Abstract
The aim of this study was to test the hypothesis that the molecular structure of cationic surfactants at the nanoparticle (NP)-interface influences the biophysical interactions of NPs with a model membrane and cellular uptake of NPs. Polystyrene NPs (surfactant free, 130 nm) were modified with cationic surfactants. These surfactants were of either dichained (didodecyldimethylammonium bromide [DMAB]) or single chained (cetyltrimethylammonium bromide [CTAB] and dodecyltrimethylammonium bromide [DTAB]) forms, the latter two with different hydrophobic chain lengths. Biophysical interactions of these surfactant-modified NPs with an endothelial cell model membrane (EMM) were studied using a Langmuir film balance. Changes in surface pressure (SP) of EMM as a function of time following interaction with NPs and in the compression isotherm (π - A) of the lipid mixture of EMM in the presence of NPs were analyzed. Langmuir-Schaeffer (LS) films, which are EMMs that have been transferred onto a suitable substrate, were imaged by atomic force microscopy (AFM), and the images were analyzed to determine the mechanisms of the NP-EMM interaction. DMAB-modified NPs showed a greater increase in SP and a shift towards higher mean molecular area (mmA) than CTAB- and DTAB-modified NPs, indicating stronger interactions of DMAB-modified NPs with the EMM. However, analysis of the AFM phase and height images of the LS films revealed that both DMAB- and CTAB-modified NPs interacted with the EMM but via different mechanisms: DMAB-modified NPs penetrated the EMM, thus explaining the increase in SP, whereas CTAB-modified NPs anchored onto the EMM's condensed lipid domains, and hence did not cause any significant change in SP. Human umbilical vein endothelial cells showed greater uptake of DMAB- and CTAB-modified NPs than of DTAB-modified or unmodified NPs. We conclude that (i) the dichained and single-chained cationic surfactants on NPs have different mechanisms of interaction with the model membrane and (ii) NPs that demonstrate greater biophysical interactions with the membrane also show greater cellular uptake. Biophysical interactions of NPs with a model membrane thus could be effectively used for developing nanocarriers with optimized surface properties for drug delivery and imaging applications.
Keywords: Atomic Force Microscopy, Drug Delivery, Polymers, Nanomedicine, Langmuir Membrane
Introduction
Interfacial properties of nanoparticles (NPs) - such as their surface charge, the presence of surface functional groups, and surface hydrophilicity/hydrophobicity - are known to influence the efficiency of NPs in transporting biotherapeutic agents to target tissue by affecting their interactions with the biological environment.1 Surface charge,1-3 surface functional groups,4, 5 and particle size6, 7 influence how well NPs interact with cells and tissues, whereas hydrophilicity is considered a critical factor for longevity of NPs in the circulation.8, 9
NPs intentionally designed with cationic surface characteristics are being extensively investigated for efficient intracellular delivery of therapeutic agents, such as genes, small interfering ribonucleic acid, oligonucleotides, or drugs with intracellular targets. It has also been shown that cationic NPs target tumor vasculature more than normal tissue vasculature because of the presence of excess anionic lipids, particularly phosphatidylserine, in tumor vasculature.10, 11 In general, a cationic surface charge is imparted to NPs by employing cationic polymers such as chitosan,12 polypeptides such as poly (L-lysine),13, 14 or cationic surfactants.15
The effects of the molecular structure of surfactants on their aggregation in solution,16 adsorption at the solid-liquid interface,17 or air-water interface18 have been extensively investigated. However, there are no reports in the literature on the effects of the molecular structure of surfactants on the biophysical interactions of the surfactant-modified NPs with a given model cell membrane or on how these interactions relate to the uptake of NPs into cells. To test these two factors, we selected cationic surfactants (dichained, didodecyldimethylammonium bromide [DMAB]; single-chained, cetyltrimethylammonium bromide [CTAB] and dodecyltrimethylammonium bromide [DTAB]). The two single-chained surfactants differed in the lengths of their hydrophobic chains. We have also used polyvinyl alcohol (PVA), a non-ionic polymeric surfactant, which is commonly used in the formulation of biodegradable, poly DL-lactide co-glycolide- and poly-lactide-based NPs (Figure 1). The biophysical interactions of surfactant-modified NPs were studied using the endothelial cell model membrane (EMM).
Figure 1.
Chemical structures of the surfactants used for surface modification of polystyrene NPs to study the interactions of the surfactant-modified NPs with endothelial cell model membrane (EMM). We coated the NPs with didodecyldimethylammonium bromide (DMAB), dodecyltrimethylammonium bromide (DTAB), cetyltrimethylammonium bromide (CTAB), and a non-ionic polymeric surfactant, polyvinyl alcohol (PVA). Cationic surfactants selected were of either dichained form (DMAB) or single-chained form with different alkyl chain lengths (DTAB and CTAB).
We used standard surfactant free polystyrene NPs (hydrodynamic diameter 130 nm), then modified them with different surfactants to ensure that the changes seen in biophysical parameters were caused by the adsorbed surfactant, not the particle size itself. It is known that NPs formulated with different surfactants form NPs of different sizes, and the size of the NPs could influence their interactions with the model membrane.19 The biophysical interactions of surfactant modified NPs were studied using a Langmuir balance: (i) changes in surface pressure (SP) of the EMM as a function of time following interaction with NPs; and (ii) the shift in the compression isotherm (π - A) of the lipid mixture of the EMM in the presence of NPs. Interactions of the surfactant modified NPs were further studied using atomic force microscopy (AFM) of Langmuir Schaeffer (LS) films. LS films are EMMs that have been transferred onto a suitable substrate. Since we looked at interactions of coated NPs with a model membrane that simulated endothelial cells, we selected human umbilical vascular endothelial cells (HUVECs) for the NP uptake study. Our results demonstrate that the molecular structure of the surfactant at the NP interface significantly influences biophysical interactions with the model membrane and the uptake of NPs into human cells.
Experimental
Materials
Surfactant free polystyrene NPs (130 nm in diameter) were purchased from Bangs Laboratories, Inc. (Fishers, IN). 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE), 1,2-dipalmitoyl-sn-glycero-3-phospho-L-serine (DPPS), L-α-phosphatidylinositol, sphingomyelin, and cardiolipin were purchased from Avanti Polar Lipid (Alabaster, AL). Dulbecco's phosphate buffered saline (D-PBS, pH 7.4, obtained from the Central Cell Services' Media Laboratory of our institution) was used as a subphase. DTAB and PVA were purchased from Sigma Aldrich (St. Louis, MO), DMAB from Aldrich (Milwaukee, WI) and CTAB, ammonium hydroxide (NH4OH), and hydrofluoric acid from Acros Organics (Fair Lawn, NJ). Hydrogen peroxide (H2O2) was obtained from Fisher Scientific (Fair Lawn, NJ). 6 Coumarin was obtained from Polysciences Inc. (Warrington, PA). Other solvents (acetonitrile, methanol, ethanol, and chloroform) used were of high performance liquid chromatography (HPLC) grade. Deionized water with 18.2 MΩ.cm resistivity was used in all the experiments (Super Q water purification system, Millipore Corporation, Billerica, MA).
Surface Coating of Polystyrene NPs
Aqueous solutions of each cationic surfactant (0.2% w/v) and PVA (2% w/v) were first prepared. For surface coating, 1 mL of a 10% suspension of NPs was mixed with 25 mL of the desired surfactant solution, and the suspension was stirred for 24 h at room temperature. NPs were centrifuged at 34,500 rpm (~81,650 g) using a Beckman Optima LE 80K Ultracentrifuge (Beckman Instruments Inc., Palo Alto, CA) for 30 min at 4 °C to remove the unbound surfactant. The supernatant was discarded, and the pellet consisting of modified NPs was resuspended in 8 mL of water; the suspension was centrifuged again as above, and the supernatant was again discarded. This washing procedure was repeated one more time. After final washing, the pellet was dispersed in 8 mL water, and the suspension was stored at 4 °C until used. One milliliter of each suspension was lyophilized (-48 °C, 3.5 Pa, using FreeZone 4.5 from Labconco Corporation, Kansas City, MI) to determine the concentration of NPs in the suspension. The average weight of the three replicates was considered for calculating the concentration of NPs in the suspension.
In preliminary experiments, we tested three concentrations of the DMAB solution (0.025, 0.1 and 0.2% w/v) for surface modification of NPs. We observed no significant change in the zeta potentials of NPs with the DMAB concentrations used. However, we noted that NPs modified with the 0.2% w/v DMAB solution showed a greater change in SP of the EMM than NPs modified with the other concentrations tested. Furthermore, NPs coated with the 0.2% DMAB solution showed a very great change in SP (up to 13 mN/m) - nearly the maximum that one can typically observe in model membrane interaction studies.20 Hence a 0.2% w/v concentration of DMAB and other cationic surfactants was used for surface modification of NPs. We also used a 2% PVA solution for modification of NPs as this is the typical concentration used in formulating biodegradable polymeric NPs.9
Size and Surface Charge of NPs
A 50 μL of 1% of each NP suspension was diluted 100-fold with water. Mean particle diameters were measured by a dynamic light-scattering technique, and mean zeta potentials were determined with a phase analysis light scattering technique using a commercial particle sizing system (PSS/NICOMP 380/ZLS, Santa Barbara, CA, USA). Particle size was also determined using transmission electron microscopy (TEM, model Philips CM12, Philips/FEI Inc., Briarcliff Manor, NY). A drop of dilute aqueous suspension of NPs was placed onto a Formvar® coated 150 mesh copper TEM grid (Ted Pella, Inc., Redding, CA), then air dried for TEM imaging. The images were analyzed for the size of NPs using Image J software (http://rsb.info.nih.gov/ij/).
Model Membrane
For the EMM, phospholipids were dissolved either in CHCl3 or a 4:1 mixture of CHCl3:CH3OH, depending upon their solubility. The lipid solutions were mixed in the following proportions: DPPC (56%), DPPE (24%), L-α-phosphatidylinositol (8 %), DPPS (4.3%), sphingomyelin (6%), and cardiolipin (1.7%). This composition is similar to the head group composition of the phospholipids in the native artery's endothelial cell membrane.21 The EMM was formed on the buffer surface using the above lipid solutions and a Langmuir film balance (Minimicro 2, KSV Instruments, Helsinki, Finland) as described in our previous study.19 In brief, 10 μL of the lipid mixture was added onto a subphase consisting of 50 mL D-PBS in a trough. A 10-min waiting period was allowed for any solvents to evaporate. Then the barriers of the balance were compressed until the SP of the membrane reached 30 mN/m (~ 85 Å2 mmA) (Figure 2). This SP is equivalent to the lateral pressure of a red blood cell membrane,22 therefore the arrangement of the phospholipid monolayer on the buffer surface at this SP would mimic that of the outer leaflet of a cell membrane.
Figure 2.
A typical isotherm of the lipids of endothelial cell model membrane (EMM). Schematic representation of the method used to investigate NP interactions with model membrane using a Langmuir balance, and the Langmuir-Schaeffer (LS) transfer technique of the model membrane on a substrate for AFM imaging.
Interaction of NPs with the EMM
A 50 μL aliquot of 1% suspension of NPs was injected over ~30 s using a 50 μL Hamilton digital microsyringe (Hamilton Company, Reno, NV) into the buffer subphase below the EMM through the injection port without causing disturbance to the membrane. Changes in SP of the EMM were recorded immediately as a function of time over a constant mmA for 20 min. To ensure that the SP changes were caused by interaction of NPs with the EMM, SP changes of the buffer alone (without membrane lipids) following injection of NPs were also measured under similar conditions for comparison.
π-A isotherm of Endothelial Cell Lipid Mixture
These experiments were carried out to investigate how well the modified NPs could penetrate the EMM. For this experiment, the lipids of the EMM were spread at an SP of 0 mN/m. A suspension of NPs was injected below the lipid mixture in the buffer subphase; NPs were allowed to interact for 20 min with the lipid mixture and then were compressed at the rate of 3 mm/min until the film collapsed.
Transfer of EMMs to LS Films for AFM Imaging
Following interaction with modified NPs for 20 min, EMMs were transferred onto a hydrophobic silicon substrate by the Langmuir-Schaeffer (LS) technique. A hydrophobic surface is necessary for the LS transfer technique, as it depends on the hydrophobic interaction between the phospholipid molecules on the buffer subphase and the silicon substrate. The lipid monolayer was transferred onto the substrate by horizontal lifting (Figure 2). In brief, the silicon (111) wafers (Ted Pella Inc, Redding, CA) were modified to generate a hydrophobic surface using the Shiraki technique, described elsewhere.23 The wafers were first placed in a solution containing 4:1:1 (v/v) H2O/H2O2/NH4OH at 80 °C for 5 min, then rinsed with deionized water, followed by washing at room temperature in a solution containing 3:1 v/v H2O/hydrofluoric acid, and finally rinsing them in deionized water to remove organic residues. Cleaned substrates were stored in double-distilled water until use. The transferred LS films were allowed to dry for at least 24 h in a desiccator at room temperature prior to AFM imaging.
AFM
LS film surface morphology before versus after interactions with NPs was studied using a Bioscope atomic force microscope (Digital Instruments, Santa Barbara, CA) in tapping mode using a 125-μm-long silicon cantilever with a resonance frequency of approximately 325 Hz and a tip radius of 10 nm (Vecco, Plainview, NY). AFM images were captured with a lateral scan frequency of 1-2 Hz and a set point ratio of 0.98. The acquired images were flattened using a second-order flattening routine (Nanoscope software, version 7.20, Vecco). Images from three different LS films for each sample were taken to ensure the reproducibility of the data.
Uptake of NPs by HUVECs
For quantification of uptake of NPs by cells, 6-coumarin was loaded into NPs. The dye loading was achieved by adding a 5 mL suspension of 10% surfactant-free polystyrene NPs to 15 mL of methanol containing 3 mg of 6-coumarin while stirring on a magnetic stir plate at room temperature for 12 h. Methanol allows diffusion of the dye into polystyrene NPs. Undissolved 6-coumarin was removed by centrifugation at 4,000 rpm (~2300 g; Sorvall Legend RT, Thermo Electron Corporation, Waltham, MA) for 10 min at 4 °C; then methanol was removed by dialysis against deionized water for 24 h using a dialysis membrane with a 10,000 molecular weight cutoff (Sigma-Aldrich, St. Louis, MO). Dialysis caused precipitation of the unloaded 6-coumarin into the dialysis bags (because of the diffusion of methanol); this unloaded portion was separated by centrifugation as above. NPs were observed under an inverted microscope in fluorescence mode (Leica DMI 6000 B, Leica Microsystems Gmbh, Wetzlar, Germany) to ensure that NPs had actually been loaded with the dye and that there were no free dye particles. Dye loading did not change the size and zeta potential of the NPs. The dye-loaded NPs were stored at 4 °C in the dark until used for surface modification with different surfactants as described above. Suspensions of the surfactant modified NPs were stored at 4 °C in the dark until they were used for the uptake studies.
HUVECs were cultured and grown in endothelial basal medium with growth factors (bovine brain extract with heparin), human epidermal growth factor, hydrocortisone, GA-1000 (gentamycin, amphotericin B), and fetal bovine serum supplied by Lonza (Walkersville, MD) in a T-75 flask in an incubator (Forma Scientific, Marietta, OH) at 37 °C in a 5% CO2 environment. The medium was changed after 24 h following subculture and subsequently every 2 days until cells reached 80-90% confluency. Cells at the 6th to 8th passage were used for the uptake experiment.
Cells were seeded at a density of 30,000 cells per well in 24-well plates (Becton Dickinson, Franklin lakes, NJ) and were allowed to attach for 2 days. Medium from each well was aspirated and cells were washed with PBS. Cells were incubated with a 50 μg/mL suspension of each formulation of NPs for 15 min. The NP suspension was prepared in endothelial basal medium only, w/o growth factors and serum to mimic the conditions used in the membrane interaction studies. So that we could focus on the effect of surface modification on cellular uptake of NPs, the incubation time was kept to a minimum of 15 min; this precaution was taken because it is known that the rate of NP uptake decreases exponentially with incubation time.24 Also, this incubation time is about the same as that used in the study of NP interaction with the EMM. Medium from each well was aspirated after incubation and cells were washed three times with cold PBS. Cells were solubilized in 1% Triton-X 100. Twenty microliters of each cell lysate was used to determine the total cell protein content using the Pierce BCA protein assay (Rockford, IL). A standard curve was obtained with bovine serum albumin solution. The remaining 80 μL of each cell lysate was lyophilized for 24 h as described above.
Lyophilized cell lysates were used to analyze the 6-coumarin content in NPs as described in our previous study.24 In brief, 6-coumarin fluorescent dye was extracted from each of the lyophilized cell lysates by incubating them at 37 °C for 18 h in 500 μL methanol. The samples were centrifuged at 14,000 rpm (~14,500 g) using a microcentrifuge (Eppendorf, Westbury, NY) for 10 min at 4 °C. A 400 μL supernatant from each tube was carefully collected for HPLC analysis. A standard curve for each formulation of NPs was constructed in the range of 1-10 μg/mL under identical conditions. The amount of 6-coumarin in the extracts was analyzed by ion-pair chromatography using an HPLC system (Shimadzu Scientific Instrument, Inc, Columbia, MD) at Ex 450 nm/Em 490 nm. The separations were achieved with a mobile phase consisting of acetonitrile:water:1-heptane sulfonic acid sodium salt (65:35:0.005 M; Fisher Scientific, Fair Lawn, NJ). A Nova-Pak® C-8 column (2×150 mm2) with 4 μm packing (Waters, Milford, MA) was used. Uptake was calculated from the corresponding standard plot for NPs and the data were normalized to per milligram of cell protein.
Molecular Modeling of DMAB
The molecular modeling for DMAB and the molecular mechanics calculations were done using an MM2 force field and MOPAC using the ChemDraw software (Cambridgesoft, Cambridge, MA). The structure of the surfactant was drawn and the minimum energy configuration was obtained by energy minimization in a vacuum using MM2 force field calculations. The minimum energy conformation in water/buffer was calculated using MOPAC with AM1 parameterization.
Results
Physical Characterization of Modified NPs
The hydrodynamic diameters of the cationic surfactant-modified NPs were similar to those of unmodified NPs but slightly higher for PVA-modified NPs because of the hydration of the PVA associated with NPs.25 Unmodified NPs measured with TEM demonstrated a mean particle size of 70.7 ± 2.5 nm (range 46.6-126.5 nm, n = 43). The diameter measured with TEM did not change significantly with surface modification. The zeta potential of unmodified NPs was slightly cationic, but that of the cationic surfactant-modified NPs was significantly more positive. The zeta potential of DMAB-modified NPs was higher than that of CTAB- and DTAB-modified NPs. The zeta potential of PVA-modified NPs was negative (Table 1).
Table 1.
Physical characteristics of surfactant-modified NPs and their effect on SP of EMM
| Sample |
Surfactant (% w/v) |
Particle size (nm; PI) |
Zeta potential (mV) |
ΔSP* (mN/m) |
|---|---|---|---|---|
| Unmodified NPs | - | 130.5 (0.01) | + 7.84 | -5.5 |
| DMAB-NPs | 0.2 | 140.4 (0.01) | +57.74 | 10.0 |
| CTAB-NPs | 0.2 | 142.0 (0.01) | +39.25 | 1.0 |
| DTAB-NPs | 0.2 | 144.0 (0.02) | +32.86 | 0.3 |
| PVA-NPs | 2.0 | 169.0 (0.01) | -14.35 | 0.2 |
Change in SP of EMM following interaction with modified NPs at 20 min.
SP, surface pressure; EMM, endothelial cell model membrane; NPs, nanoparticles; DMAB, didodecyldimethylammonium bromide; CTAB, cetyltrimethylammonium bromide; DTAB, dodecyltrimethylammonium bromide; PI, polydispersity index.
Interactions of NPs with the EMM
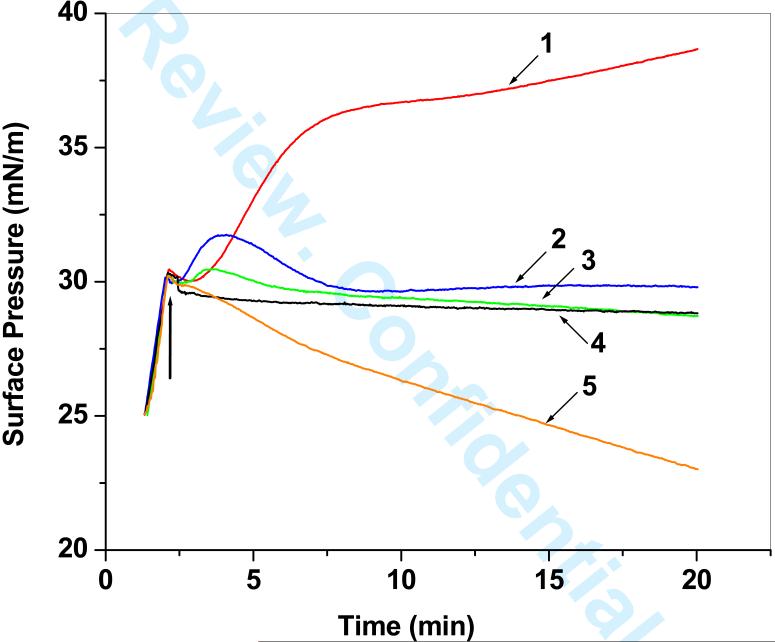
The pattern of interaction of NPs with the EMM varied with the molecular structure of the cationic surfactants used (Figure 3). The initial increase in SP for DMAB-modified NPs was sharp, reaching 37 mN/m within 5 min, but the increase thereafter was gradual. CTAB-modified NPs also showed an initial sharp increase in SP, but this increase was significantly lower than that seen with DMAB-modified NPs. SP with CTAB-modified NPs declined slowly after an initial increase, going back to baseline levels, and then showing a marginal increase again. DTAB-modified NPs also showed almost the same pattern of SP change as CTAB-modified NPs, but the initial increase was marginal and levels gradually decreased, reaching a SP level similar to that of the membrane without NPs. PVA-modified NPs did not cause any change in SP, whereas unmodified NPs showed a gradual decrease in SP, ending up below the SP of the membrane without NPs. Injection of water alone, which was used as a vehicle for making NP suspensions, did not show any change in SP. In the experiment in which buffer alone (without lipids) was used, DMAB- and PVA-modified NPs showed a sharp increase in SP up to 12 mN/m, whereas CTAB-modified NPs showed an increase in SP only up to 2 mN/m. DTAB-modified and unmodified NPs showed no change in SP.
Figure 3.
Changes in surface pressure (SP) of the EMM with time caused by interaction of the membrane with different surfactant-modified NPs. Fifty microliters of a 1% NP suspension was injected into the subphase below the EMM without causing a disturbance to the membrane itself, and any change in surface pressure over time was immediately recorded. Nanoparticle concentration in buffer = 10 μg/mL. Key: 1, DMAB-modified NPs; 2, CTAB-modified NPs; 3, DTAB-modified NPs; 4, PVA-modified NPs; 5, unmodified-NPs. Representative data from at least three repeats are shown.
Isotherm of Endothelial Phospholipid Mixture in the Presence of NPs
The shape of the compression isotherm (π - A) of the EMM's phospholipid mixture showed two distinct regions (Figure 4). The SP increased, reaching a mmA of 100 Å2, and then showed a small “kink” at ~84 Å2 prior to a rapid increase until the membrane collapsed at 55 Å2. In the presence of DMAB modified NPs, the isotherm differed significantly from the lipid mixture alone or in the presence of other NPs. Specifically, the initial SP was 12 mN/m in the presence of DMAB modified NPs, which was significantly higher than the SP of 0 mN/m for the lipid mixture alone. With further compression, the SP increased gradually prior to membrane collapse at an SP of 46 mN/m (corresponding to 112 Å2 mmA), which for the membrane lipids alone was at 55 Å2. Unlike DMAB modified NPs, the shape of the isotherms remained mostly unaltered in the presence of CTAB- and DTAB-modified NPs, except with a slight shift toward a higher mmA (Figure 4).
Figure 4.
Surface pressure-area (π - A) isotherm of the membrane lipid mixture in the presence of different surfactant-modified NPs. The lipid mixture of the EMM was spread on the buffer subphase at high mmA (220 Å2 or 0 mN/m surface pressure), and 50 μL of a 1% NP suspension was injected into the trough. The lipid mixture was compressed for 20 min following NP injection until the EMM collapsed. Nanoparticle concentration in buffer = 10 μg/mL. Inset shows the changes in mmA of the membrane lipid mixture in the presence of different surfactant-modified NPs. Key: 1, DMAB-modified NPs; 2, CTAB-modified NPs; 3, DTAB-modified NPs; 4, PVA-modified NPs; 5, unmodified NPs; 6, EMM lipid alone. Representative data from three repeats are shown.
AFM Imaging of LS Films
Surface morphology of the LS films prior to and after interaction with different surfactant-modified NPs varied significantly (Figure 5). A phase image analysis of the LS films' lipids alone showed bright and dark regions, corresponding to the liquid-condensed and liquid-order phases (Figure 5a), whereas following interaction with DMAB-modified NPs, the LS films showed a highly condensed lipid membrane with spherical structures (Figure 5b). Phase images of the LS films following interaction with CTAB modified NP showed bright lipid condensed domains with spherical structures attached to the condensed lipid domain structures of the membrane (Figure 5c). DTAB-modified NPs showed no change in the phase image of the LS films (Figure 5d). A phase image of the LS films following interaction with PVA-modified NPs was also similar to that of the LS films of lipids alone but with few spherical structures anchored to the film (Figure 5e).
Figure 5.
Changes in EMM Langmuir-Schaeffer film morphology following interactions with different surfactant-modified NPs. LS films were transferred onto a silicon substrate following interaction with NPs for 20 min, and the imaging was carried out using tapping mode AFM in air. a. EMM transferred at SP 30 mN/m. Panels b, c, d, and e show images of the EMM following interaction with DMAB-, CTAB-, DTAB-, and PVA-modified NPs, respectively. The phase angle scale for all images was 50°. Height scales for the images were as shown: a, d = 3 nm; b, c, f = 50 nm; e = 200 nm. The section analysis was carried out on AFM height images across the white line. The height scale for 3-D height images is 50 nm; scan size was 2 μm. At least three to five images were scanned to obtain mean height.
Height images were used for quantitative analysis of the changes observed in LS film morphology following interaction with modified NPs. Section analysis of the 2D height images of the LS film for lipids alone showed a small step in between liquid order and liquid-condensed domains. This height difference corresponds to the typical difference in the arrangement of lipids (Figure 5a). The 2D height images of the LS films following interaction with DMAB- and CTAB-modified NPs showed spherical structures (Figure 5b and 5c), whereas the LS film that had contact with DTAB-modified NPs showed no spherical structures (Figure 5d). The LS film that had interacted with PVA-modified NPs also showed relatively few large spherical structures (Figure 5e).
Analysis of the height images of the LS films for the number of NPs anchored and a width and height analysis of the anchored NPs showed significant variations with surface modification. As shown in Table 2, DMAB- and CTAB-modified NPs showed almost the same number of NPs anchored to the LS film, but their height scales were different. The range of height for DMAB-modified NPs was 5-15 nm, whereas that for CTAB-modified NPs was 20-35 nm. For PVA-modified NPs, the height range was greater (50-73 nm).
Table 2.
Quantitative analysis of interactions of NPs with EMM based on the AFM height images
| Sample |
Average height (range)* nm) |
Number of NPs per 5 μm2 |
|---|---|---|
| EMM | 0 | |
| DMAB-NPs | 7 (5-15) | 96 |
| CTAB-NPs | 20 (20-35) | 86 |
| DTAB-NPs | - | 0 |
| PVA-NPs | 58 (50-73) | 14 |
Range (minimum height-maximum height) of spherical features observed in AFM images.
EMM, endothelial cell model membrane; AFM, atomic force microscopy; NPs, nanoparticles; DMAB, didodecyldimethylammonium bromide; CTAB, cetyltrimethylammonium bromide; DTAB, dodecyltrimethylammonium bromide; PVA, polyvinyl alcohol.
Uptake of NPs by HUVECs
The extent of uptake of NPs by HUVECs varied, depending on the molecular structure of the surfactants used for surface modification (Figure 6i). DMAB- and CTAB-modified NPs showed greater uptake than DTAB-modified NPs. The uptake of PVA-modified NPs was higher in comparison to unmodified NPs but significantly less in comparison to the uptake of cationic surfactant-modified NPs.
Figure 6.
Correlation between biophysical interactions of different surfactant-modified NPs with EMM and uptake of NPs by HUVECs. (i) The effects of different surfactant coatings on the uptake of NPs into human cells was studied by incubating 50 μg/mL of surfactant-modified NPs with HUVECs (3 × 104 cells per well in 24-well plates) for 15 min. Data are expressed as mean ± s.e.m (n = 3). (ii) Change in SP of the EMM following interaction with surfactant-modified NPs after 20 min. (iii) Number of NPs transferred onto LS films following NP interaction for 20 min. These data were taken from the AFM height image; average number of NPs was determined from three images.
Discussion
We investigated the biophysical interactions of NPs with EMMs using Langmuir film balance and AFM of the LS films to understand the effect of the molecular structures of the coated surfactant on these interactions and to determine whether such interactions could predict cellular uptake of NPs. In brief, the phospholipid composition that represents the phospholipid head group composition of the arterial endothelial cell membrane was used to form the EMM, and HUVECs used for comparison were used to determine the uptake of NPs into cells.
Biophysical interactions of NPs with a model membrane can be investigated by injecting the NPs into the buffer subphase below the membrane. Changes in the SP of the membrane over time for a constant mmA reflect the interactions of NPs with the membrane. Typically, any change in the EMM's SP, either positive or negative, indicates interaction of the injected molecules with the membrane. A positive change in SP indicates the condensation of the membrane's phospholipids, which could be caused either by penetration of the injected molecules into the membrane or by their electrostatic interactions with the phospholipid head groups.26-28 A negative change in SP indicates the loss of the membrane's phospholipids from the surface into the subphase. This result appears to be the case with the unmodified NPs in our study, which showed a decrease in SP over time (Figure 3). appears to be the case with the unmodified NPs in our study, which showed a decrease in SP over time (Figure 3).
In our study, changes in SP of the EMM following interaction with various modified NPs, as calculated from the difference in the SP at 20 min, shows that the cationic surfactants coating the NPs facilitate their interactions in the following order: DMAB > CTAB > DTAB, with PVA modified NPs not showing any membrane interaction (Figure 3, Table 1). Interestingly, this order is in accordance with the decreasing hydrophobicity of cationic surfactants,29 31 i.e., DMAB has a greater hydrophobicity in comparison to CTAB and DTAB because of its dichain nature. In between CTAB and DTAB, CTAB is more hydrophobic than DTAB because of its longer hydrophobic chain (Figure 1). This pattern of change in SP was distinctly different compared to that seen in the absence of the membrane. For instance, PVA modified NPs caused an increase in SP of the buffer, but not of the membrane. This finding clearly suggests that changes in SP arise from NP membrane interactions and not solely their intrinsic surface active behavior.
Changes in SP of the EMM may not allow us to distinguish whether the increase in SP is caused by penetration of NPs into the membrane or by the electrostatic interactions between the phospholipid head groups of the membrane and the cationic head groups of the surfactant coating of the NPs. Therefore, we further investigated changes in the isotherms of the lipid mixtures of the EMM in the presence of modified NPs (Figure 4). In our previous study, we saw an increase in SP caused by penetration of small (20 nm) polystyrene NPs into a model membrane.19 Others also have shown that penetration of polymeric non ionic surfactant (Pluronic)32 into DPPC and DPPE model membranes causes an increase in SP. Thus, changes in SP and isotherm of the lipid mixture could predict the interaction of NPs with model membranes.
The isotherm experiment demonstrated that all the cationic surfactants used for NP modification facilitate the penetration of NPs into the lipid mixture monolayer at lower lipid densities (<10 mN/m SP); however, at higher lipid densities (~30 mN/m SP, which is equivalent to the lateral pressure in a biological cell membrane), only the DMAB modified NPs seem to remain in the monolayer. The evidence for this finding comes from the shift in the mmA of the EMM in the presence of DMAB NPs (Figure 4). Other surfactant modified NPs seem to squeeze out of the monolayer at 30 mN/m SP; hence the mmA of the membrane in their presence at this SP is similar to that of the lipid mixture alone (inset of Figure 4).
A phase image of the LS films in AFM for lipids only shows bright and dark regions. Bright structures correspond to the liquid condensed phase, and dark areas correspond to the liquid expanded phase of phospholipids in the model membrane.20 Phase and height images of the LS films following interactions with DMAB, DTAB, and PVA modified NPs (Figure 5b-e) are consistent with the analysis of the results from the Langmuir studies. For instance, the phase image of the DMAB modified NP interacted LS film shows (Figure 5b) condensed lipid domains with embedded spherical structures, which is in accordance with the assumption that DMAB modified NPs penetrated the membrane and caused its condensation. A phase image of the CTAB modified NP interacted LS film shows relatively more condensed lipid domains than before the interaction (Figure 5c) but significantly fewer than those seen in the DMAB modified NP interacted LS film. This pattern of lipid condensation seen in the phase images is in agreement with the SP changes seen in the Langmuir studies, which showed greater changes for DMAB modified NPs than for CTAB modified NPs. Surprisingly, the number of CTAB modified NPs anchored to the LS films is similar to that for DMAB modified NPs (Table 2). This anchoring of CTAB modified NPs to the condensed part of the membrane was not evident from the Langmuir studies, as it did not show a significant change in SP of the EMM. Thus, the data from the interaction of the CTAB modified NPs with the EMM could reflect the combined effect of the NPs' surface active nature and ionic interactions of the surfactant head group with the condensed lipid domain of the membrane. Similar specific interactions of polyamidoamine (PAMAM) dendrimer and MSI 78, a peptide with 22 amino acids, have been reported with liquid crystalline fluid phases of the lipid bilayers.33 Thus our study reveals that DMAB and CTAB modified NPs have different patterns of interactions with the EMM: DMAB modified NPs show nonspecific interactions, whereas CTAB modified NPs seem to interact only with the liquid condensed phases of the EMM.
DTAB modified NPs, despite a surface charge similar to that of CTAB modified NPs, showed minimal interaction with the EMM, which could be because of the lack of surface activity of the DTAB modified NPs. A phase image of the DTAB modified NP interacted membrane appears similar to that of the membrane alone (Figure 5d vs 5a), further confirming a minimal interaction of these modified NPs with the EMM. From height analysis of the LS film, we conclude that DMAB modified NPs must be embedded in the EMM, since the average height of the NPs in the membrane is three times less than that of CTAB modified NPs and eight times less than that of PVA modified NPs. The greater height of PVA modified NPs could arise from their anchoring to the EMM through long PVA polymer chains that coat the NPs (Table 2).
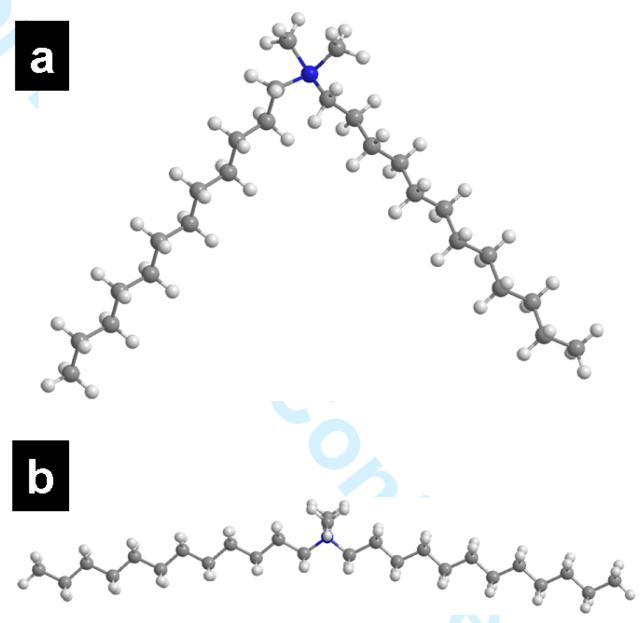
The correlation seen in our studies between cellular uptake of NPs and their biophysical interactions with the EMM illustrates the significance of such studies in predicting interactions of NPs with cells (Figure 6). DMAB-modified NPs showed a significant change in SP of the EMM (Figure 3), which could most likely to be caused by the interaction of the surfactant coating of the NPs with the EMM. The unique molecular structure of DMAB, which consists of a single head group with two hydrophobic tails, gives it almost a linear energy minimized conformation. MM2 force field calculations in a vacuum and MOPAC minimum energy calculations in water/buffer show that the linear conformation is preferred over the angled conformation (Figure 7). The linear conformation confers DMAB the unique adsorption mechanism on NPs, i.e., one hydrophobic chain is adsorbed onto the particle surface while the other remains free for interaction and penetration of the membrane (Figure 8). This quality of DMAB explains the significant increase in SP of the membrane in the presence of DMAB-modified NPs and the shift in the isotherm toward a higher mmA. Bakshi et al.34 have reported that a PAMAM-DMAB dendrimer-surfactant complex is more surface active than a PAMAM-DTAB complex because of the dichained nature of DMAB. In the above case, a strong electrostatic interaction between the head groups of the surfactant and PAMAM expels the hydrophobic tails to the interface, thus making the complex surface active. In another study, a cationic double-chain surfactant, (bis(N,N-dimethyl-N-tetradecylammonio-11-undecanoylaminoethyl) disulfide), attached to gold NPs showed greater interaction with a DPPC model membrane than gold NPs attached to cationic single-chain surfactants.35 These studies and our data thus suggest that the dichained surfactants anchor differently to the solid interface than single-chained surfactants (Figure 8), which confers on the dichained surfactant-modified NPs greater surface activity than single-chained surfactant-modified NPs and the ability to penetrate the EMM.
Figure 7.
Energy minimized conformation of DMAB. Minimum energy calculated using an MM2 force field in a vacuum for the DMAB conformation shown in (a) is 156.50 kcal/mol and (b) 26.13 kcal/mol. Heat of formation calculated using MOPAC with AM1 parameterization in water and buffer for the conformation shown in (a) is 6.19 kcal/mol and (b) is 4.78 kcal /mol.
Figure 8.
(i) Schematic representation of adsorption of different surfactants onto NPs. (ii) (a) EMM alone and schematic representation of interaction of (b) DMAB-, (c) CTAB- (d) DTAB-, and (e) PVA-modified NPs with model membrane. DMAB-modified NPs penetrate the EMM, whereas CTAB-modified NPs interact with phospholipid liquid-condensed domains through electrostatic interactions. DTAB- and PVA-modified NPs did not interact. Corresponding AFM phase images of LS films show that DMAB-modified NPs become embedded in between phospholipids, whereas CTAB-modified NPs attach to the condensed phospholipid domains.
Comparing CTAB-to DTAB-modified NPs, we found that CTAB-modified NPs showed slight surface activity, but DTAB-modified NPs did not. Considering that both CTAB and DTAB adsorb onto NPs through hydrophobic interactions (Figure 8), it is not clear how a small increase in the alkyl chain length of CTAB can impart surface activity to NPs. However, it has been suggested that the difference in alkyl chain length significantly influences surfactant adsorption at the solid interface, with small-chain surfactants anchoring perpendicular to the surface and long-chain surfactants anchoring at an angle, thus partly exposing the hydrophobic chains of the surfactant to the surrounding environment.17, 36 This difference in arrangement of surfactants at the NP surface may be the reason for the relatively higher surface activity of CTAB-modified NPs over DTAB-modified NPs (increase in SP = 2 mN/m vs 0 mN/m, respectively), although it is significantly lower than that observed for DMAB-modified NPs (increase in SP =12 mN/m).
In general, in drug delivery applications, cationic surfactants are commonly used to impart a positive surface charge to NPs, but the potential effect of the surfactant molecular structure and its role in interaction with cells is generally ignored. In this study, we show that surface charge alone cannot be taken as a primary criterion, as all the cationic surfactants used in our study impart a positive charge to NPs, but the results of our studies suggest that each surfactant's molecular structure plays a significant role in how efficiently NPs are transported across the cell membrane. Figure 8 illustrates the proposed mechanisms of interactions with different surface-modified NPs with the EMM based on the change in SP, (π - A) compression isotherm, and analysis of the LS films using AFM.
In our study, although PVA-modified NPs did not show any significant interaction with the EMM and unmodified NPs showed a decrease in SP, these two types of NPs were taken up into HUVECs, although to a lesser extent than the cationic surfactant-modified NPs. This result suggests that the biophysical interactions with the EMM did not exactly replicate all aspects of the cellular uptake process of NPs. This dissimilarity could be because of the lack of typical cell membrane characteristics in the EMM, such as the absence of anchoring membrane proteins, receptors, the bilayer lipid structure of cell membrane, and the active process of endocytosis. Despite this shortcoming, we found a good correlation between biophysical interactions and cellular uptake, suggesting that the interaction of NPs with lipids plays a critical role in triggering the process of uptake of such NPs into cells. Recently, we have shown that the force of adhesion of NPs with cell membrane determines cellular uptake of NPs.37 In that study, poly-L-lysine functionalized NPs demonstrated a greater force of adhesion and greater cellular uptake than unmodified NPs. The role of the lipid bilayer membrane thus goes beyond simply encapsulating and protecting the cellular content; it is also involved in cell signaling and controlling various cellular functions.
The current study was carried out with NPs of 130 nm diameter because most drug delivery systems use NPs of this average particle diameter, but it would be interesting to determine if and how either particle size or the concentration of surfactants used for coating influences NP-EMM interactions. Further studies with different cationic surfactants varying in chain lengths and structures could be useful in establishing a correlation between the surfactant's molecular structure and biophysical interactions with the EMM. Apart from understanding the basic mechanisms of interaction of NPs with the cell membrane, biophysical interactions could be helpful in choosing surface properties to modulate the characteristics of NPs for specific applications. For example, one could foresee developing modified NPs based on biophysical interactions that could target the specific lipid domains expressed in cells in a particular tissue or in disease conditions for targeted delivery of therapeutics or an imaging agent.
Conclusions
We have demonstrated that the molecular structure of cationic surfactants significantly influences both the biophysical interactions of the surfactant modified NPs with the model membrane and the extent of cellular uptake of the NPs. Our study has also shown that modified NPs that had greater interactions with the model membrane also demonstrated greater cellular uptake of NPs. Thus, biophysical interactions with a model membrane could be useful in developing efficient nanocarrier systems for biomedical applications.
Acknowledgment
The study reported here is funded by grant 1R01 EB 003975 from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (to VL).
References
- 1.Chung TH, Wu SH, Yao M, Lu CW, Lin YS, Hung Y, Mou CY, Chen YC, Huang DM. Biomaterials. 2007;28:2959–2966. doi: 10.1016/j.biomaterials.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Kumar MN, Mohapatra SS, Kong X, Jena PK, Bakowsky U, Lehr CM. J. Nanosci. Nanotechnol. 2004;4:990–994. doi: 10.1166/jnn.2004.130. [DOI] [PubMed] [Google Scholar]
- 3.Lockman PR, Koziara JM, Mumper RJ, Allen DD. J. Drug Target. 2004;12:635–641. doi: 10.1080/10611860400015936. [DOI] [PubMed] [Google Scholar]
- 4.Hauck TS, Ghazani AA, Chan WC. Small. 2008;4:153–159. doi: 10.1002/smll.200700217. [DOI] [PubMed] [Google Scholar]
- 5.Pan J, Feng SS. Biomaterials. 2008;29:2663–2672. doi: 10.1016/j.biomaterials.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Balogh L, Nigavekar SS, Nair BM, Lesniak W, Zhang C, Sung LY, Kariapper MS, El-Jawahri A, Llanes M, Bolton B, Mamou F, Tan W, Hutson A, Minc L, Khan MK. Nanomedicine. 2007;3:281–296. doi: 10.1016/j.nano.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Tabata Y, Ikada Y. Biomaterials. 1988;9:356–362. doi: 10.1016/0142-9612(88)90033-6. [DOI] [PubMed] [Google Scholar]
- 8.Sahoo SK, Panyam J, Prabha S, Labhasetwar VJ. Controlled Release. 2002;82:105–114. doi: 10.1016/s0168-3659(02)00127-x. [DOI] [PubMed] [Google Scholar]
- 9.Woodle MC. Adv. Drug Del. Rev. 1998;32:139–152. doi: 10.1016/s0169-409x(97)00136-1. [DOI] [PubMed] [Google Scholar]
- 10.Ran S, Downes A, Thorpe PE. Cancer Res. 2002;62:6132–6140. [PubMed] [Google Scholar]
- 11.Ran S, He J, Huang XM, Soares M, Scothorn D, Thorpe PE. Clin. Cancer Res. 2005;11:1551–1562. doi: 10.1158/1078-0432.CCR-04-1645. [DOI] [PubMed] [Google Scholar]
- 12.Nafee N, Taetz S, Schneider M, Schaefer UF, Lehr CM. Nanomedicine. 2007;3:173–183. doi: 10.1016/j.nano.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Minigo G, Scholzen A, Tang CK, Hanley JC, Kalkanidis M, Pietersz GA, Apostolopoulos V, Plebanski M. Vaccine. 2007;25:1316–1327. doi: 10.1016/j.vaccine.2006.09.086. [DOI] [PubMed] [Google Scholar]
- 14.Zhu SG, Xiang JJ, Li XL, Shen SR, Lu HB, Zhou J, Xiong W, Zhang BC, Nie XM, Zhou M, Tang K, Li GY. Biotechnol. Appl. Biochem. 2004;39:179–187. doi: 10.1042/BA20030077. [DOI] [PubMed] [Google Scholar]
- 15.Labhasetwar V, Song C, Humphrey W, Shebuski R, Levy RJ. J. Pharm. Sci. 1998;87:1229–1234. doi: 10.1021/js980021f. [DOI] [PubMed] [Google Scholar]
- 16.Mitra D, Bhattacharya SC, Moulik SP. J. Phys. Chem. B. 2008;112:6609–6619. doi: 10.1021/jp800320a. [DOI] [PubMed] [Google Scholar]
- 17.Wang JB, Han BX, Dai M, Yan HK, Li ZX, Thomas RK. J. Colloid Interface Sci. 1999;213:596–601. doi: 10.1006/jcis.1999.6147. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Taylor DJF, Li PX, Thomas RK. Langmuir. 2008;24:1863–1872. doi: 10.1021/la7021566. [DOI] [PubMed] [Google Scholar]
- 19.Peetla C, Labhasetwar V. Mol. Pharmaceutics. 2008;5:418–429. doi: 10.1021/mp700140a. [DOI] [PubMed] [Google Scholar]
- 20.Deshayes S, Morris MC, Divita G, Heitz F. Interactions of cellpenetrating peptides with model membranes. In: Langel U, editor. Handbook of Cell-Penetrating Peptides. CRC/Taylor & Francis; Boca Raton: 2007. pp. 139–160. [Google Scholar]
- 21.Leray C, Andriamampandry M, Gutbier G, Cavadenti J, KleinSoyer C, Gachet C, Cazenave JP. J. Chromatogr. B. 1997;696:33–42. doi: 10.1016/s0378-4347(97)00230-2. [DOI] [PubMed] [Google Scholar]
- 22.Zwaal RFAD,RA, Roelofsen B, Deenen LLM. Trends Biochem. Sci. 1976;1:112–114. [Google Scholar]
- 23.Peetla C, Graf K, Kressler J. Colloid. Polym. Sci. 2006;285:27–37. [Google Scholar]
- 24.Davda J, Labhasetwar V. Int. J. Pharm. 2002;233:51–59. doi: 10.1016/s0378-5173(01)00923-1. [DOI] [PubMed] [Google Scholar]
- 25.Murakami H, Kawashima Y, Niwa T, Hino T, Takeuchi H, Kobayashi M. Int. J. Pharm. 1997;149:43–49. [Google Scholar]
- 26.Larios C, Minones J, Haro I, Alsina MA, Busquets MA, Trillo JM. J. Phys. Chem. B. 2006;110:23292–23299. doi: 10.1021/jp0628582. [DOI] [PubMed] [Google Scholar]
- 27.Santos HA, Garcia-Morales V, Roozeman RJ, Manzanares JA, Kontturi K. Langmuir. 2005;21:5475–5484. doi: 10.1021/la046825u. [DOI] [PubMed] [Google Scholar]
- 28.Zhang LJ, Rozek A, Hancock REW. J. Biol. Chem. 2001;276:35714–35722. doi: 10.1074/jbc.M104925200. [DOI] [PubMed] [Google Scholar]
- 29.Atkin R, Craig VSJ, Wanless EJ, Biggs S. Adv. Colloid Interface Sci. 2003;103:219–304. doi: 10.1016/S0001-8686(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 30.Esumi K, Matoba M, Yamanaka Y. Langmuir. 1996;12:2130–2135. [Google Scholar]
- 31.Esumi K, Taguma K, Koide Y. Langmuir. 1996;12:4039–4041. [Google Scholar]
- 32.Maskarinec SA, Lee KYC. Langmuir. 2003;19:1809–1815. [Google Scholar]
- 33.Mecke A, Lee DK, Ramamoorthy A, Orr BG, Holl MM. Langmuir. 2005;21:8588–8590. doi: 10.1021/la051800w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bakshi MS, Kaura A, Mahajan RK, Yoshimura T, Esumi K. Colloids Surf. Physicochem. Eng. Aspects. 2004;246:39–48. [Google Scholar]
- 35.Bhattacharya S, Srivastava A. Langmuir. 2003;19:4439–4447. [Google Scholar]
- 36.Chen X, Wang J, Liu M. J. Colloid Interface Sci. 2005;287:185–190. doi: 10.1016/j.jcis.2005.01.065. [DOI] [PubMed] [Google Scholar]
- 37.Vasir JK, Labhasetwar V. Biomaterials. 2008;29:4244–4252. doi: 10.1016/j.biomaterials.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]