Abstract
Recently, it was shown that d-cycloserine (DCS; a NMDA partial agonist) facilitated extinction of fear as well as cocaine conditioned place preference (CPP) in rats.
Methods
The present study examined the effects of DCS (15mg/kg i.p and 30 mg/kg i.p) on extinction and renewal of cocaine-induced CPP in C57bL/c mice. In parallel we examined the effects of DCS on locomotor activity.
Results
Extinction to cocaine CPP was significantly faster with DCS than with vehicle treatment (3 sessions, versus 6 sessions respectively). After extinction was achieved; mice were retested for CPP 1 and 2 weeks later. All animals maintained extinction to CPP one week later but at 2 weeks the vehicle and the 15mg/kg DCS treated animals maintained the extinction but the 30mg/kg DCS treated mice had renewed CPP. During induction of cocaine CPP, mice displayed enhanced locomotor activity following treatment with cocaine, as expected based on previous literature. During extinction, there were no differences in locomotor activity between the vehicle and the 15mg/kg DCS treated mice whereas the 30mg/kg DCS treated animal showed significant locomotor activity inhibition. These results corroborate in mice the previously reported acceleration of extinction to cocaine induced CPP by DCS in rats. However we also show that the higher DCS doses facilitated CPP reestablishment after extinction. Thus while DCS could be beneficial in accelerating the extinction to conditioned responses in addiction it could, at higher doses, increase the risk of relapse. Thus studies evaluating the beneficial therapeutic effects of DCS should assess not only the short-term effects but also the potential of longer lasting undesirable effects.
Keywords: Learning, glutamate, withdrawal, abstinence, drug abuse, NMDA, addiction, substance abuse
1. Introduction
Exposure to conditioned cues (stimuli associated with the drug) is a key contributor to relapse in addicted individuals. In the case of cocaine, both preclinical and clinical studies have shown that when the addicted animal or person is exposed to stimuli or environments associated with cocaine (cocaine cues) there is an increase in the concentration of dopamine - neurotransmitter associated with reward and prediction of reward - in the striatum. This is believed to reflect a learning process mediated in part through the amygdala and the prefrontal cortex that involves neuroplastic changes mediated in part by glutamatergic neurotransmission (Kalivas and Volkow 2005).
Particularly relevant are AMPA (alpha-amino-3-hydroxy-5-methyl-isoxazole propionic acid) and NMDA (N-methyl-D-asparate) receptors, which participate in the neuroplastic processes associated with learning and memory including long term potentiation (Rao and Finkbeiner 2007). Indeed acute and chronic cocaine potentiated synaptic strength in the ventral tegmental area (VTA) through changes in AMPA receptors, which is an effect blocked by NMDA receptor antagonist (Borgland et al 2004). There is also evidence that NMDA and AMPA receptors are involved in cocaine-seeking behavior controlled in part by drug-associated cues (Di Ciano and Everitt 2001). This has generated interest on the therapeutic potential of medications that interact through NMDA as well as AMPA receptors for the treatment of cocaine addiction. D-cycloserine, (DCS), a partial NMDA receptor agonist (Klodzinska and Chojnacka-Wojcik 2000) has been shown to facilitate extinction to conditioned place preference (CPP) to cocaine in rats (Botreau et al 2006). Facilitated extinction was interpreted to reflect the ability of DCS to enhance memory consolidation processes during the extinction conditions, via its effects in NMDA receptors (Botreau et al 2006). Here we extend these findings by evaluating the effects of two doses of DCS (15 and 30 mg/kg ip) in the extinction of cocaine CPP and assessing the stability of this facilitated extinction by examining CPP one and two weeks after extinction has been established; furthermore, locomotor behavior during cocaine CPP as well as extinction under test and control conditions will also be evaluated.
2. Materials and Methods
2.1 Subjects
Thirty six, adolescent (5 week old), male C57bl/c mice (Charles River Laboratories) weighing 20–25 grams were used in this study. Mice were allowed seven days to habituate to experimental conditions: temperature (72±2°F); controlled humidity (40–60%); a twelve hour reverse light cycle (lights off at 08:00 hours). Mice were individually housed and kept on an ad lib diet (in an accredited animal husbandry facility that was approved by the Association for Assessment of Laboratory Animal Care, and the Institutional Animal Care and Use Committee of Brookhaven National Laboratory).
2.2 Apparatus
The CPP apparatus (Habitest - Coulbourn Instruments; Allentown, PA) was composed of two compartments (30.5cm length × 26.5cm width × 37cm height) that were connected by a central corridor (12.75cm length × 23cm width × 15.25cm height). The compartment on the left had a striped black/white wall color with a perforated stainless steel floor with round holes on staggered centers, the central corridor was transparent with a smooth flexi-glass floor and the left compartment had a white wall color with a stainless steel mesh floor. Four infrared beams were used to assess the animal’s location, preference and locomotor activity. Two infrared beams on the ceiling of the black and white compartments map the area; each movement made by the animal was counted as a beam break. The infrared beams were connected to a Cobalt Computer and data was acquired with Graphic State version II software (Coulbourn Instruments, Allentown, PA).
2.3 Procedures: Conditioned Place Preference, Extinction and Locomotor Activity
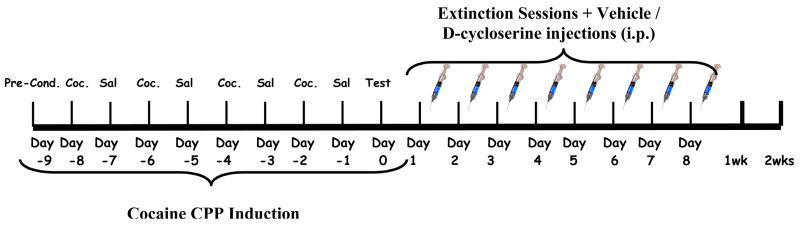
The procedure of this study consisted of several phases as outlined in figure 1.
Figure 1.
Timeline of the study.
2.3.1 Preconditioning Phase
On day one all mice were placed in the center corridor; the automated sliding doors leading to both compartments were then opened giving the animal free access to both compartments for a total of a 15 minute session. During this period locomotor activity (measured in beam breaks) and the time spent in each compartment (measured in milliseconds) was calculated. Mice remained in the pre-conditioning phase until they had reached our protocol criteria of non-preference for either compartment. This criterion was met once the animals demonstrated no preference for either compartment. Mice generally showed no preference between the two compartments on the first day or two of the study. Mice reached this preconditioning criterion in one or two sessions.
2.3.2 Conditioning Phase
Mice where randomly assigned and counterbalanced to receive four drug-paired sessions in one compartment (every other day, ie. day 2, 4, 6 and 8) and four saline-paired sessions in the opposite compartment on alternate days (see figure 1). This phase took place over a period of eight days and each session was for 40 minutes. Locomotor activity (measured in beam breaks) was assessed daily in this phase for both the drug and saline sessions.
2.3.3 Test Day
On day 10 the mice were placed in the center corridor where they had access to both compartments for a total of 15 minutes (figure 1). Total % preference was assessed for each of the two compartments by measuring the time spent in each compartment (measured in milliseconds).
2.3.4 Extinction Phase
On day 11, extinction sessions began. Mice were administered a saline injection (i.p.) and placed in the previously drug-paired compartment for forty minutes; on alternate days the animals are placed in the previously saline-paired compartment, again for forty minutes, after receiving a saline injection (i.p) for forty minutes each.
2.3.5 Extinction Test
After each extinction session, mice were placed back into the center corridor and allowed access to the entire apparatus for fifteen minutes (adapted from (Botreau et al 2006). The extinction phase was continuous until the mice returned to and maintained baseline behavior.
2.4 Drugs
Cocaine HCl (Sigma-Aldrich, St. Louis) was used in the dose of 20mg/kg (2 M; 10 ml/kg i.p.) during the induction of conditioned place preference. D-cycloserine (Sigma-Aldrich, St. Louis) was used in a 15mg/kg dose (1.5 M; 10 ml/kg i.p.) and a 30mg/kg dose (3 M; 10 ml/kg i.p.) during extinction. Saline (0.9% NaCl) was used (10 ml/kg i.p) as the vehicle solution.
2.5 Groups
Three groups of randomly selected male naïve mice were administered cocaine, at a dose of 20 mg/kg (i.p.), during drug days of the conditioning period. Upon completion of CPP induction, these animals received extinction training. Immediately after the end of each session, mice were injected (i.p) with either: a) vehicle (N = 10) b) 15 mg/kg DCS or 30 mg/kg DCS (N=12). Mice were then tested again for CPP one and two weeks after the last extinction session.
2.6. Statistical Analysis
One way repeated-measure ANOVAs were used to analyze the preference and locomotor activity data, respectively. This was followed by pair-wise comparisons (Holm-Sidak method) were also performed and reported. All statistical comparisons were performed using the SigmaStat 3.1 statistical software.
3. Results
3.1 Conditioned Place Preference and Extinction
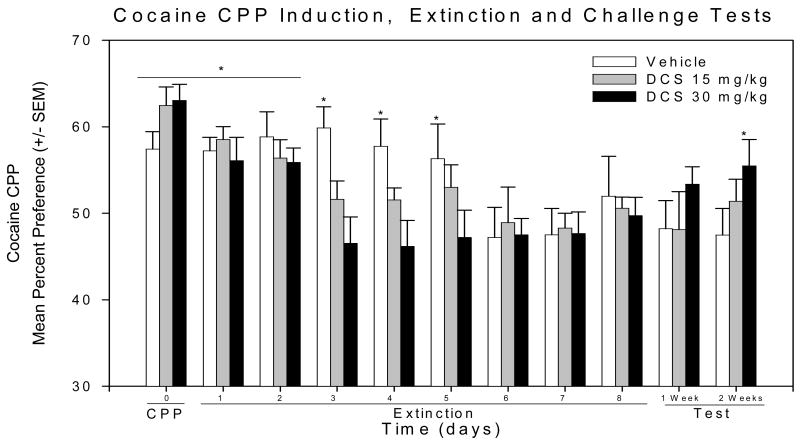
CPP Induction (Day 0/pre-extinction)
A one-way ANOVA was conducted and revealed significant preference for the 20 mg/kg i.p. cocaine-paired compartment on day zero (0) – day prior to extinction - [F (1, 33) = 170.017; P < 0.001] for all mice (see figure 2). After test-day, mice were randomly divided into one of three treatment groups (vehicle, DCS 15 mg/kg and DCS 30 mg/kg). A one-way ANOVA was used to examine CPP across treatment groups. As a result; analysis on the three groups indicated a significance for the cocaine-paired compartment [F(1, 33) = 176.682; p < 0.001] in all three groups (Figure 2). Pair-wise comparisons (Holm-Sidak method) between the saline-paired compartment and the cocaine-paired compartment were performed, within each treatment group, for CPP. Significant CPP to the cocaine-paired compartment was observed in all three groups; vehicle (t = 4.963; P < 0.05), 15 mg/kg i.p. DCS (t = 8.741; P < 0.05) and 30 mg/kg i.p. DCS (t = 9.548; P < 0.05).
Figure 2.
Cocaine conditioned place preference: (*) Indicates significant preference for the cocaine paired compartment.
3.2 Extinction of CPP (Days 1–8)
After cocaine CPP (figure 2) mice were separated into their respective treatment groups, underwent a series of extinction sessions, adapted from (Botreau et al 2006;Itzhak 2001). A series of one-way repeated measure ANOVAs were conducted across treatment groups examining the progression of extinction of cocaine CPP over time; specifically, each treatment group showed the following:
a) Vehicle
A statistical significance was found over time during extinction of cocaine CPP in the vehicle treated mice (F (9,10) = 3.695; P < 0.001). Pairwise comparison (Holm-Sidak method) revealed significant cocaine –paired CPP during the extinction phase (t = 2.496; P <0.05; fig. 2) for five days. At day 6 of extinction training session, CPP was lost (t = 1.789; P > 0.05; fig 2); and this lack of significant CPP was observed to last through the end of extinction training. Tests for CPP were also performed one and two weeks later and again no significant CPP was found at both times (t = 0.798; P > 0.05 and t = 1.084; P > 0.05; respectively; fig 2).
b) D-cycloserine 15mg/kg
The 15 mg/kg i.p. DCS treated mice revealed statistical significance in the extinction of CPP [F (1, 11) = 5.716; P < 0.05; figure 2]. Pair-wise comparisons (Holm-Sidak method) revealed that cocaine CPP was present during the initial days of extinction training with15 mg/kg i.p. DCS (t = 2.460; P < 0.05; fig 2). However, no significant CPP, extinction, was observed (t = 0.623; P > 0.05; fig 2) by day 3 of extinction. CPP performed one and two weeks later also revealed no significant CPP (t = 0.726; P > 0.05 and t = 0.534; P > 0.05; respectively; fig 2).
c) D-cycloserine 30 mg/kg
The 30 mg/kg DCS treated mice showed significant effects on extinction to cocaine CPP [F (9, 12) = 2.721; P < 0.01]. Pair-wise comparisons (Holm-Sidak method) revealed cocaine CPP was present during the first two days of extinction (t = 2.266; P < 0.05; fig 2). However, by day 3 of extinction no significant CPP was observed (t = 1.478; P > 0.05; fig 2). CPP testing performed one and two weeks after the end of extinction training showed no significant CPP found on the one week (t = 1.294; P > 0.05; fig 2). In contrast, a statistically significant CPP to the cocaine-paired compartment had returned at the two weeks post-extinction test (t = 2.114; P < 0.05; fig 2).
3.3. Locomotor Activity
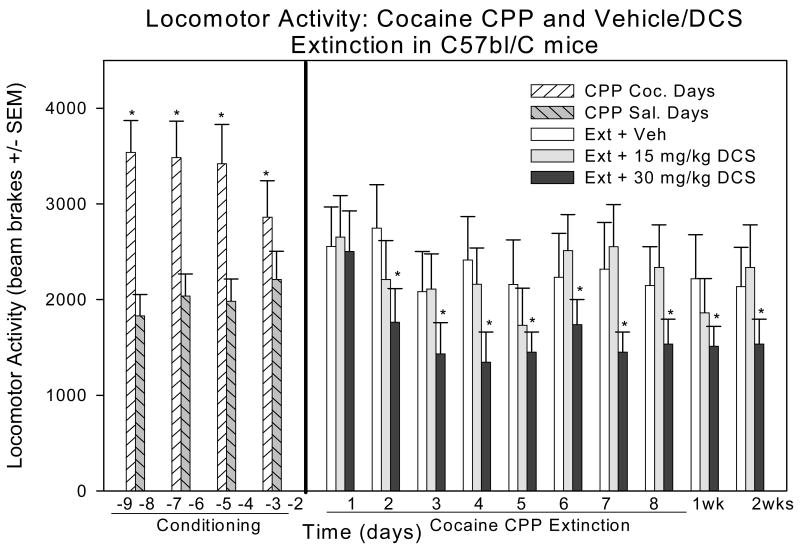
a) During Cocaine CPP
As expected, a one-way repeated measures ANOVA, for the conditioning days, revealed a statistically significant difference in locomotor activity between the cocaine-paired versus the saline-paired compartments across all mice [F(7, 33) = 14.265; P < 0.05; Figure 3].
Figure 3.
Locomotor activity during cocaine CPP, Extinction and Post-extinction testing: (*) indicates significant difference in locomotor activity between Vehicle and 30 mg/kg i.p. DCS; (**) indicates significant difference in locomotor activity between 15 mg/kg i.p. DCS and 30 mg/kg i.p. DCS.
b) During Extinction of Cocaine CPP
A 2-way ANOVA was used to analyze the locomotor activity during the extinction training sessions. Comparison between treatment groups indicated statistical significance in locomotor activity [F (2, 33) = 8.468; P < 0.001]. Time, however, did not have a significant difference in locomotor activity during extinction [F (2, 33) = 1.187; P > 0.05; fig 3].
Pair-wise comparisons (Holm-Sidak method) revealed significant locomotor activity differences in the comparison of treatment groups: locomotor activity was lower for the 30 mg/kg i.p. DCS than for vehicle (t = 3.601; P < 0.001), for the 30 mg/kg i.p. DCS than the 15 mg/kg i.p. DCS (t = 3.442; P < 0.001; fig 3); but no significant difference was found between the vehicle and 15 mg/kg i.p. DCS treatment groups (t = 0.241; P > 0.05; fig 3).
4. Discussion
The present findings replicate the facilitative effects of treatment with 15 mg/kg of DCS on the extinction of cocaine CPP in rats (Botreau et al 2006), and in mice (Kelley et al 2007). In addition, the present study examined more than one DCS dose. Specifically a higher DCS dose (30 mg/kg) was tested and similarly shown to facilitate extinction. Furthermore this study examined the long-term effects of DCS on extinction of cocaine CPP. Results showed that dose did have a major impact on long-term efficacy of extinction of cocaine CPP. Specifically, 30mg/kg DCS failed to maintain extinction to cocaine CPP when tested two weeks later and actually increased cocaine CPP. The facilitation of extinction of cocaine CPP by DCS was consistent with findings from preclinical and clinical studies indicating the facilitative effects of DCS on extinction to conditioned fear and that it accelerates desensitization training to acrophobia (Davis et al 2006; Guastella et al 2007; Parnas 2005; Woods and Bouton 2006). It is believed that the extinction reflects new learning rather than erasure of the conditioned memory. In this respect DCS is believed to act by facilitating the creating of a new memory via its effects on NMDA receptors (Vervliet 2008). This new memory then interferes with the expression of the conditioned memory driving CPP.
4.1 Conditioned Place Preference
Induction
Cocaine (20 mg/kg i.p.) induced significant CPP, consistent with the literature (Brabant et al 2005a; Itzhak and Martin 2002).
a) Extinction - Vehicle
Extinction occurred more rapidly in the DCS-paired groups, at both doses, in comparison to the vehicle group. Vehicle treated mice required 6 days to show extinction of the cocaine CPP; these results are comparable to previous reports on cocaine extinction in mice [20mg/kg cocaine; Swiss Webster mice; (Itzhak and Martin 2002)].
b) Extinction – DCS 15mg/kg and 30mg/kg
DCS (15 and 30mg/kg) demonstrated to have an effect on accelerating the rate of extinction of cocaine CPP. This was in agreement with prior findings in rats (Botreau et al 2006) and mice (Kelley et al 2007). Extinction of cocaine CPP was achieved after 3 days in mice treated with DCS compared to 6 days in the vehicle treated mice. While similar results were observed for both the 15 and 30 mg/kg DCS treated mice; there was a dose dependent pattern in accelerating the extinction of cocaine CPP.
Renewal of CPP
In the present study we also examined the duration of the effects of DCS on the acquired extinction of cocaine CPP. All mice were presented with a challenge CPP test one and two weeks after the end of extinction training. One week following extinction, all the DCS treated mice still showed extinction. At two weeks, the vehicle and 15 mg/kg DCS treated mice still showed extinction of cocaine CPP; whereas the 30 mg/kg DCS treated mice no longer did. To our knowledge this is the first time that a facilitation of renewal to cocaine CPP has been noted after DCS treatment at this dose (30 mg/kg i.p. DCS). Studies on fear extinction with DCS have reported that DCS reduces some forms of relapse (reduced renewal), but not others (contextual renewal, rapid reacquisition) (Vervliet 2007). To the extent that CPP renewal serves as a model to relapse in drug addiction our findings of an enhanced renewal with the higher dose of DCS would suggest that higher doses could be potentially deleterious. Thus, further studies are required to determine the extent to which DCS while facilitating initial extinction over longer time periods could facilitate renewal (including assessments longer than 2 week time periods) and the effects of doses in these responses.
4.2 Locomotor Activity
Locomotor activity of rats during cocaine CPP induction has been previously studied; however, in this study we also assessed this locomotor activity following treatment with DCS and during cocaine CPP extinction, which has not been previously examined. The effects of cocaine (20mg/kg i.p.) on locomotor activity during the cocaine CPP, in the present study supported previous findings (Brabant et al 2005b; Orsini et al 2005).
Locomotor activity post DCS treatment, that is, during extinction of the cocaine CPP, showed that mice treated with a high dose of DCS (30 mg/kg ip) significantly reduced their locomotor activity and this was maintained up to two weeks later (post-extinction). Prior studies had shown that NMDA receptor antagonist inhibit hypermotility in rats (Dall’Olio et al 1994), thus suggesting that the 30 mg/kg i.p. DCS (a partial-antagonist) may have shown similar properties here. Indeed prior studies had shown the DCS at high doses behaves like an NMDA antagonist (Klodzinska and Chojnacka-Wojcik 2000).
Studies on the effects of DCS and extinction have previously been well examined on conditioned fear and anxiety. Much less research however exists on the effect of DCS on extinction of drugs of abuse behavior, such as cocaine CPP in rats (Botreau et al 2006). The potential of DCS in treatment of drug seeking and preference remains novel and has not been well examined; particularly its long-term effects. In addition, the duration of this extinction of cocaine CPP has not been examined nor in terms of the optimal dose of DCS as in a dose response study for such an effect.
Limitations
The protocol used in this CPP study, although widely used in the literature it had some limitations. Specifically, because the session length was different on test day compared to other days (15 minute long sessions compared to 40 minutes) we could not make that specific comparison in terms of locomotor activity on test day. Furthermore, the effect of different doses of DCS on locomotor activity are not well characterized in mice, therefore it is difficult to provide an accurate comparison with the literature in terms of baseline effects. Future work needs to further examine the acute and long-term effects of DCS on locomotor activity. Such studies could provide a greater understanding of the effects of DCS on locomotor activity.
Summary
The present findings supported the further evaluation of NMDA partial agonists as potential treatments to facilitate extinction to conditioned responses in cocaine addicted subjects. Recently, a study on the effects of antagonism of NMDA receptors on reconsolidation of drug addiction memories has produced strong data revealing that drugs with the potential to modulate glutamatergic transmissions at the NMDA receptor, such as DCS; may have potential use in the treatment of relapse of drug addiction (Milton et al 2008). The documented interactions of DCS at the NMDA receptor site, along with the data obtained from this and previous literature on extinction of cocaine CPP and conditioned fear, further aid in strengthening this approach. Finally the present results suggested that future research should examine further the duration of DCS effects as well as the dose-dependent effects of DCS on cocaine CPP as well as any potential long-term effects on behavior.
Acknowledgments
This work was supported by the Intramural Research Program of NIAAA at the National Institute of Health (NIH), and the U.S. Department of Energy under contract DE-AC02-98CH108886 and the Behavioral Pharmacology and Neuroimaging Lab (http://www.bnl.gov/thanoslab). Carlos Bermeo was partially supported by the NIDA summer research program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botreau F, Paolone G, Stewart J. d-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172:173–178. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Brabant C, Charlier Y, Quertemont E, Tirelli E. The H3 antagonist thioperamide reveals conditioned preference for a context associated with an inactive small dose of cocaine in C57BL/6J mice. Behavioural brain research. 2005a;160:161–168. doi: 10.1016/j.bbr.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Brabant C, Quertemont E, Tirelli E. Evidence that the relations between novelty-induced activity, locomotor stimulation and place preference induced by cocaine qualitatively depend upon the dose: a multiple regression analysis in inbred C57BL/6J mice. Behavioural brain research. 2005b;158:201–210. doi: 10.1016/j.bbr.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Dall’Olio R, Rimondini R, Gandolfi O. The NMDA positive modulator D-cycloserine inhibits dopamine-mediated behaviors in the rat. Neuropharmacology. 1994;33:55–59. doi: 10.1016/0028-3908(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on extinction and fear conditioning in humans. Behav Res Ther. 2007;45:663–672. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL. Cocaine-induced conditioned place preference in mice: induction, extinction and reinstatement by related psychostimulants. Neuropsychopharmacology. 2002;26:130–134. doi: 10.1016/S0893-133X(01)00303-7. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kelley JB, Anderson KL, Itzhak Y. Long-term memory of cocaine-associated context: disruption and reinstatement. Neuroreport. 2007;18:777–780. doi: 10.1097/WNR.0b013e3280c1e2e7. [DOI] [PubMed] [Google Scholar]
- Klodzinska A, Chojnacka-Wojcik E. Anticonflict effect of the glycineB receptor partial agonist, D-cycloserine, in rats. Pharmacological analysis. Psychopharmacology (Berl) 2000;152:224–228. doi: 10.1007/s002130000547. [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J Neurosci. 2008;28:8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini C, Bonito-Oliva A, Conversi D, Cabib S. Susceptibility to conditioned place preference induced by addictive drugs in mice of the C57BL/6 and DBA/2 inbred strains. Psychopharmacology. 2005;181:327–336. doi: 10.1007/s00213-005-2259-6. [DOI] [PubMed] [Google Scholar]
- Marianne Parnas ASW, Rick Richardson. Effects of Multiple Exposures to D-Cycloserine on Extinction of Conditioned Fear in Rats. Neurobiology of Learning and Memory. 2005;83:224–231. doi: 10.1016/j.nlm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Rao VR, Finkbeiner S. NMDA and AMPA receptors: old channels, new tricks. Trends Neurosci. 2007;30:284–291. doi: 10.1016/j.tins.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Vervliet B. Learning and memory in conditioned fear extinction: Effects of d-cycloserine. Acta Psychol (Amst) 2007 doi: 10.1016/j.actpsy.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Vervliet B. Learning and memory in conditioned fear extinction: effects of D-cycloserine. Acta Psychol (Amst) 2008;127:601–613. doi: 10.1016/j.actpsy.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behav Neurosci. 2006;120:1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]