. Approximately 50% of naïve male mice did not attack young, but exhibited parental behavior on their initial exposure. Further investigations are necessary to determine what distinguishes the naïve males that express parental behaviors from those that do not. For example, it is possible that these spontaneously parental mice represent animals that were exposed to a different in utero environment than mice that ignored or attacked young [43]. For the other 50% of naïve males, prior exposure to P in adulthood increases the proportion of males that attack pups rather than ignore them. It will also be informative to follow males for longer after the prolonged P exposure to see if these behaviors persist. If so, then it is possible that P exerts prolonged organizational effects on infant-directed aggression. Additional studies are necessary to determine the mechanism by which P exerts prolonged effects resulting in the increased expression of infant-directed aggression.
Acknowledgments
We gratefully acknowledge the technical expertise of Ms Brigitte Mann. Supported by NIH U54 HD041859 (JEL) and P50 HD4440 (JEL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bridges R, Rosenblatt J, Feder H. Serum progesterone concentrations and maternal behavior in rats after pregnancy termination: behavioral stimulation after progesterone withdrawal and inhibition by progesterone maintenance. Endocrinology. 1978;102:258–267. doi: 10.1210/endo-102-1-258. [DOI] [PubMed] [Google Scholar]
- 2.Bridges R, Rosenblatt J, Feder H. Stimulation of maternal responsiveness after pregnancy termination in rats: effect of time of onset of behavioral testing. Hormones and Behavior. 1978;10:235–245. doi: 10.1016/0018-506x(78)90067-3. [DOI] [PubMed] [Google Scholar]
- 3.Bridges R, Zarrow M, Denenberg V. The Role of Neonatal Androgen in the Expression of Hormonally Induced Maternal Responsiveness in the Adult Rat. Hormones and Behavior. 1973;4:315–322. [Google Scholar]
- 4.Brown RE. Social and hormonal factors influencing infanticide and its suppression in adult male Long-Evans rats (Rattus norvegicus) J Comp Psychol. 1986;100:155–161. [PubMed] [Google Scholar]
- 5.Clark MM, Galef BG., Jr A testosterone-mediated trade-off between parental and sexual effort in male mongolian gerbils (Meriones unguiculatus) J Comp Psychol. 1999;113:388–395. doi: 10.1037/0735-7036.113.4.388. [DOI] [PubMed] [Google Scholar]
- 6.Curtin R. Females, male competition and gray langur troop structure. Folia Primatol (Basel) 1982;37:216–227. doi: 10.1159/000156034. [DOI] [PubMed] [Google Scholar]
- 7.Curtin R, Dolhinow P. Primate social behavior in a changing world. American scientist. 1978;66:468–475. [Google Scholar]
- 8.de Vries G, Boyle P. Double duty for sex differences in the brain. Behav Brain Res. 1998;92:205–213. doi: 10.1016/s0166-4328(97)00192-7. [DOI] [PubMed] [Google Scholar]
- 9.Dewsbury DA. An exercise in the prediction of monogamy in the field from laboratory data on 42 species of muroid rodents. The Biologist. 1981;63:138–162. [Google Scholar]
- 10.Gibber JR, Piontkewitz Y, Terkel J. Response of male and female Siberian hamsters towards pups. Behav Neural Biol. 1984;42:177–182. doi: 10.1016/s0163-1047(84)91033-1. [DOI] [PubMed] [Google Scholar]
- 11.Gibber JR, Terkel J. Effect of postweaning social experience on response of Siberian hamsters (Phodopus sungorus sungorus) toward young. J Comp Psychol. 1985;99:491–493. [PubMed] [Google Scholar]
- 12.Gubernick DJ, Nelson RJ. Prolactin and paternal behavior in the biparental California mouse, Peromyscus californicus. Horm Behav. 1989;23:203–210. doi: 10.1016/0018-506x(89)90061-5. [DOI] [PubMed] [Google Scholar]
- 13.Gubernick DJ, Schneider KA, Jeannotte LA. Individual differences in the mechanisms underlying the onset and maintenance of paternal behavior and the inhibition of infanticide in the monogamous biparental California mouse, Peromyscus californicus. Behavioral Ecology and Sociobiology. 1994;34:225–231. [Google Scholar]
- 14.Hrdy S. Infanticide among animals: a review, classification, and examination of the implications for the reproductive strategy of females. Ethol Sociobiol. 1979;1:13–40. [Google Scholar]
- 15.Hume JM, Wynne-Edwards KE. Castration reduces male testosterone, estradiol, and territorial aggression, but not paternal behavior in biparental dwarf hamsters (Phodopus campbelli) Horm Behav. 2005;48:303–310. doi: 10.1016/j.yhbeh.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Jakubowski M, Terkel J. Incidence of pup killing and parental behavior in virgin female and male rats (Rattus norvegicus): differences between Wistar and Sprague-Dawley stocks. J Comp Psychol. 1985;99:93–97. [PubMed] [Google Scholar]
- 17.Jones J, Wynne-Edwards K. Paternal behaviour in biparental hamsters, Phodopus campbelli, does not require contact with the pregnant female. Animal Behaviour. 2001;62:453–464. [Google Scholar]
- 18.Kleiman DG, Malcolm JR. The evolution of male parental investment. In: Gubernick DJ, Klopfer PH, editors. Parental Care in Mammals. Plenum; New York: 1981. pp. 347–387. [Google Scholar]
- 19.Labov J, Huck U, Elwood R, Brooks R. Current problems in the study of infanticidal behavior of rodents. Quarterly review of biology. 1985;60:1–20. [Google Scholar]
- 20.Labov JB. Factors influencing infanticidal behavior in wild male house mice (Mus musculus) Behavioral Ecology and Sociobiology. 1980;6:297–303. [Google Scholar]
- 21.Lonstein J, De Vries G. Sex differeences in the parental behavior of rodents. Neurosci Biobehav Rev. 2000;24:669–686. doi: 10.1016/s0149-7634(00)00036-1. [DOI] [PubMed] [Google Scholar]
- 22.Lonstein JS, De Vries GJ. Sex differences in the parental behaviour of adult virgin prairie voles: independence from gonadal hormones and vasopressin. J Neuroendocrinol. 1999;11:441–449. doi: 10.1046/j.1365-2826.1999.00361.x. [DOI] [PubMed] [Google Scholar]
- 23.McCarthy MM. Short-term early exposure to pups alters infanticide in adulthood in male but not in female wild house mice (Mus domesticus) J Comp Psychol. 1990;104:195–197. doi: 10.1037/0735-7036.104.2.195. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy MM, vom Saal FS. The influence of reproductive state on infanticide by wild female house mice (Mus musculus) Physiol Behav. 1985;35:843–849. doi: 10.1016/0031-9384(85)90248-3. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy MM, Vom Saal FS. Inhibition of infanticide after mating by wild male house mice. Physiol Behav. 1986;36:203–209. doi: 10.1016/0031-9384(86)90004-1. [DOI] [PubMed] [Google Scholar]
- 26.Milligan SR, Cohen PE. Silastic implants for delivering physiological concentrations of progesterone to mice. Reprod Fertil Dev. 1994;6:235–239. doi: 10.1071/rd9940235. [DOI] [PubMed] [Google Scholar]
- 27.Numan M, Insel T. The Neurobiology of Parental Behavior. Springer-Verlag; New York: 2003. [Google Scholar]
- 28.Perrigo G, Belvin L, vom Saal FS. Individual variation in the neural timing of infanticide and parental behavior in male house mice. Physiol Behav. 1991;50:287–296. doi: 10.1016/0031-9384(91)90068-y. [DOI] [PubMed] [Google Scholar]
- 29.Phoenix C, Belvin L, Vom Saal F. Time and sex in the male mouse: temporal regulation of infanticide and parental behavior. Chronobiol Int. 1992;9:421–433. doi: 10.3109/07420529209064554. [DOI] [PubMed] [Google Scholar]
- 30.Quadagno DM. Maternal behavior in the rat: aspects of concaveation and neonatal androgen treatment. Physiol Behav. 1974;12:1071–1074. doi: 10.1016/0031-9384(74)90157-7. [DOI] [PubMed] [Google Scholar]
- 31.Quadagno DM, McCullough J, Ho GK, Spevak AM. Neonatal gonadal hormones: effect on maternal and sexual behavior in the female rat. Physiol Behav. 1973;11:251–254. doi: 10.1016/0031-9384(73)90357-0. [DOI] [PubMed] [Google Scholar]
- 32.Quadagno DM, Rockwell J. The effect of gonadal hormones in infancy on maternal behavior in the adult rat. Horm Behav. 1972;3:55–62. doi: 10.1016/0018-506x(72)90007-4. [DOI] [PubMed] [Google Scholar]
- 33.Romeo R, Bellani R, McEwen B. Stress-induced progesterone secretion and progesterone receptor immunoreactivity in the paraventricular nucleus are modulated by pubertal development in male rats. Stress. 2005;8:265–271. doi: 10.1080/10253890500489320. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg KM, Denenberg VH, Zarrow MX, Frank BL. Effects of neonatal castration and testosterone on the rat’s pup-killing behavior and activity. Physiol Behav. 1971;7:363–368. doi: 10.1016/0031-9384(71)90315-5. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg KM, Sherman GF. The role of testosterone in the organization, maintenance and activation of pup-killing behavior in the male rat. Horm Behav. 1975;6:173–179. doi: 10.1016/0018-506x(75)90033-1. [DOI] [PubMed] [Google Scholar]
- 36.Schneider JS, Burgess C, Sleiter NC, DonCarlos LL, Lydon JP, O’Malley B, Levine JE. Enhanced sexual behaviors and androgen receptor immunoreactivity in the male progesterone receptor knockout mouse. Endocrinology. 2005;146:4340–4348. doi: 10.1210/en.2005-0490. [DOI] [PubMed] [Google Scholar]
- 37.Schneider JS, Stone MK, Wynne-Edwards KE, Horton TH, Lydon J, O’Malley B, Levine JE. Progesterone receptors mediate male aggression toward infants. Proc Natl Acad Sci U S A. 2003;100:2951–2956. doi: 10.1073/pnas.0130100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schum J, Wynne-Edwards K. Estradiol and progesterone in paternal and non-paternal hamsters (Phodopus) becoming fathers: conflict with hypothesized roles. Hormones and Behavior. 2005;47:410–418. doi: 10.1016/j.yhbeh.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Siegel HI, Rosenblatt JS. Progesterone inhibition of estrogen-induced maternal behavior in hysterectomized-ovariectomized virgin rats. Horm Behav. 1975;6:223–230. doi: 10.1016/0018-506x(75)90009-4. [DOI] [PubMed] [Google Scholar]
- 40.Trainor BC, Bird IM, Alday NA, Schlinger BA, Marler CA. Variation in aromatase activity in the medial preoptic area and plasma progesterone is associated with the onset of paternal behavior. Neuroendocrinology. 2003;78:36–44. doi: 10.1159/000071704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trainor BC, Marler CA. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc Biol Sci. 2002;269:823–829. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trainor BC, Marler CA. Testosterone, paternal behavior, and aggression in the monogamous California mouse (Peromyscus californicus) Horm Behav. 2001;40:32–42. doi: 10.1006/hbeh.2001.1652. [DOI] [PubMed] [Google Scholar]
- 43.vom Saal F. Variation in infanticide and parental behavior in male mice due to prior intrauterine proximity to female fetuses: elimination by prenatal stress. Physiol Behavior. 1983;30:675–681. doi: 10.1016/0031-9384(83)90162-2. [DOI] [PubMed] [Google Scholar]
- 44.vom Saal FS, Howard LS. The regulation of infanticide and parental behavior: implications for reproductive success in male mice. Science. 1982;215:1270–1272. doi: 10.1126/science.7058349. [DOI] [PubMed] [Google Scholar]
- 45.Wagner C. The many faces of progesterone: A role in adult and developing male brain. Front Neuroencocrinol. 2006;27:340–359. doi: 10.1016/j.yfrne.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z, De Vries GJ. Testosterone effects on paternal behavior and vasopressin immunoreactive projections in prairie voles (Microtus ochrogaster) Brain Res. 1993;631:156–160. doi: 10.1016/0006-8993(93)91203-5. [DOI] [PubMed] [Google Scholar]
- 47.Witt DM, Young LJ, Crews D. Progesterone modulation of androgen-dependent sexual behavior in male rats. Physiol Behav. 1995;57:307–313. doi: 10.1016/0031-9384(94)00247-3. [DOI] [PubMed] [Google Scholar]