Abstract
The origin of circulating DHEA and adrenal-derived androgens in humans and nonhuman primates is largely distinct from other mammalian species. In humans and many Old world primates, the fetal adrenal gland and adult zona reticularis (ZR) are known to be the source for production of DHEA (and DHEAS) in mg quantities. In spite of similarities there are also some differences. Herein, we take a comparative endocrine approach to the diversity of adrenal androgen biosynthesis and its developmental timing in three primate species to illustrate how understanding such differences may provide unique insight into mechanisms underlying adrenal androgen regulation and its pathophysiology in humans. We contrast the conventional developmental onset of adrenal DHEA biosynthesis at adrenarche in humans with (1) an earlier, peri-partutrition onset of adrenal DHEA synthesis in rhesus macaques (Old World primate) and (2) a more dynamic and reversible onset of adrenal DHEA biosynthesis in female marmosets (New World primate), and further consider these events in terms of the corresponding developmental changes in expression of CYP17, HSD3B2 and CYB5 in the ZR. We also integrate these observations with recently described biochemical characterization of CYP17 cDNA cloned from each of these nonhuman primate species and the corresponding effects of phosphorylation versus CYB5 coexpression on 17,20 lyase versus 17-hydroxylase activity in each case. In addition, female rhesus macaques exposed in utero to exogenous androgen excess, exhibit symptoms of adrenal hyperandrogenism in adult females in a manner reminiscent of that seen in the human condition of PCOS. The possible mechanisms underlying such adrenal hyperandrogenism are further considered in terms of the effects of altered relative expression of CYP17, HSD3B2 and CYB5 as well as the altered signaling responses of various kinases including protein kinase A, or the insulin sensitive PI3-kinase/AKT signaling pathway which may impact on 17,20 lyase activity. We conclude that while the triggers for the onset of ZR function in all three species show clear differences (age, stage of development, social status, gender), there are still common mechanisms driving an increase in DHEA biosynthesis in each case. A full understanding of the mechanisms that control 17,20 lyase function and dysfunction in humans may best be achieved by comparative studies of the endocrine mechanisms controlling adrenal ZR function and dysfunction in these nonhuman primate species.
Introduction
The adrenal cortex is considered a compound endocrine organ giving rise to production of mineralocorticoids, glucocorticoids and dehydroepiandrosterone and its sulfate (DHEA and DHEAS, collectively termed adrenal androgens, or as we refer to here, the C19 steroids). These steroids arise in humans from three distinct zones namely the zonae glomerulosa, fasciculata and reticularis respectively. We have previously reviewed the structure and function of the mammalian adrenal cortex and how adrenal and gonadal function are compartmentalized through not only physical but also biochemical mechanisms (Conley and Bird 1997), and we have also given an overview of primates as a model for human adrenal function (Conley, Pattison and Bird 2004). In this review, we focus on zona reticularis (ZR) function and dysfunction in primates. We begin with how the adrenal ZR achieves C19 steroid biosynthesis with a particular emphasis on recent findings in rhesus (Old World primate) and marmoset (New World primate). The second area of emphasis involves altered rhesus adrenal C19 steroid biosynthesis under conditions which give rise to the hyperandrogenic and infertility condition of polycystic ovary syndrome (PCOS) and how that further informs us about human adrenal ZR function and dysfunction.
Requirements for Adrenal C19 Steroid production
Whereas there is a great deal known of both the mechanism of adrenal aldosterone and cortisol production, and the endocrine control of the respective zonae glomerulosa and fasciculata that make them, comparatively little is known of the mechanisms controlling C19 steroid production by the human and nonhuman primate ZR or the human fetal adrenal zone. It is clear that once cholesterol has been transported by StAR and converted to pregnenolone by cytochrome P450scc (CYP11A1), the actual nature of the end product of a steroid secreting cell is a complement of the other P450s and hydroxysteroid dehydrogenases (HSDs) expressed therein (Conley and Bird 1997). Since the cloning of the respective P450s and 3βHSD enzymes beginning in the 1980’s, it is also clear that the ability of the adrenal zona fasciculata to predominantly synthesize cortisol to physiologic levels without significant C19 steroid byproducts is largely dependent on an abundant expression of P450c17 (CYP17) matched by abundant expression of 3βHSD type II (HSD3B2) (Conley and Bird 1997) (See Figure 1A). Under these conditions the commitment of the pathway to 17-hydroxyprogesterone biosynthesis is in itself a commitment in humans and nonhuman primates (and indeed sheep cows and goats) to cortisol biosynthesis (Conley and Bird 1997, and see below). On the other hand, for the human ZR or fetal zone to efficiently synthesize C19 steroids, and do so in mg quantities, more extreme conditions are required to avoid 17-hydroxyprogesterone biosynthesis and instead favour DHEA biosynthesis. Histologic studies suggest in humans that in addition to high CYP17 expression, there is also a requirement for little or no expression of the otherwise competing HSD3B2 (Gell et al 1996, 1998), together with high expression levels of cytochrome b5 (CYB5)(Suzuki et al 2000, Dharia et al 2004) (See Figure 1B). These are simple and even reliable markers of zonal function necessary for DHEA production, with high CYB5 being the clearest marker. The fetal zone of the human fetal adrenal gland and the post adrenarche (usually by 6–8 years of age) ZR both demonstrate these attributes (reviewed in Havelock et al 2004).
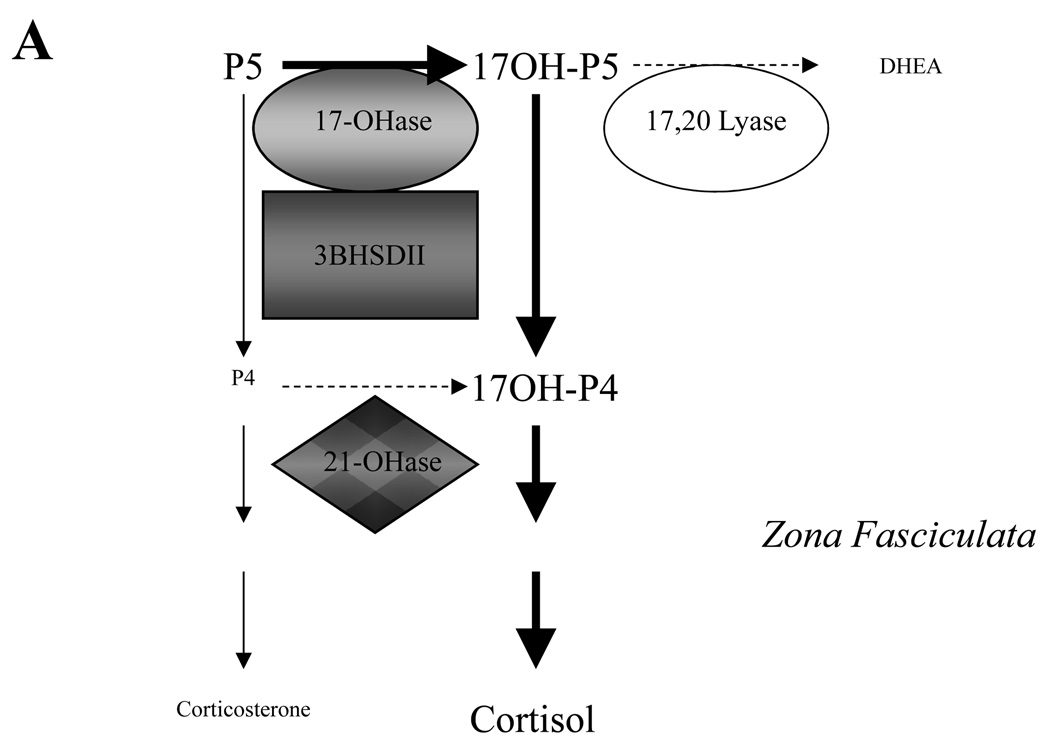
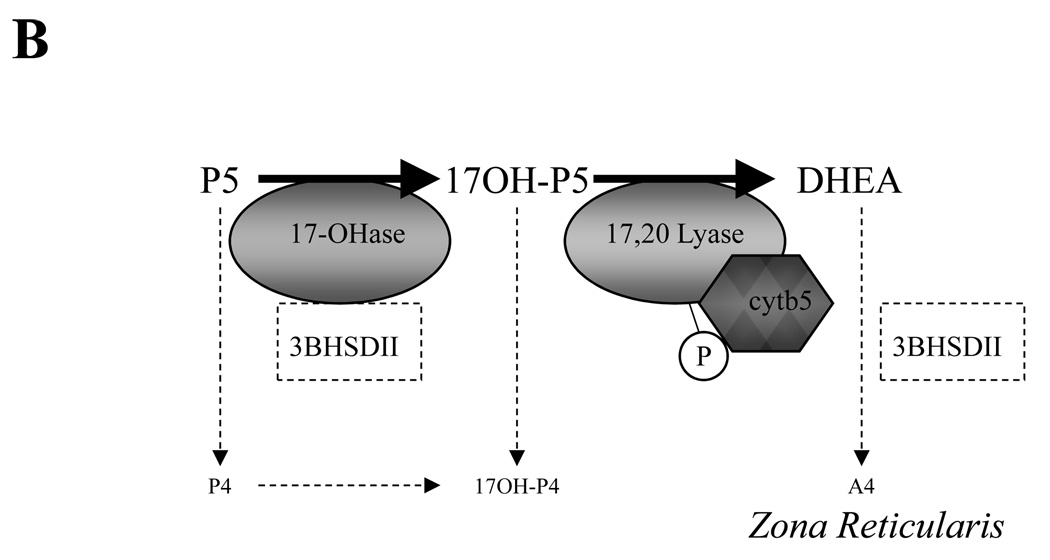
Figure 1. Relationship between relative expression levels of CYP17, HSD3BII and CYB5 in the adrenal zona fasciculata and ZR as suggested by histologic studies of human and nonhuman primate adrenals, and the resulting predominant direction of pregnenolone metabolism.
In the Zona fasciculata (Panel A) the higher affinity of CYP17 17-hydroxylase activity (17-OHase) for pregnenolone directs pregneolone metabolism first to 17-hydroxypregnenolone (17-OHP5), and subsequent abundant levels of 3BHSDII then directs further conversion to 17-hydroxyprogesterone (17-OHP4). In the absence of any significant CYP17 delta 4 lyase activity, the highly efficient CYP21 enzyme also present in the same membranes can then undertake 21-hydroxylation (21-Ohase) to make deoxycortisol and so commit to cortisol production. However, in the ZR (Panel B) where CYP17 is still highly expressed but 3BHSDII is expressed at a lower level or absent, high levels of CYB5 unique to this zone bind CYP17 and (together with CYP17 phosphorylation?) enhance the relative 17–20 lyase activity. In the presence of CYB5 and the relative absence of 3BHSDII, the net direction of pregnenolone metabolism is now 17-OHase activity to 17-hydroxypregnenolone and then 17–20 lyase conversion to DHEA. In the presence of low levels of 3BHSDII, some additional conversion to androstenedione (A4) is possible. Other abbreviations: Progesterone, P4.
An understanding of the reasons behind the histologic patterns of gene expression necessary for efficient DHEA synthesis in human ZR and indeed the fetal zone is apparent when one considers the molecular and biochemical determinants of C19 secretion by CYP17 and its competing HSD3BII. In addition to the previously described considerations of CYP17 substrate preference, and poor capability of delta 4 lyase (i.e., conversion of 17-hydroxyprogesterone to androstenedione) there is also the possible influence of altered CYP17 phosphorylation through signaling kinases, and the influence of coexpressed CYB5. Some of this has been reviewed in detail previously (Conley and Bird 1997), but we need to reemphasize here the physiologic consequence of the inability of CYP17 to efficiently use 17-hydroxyprogesterone as a substrate for significant androstenedione production. This relatively low delta 4 lyase activity which occurs in humans and many nonhuman primates studied to date (as well as sheep, cows and goats) means DHEA is an obligatory intermediate in any C19 biosythetic pathway in these species. In addition, a preference of CYP17 for pregnenolone as a substrate over progesterone in species such as humans and nonhuman primates (Conley and Bird, 1997) will significantly favor DHEA production even if a small amount of HSD3B2 is present.
Note that while comparative histology between humans and nonhuman primates shows much similarity (particularly Great Apes and Old World monkeys), on comparison to other species the similarity deteriorates. Rat and mouse adrenals do not express CYP17 at all, and as a result the major glucocorticoid is corticosterone and there is no significant adrenal C19 steroid production (Ishimura and Fujita 1997, Pelletier et al 2001). These differences make these species extremely poor models for human steroidogenesis when we are considering factors controlling adrenal C19 and particularly DHEA/DHEAS steroid biosynthesis. Cows and sheep are closer to humans in terms of steroid biosynthesis regulation, yet there are still some limitations. Ovine and bovine CYP17 do show relative delta 4 lyase deficiency yet their CYP17 also has a greater ability to hydroxylate progesterone with even a substrate preference for progesterone over pregnenolone (Estabrook et al 1991, Waterman et al 1993, Shet et al 1994, Swart et al 2003, Shet et al 2007). The expression of an abundance of CYP17 and HSD3B2 in the zona fasciculata still favors cortisol production over corticosterone Bovine (Ishimura and Fujita 1997, ovine Tangalakis et al 1990, Bird et al 1996). Indeed, during late gestation in sheep, a rise in expression of CYP17 relative to HSD3B2 coincides with a dramatic rise in the cortisol:corticosterone ratio in vivo which is even more apparent when the adrenal is driven hard by ACTH infusion (Wintour et al 1975, Challis et al 1982). Nonetheless, the greater preference of ovine or bovine CYP17 for progesterone than in humans or nonhuman primates does explain differences in localization of ovarian expression of CYP17 vs HSD3B2. In human wherein pregnenolone is the preferred substrate for CYP17, during the follicular phase CYP17 is relatively high in the theca (Asakura et al 1997, Dharia et al 2004) and HSD3B2 is clearly found in the theca as well as the granulosa (Sassano et al 1990). Nonetheless in ovine and bovine where progesterone is the preferred substrate for CYP17, during the follicular phase the ovarian theca expresses CYP17 but little or no competing HSD3B2 (Conley et al 1995), so protecting DHEA biosysnthesis. Further major differences between human and the ovine/bovine models are revealed in terms of developmental studies, whereby the cow and sheep do not develop an androgenic fetal zone in the adrenal during gestation nor a significant ZR during postnatal life, and consequently circulating levels of DHEA/DHEAS remain comparatively low (Tangalakis et al 1989, Moritz et al 1998, Conley et al 1992, Marinelli et al 2007).
One other biochemical consideration in understanding C19 production is the more recent area of investigation concerning CYP17 phosphorylation in comparison to the role of CYB5 as a determinant of C19 steroid biosynthesis. Studies have shown or implied that phosphorylation of CYP17 in response to a cAMP analogue for just a few hours (i.e., insufficient time to alter expression levels of CYP17) can enhance lyase activity relative to hydroxylase activity (Zhang et al 1995, Biason Lauber et al 2000b, Pandey et al 2003). There is some debate, however, as to which kinase (Protein Kinase A, Protein Kinase B (Akt) or an alternative kinase such as Rho-associated, coiled-coil containing protein kinase (ROCK)) is actually phosphorylating the serine/threononine residues on CYP17 itself, if the residue in question is the same in each case, and if it is an activating event (Tee et al 2008). While further studies into the role of phosphorylation as a regulator of lyase activity are needed, the ability of CYB5 to activate lyase activity is more clearly established. CYB5 can enhance the lyase activity of human CYP17 relative to hydroxylase activity (Katagiri et al 1995, Lee Robichaud et al 1995, Auchus et al 1998, Brock and Waterman 1999). This effect appears to be allosteric given that the addition of both biologically active or inactive (in terms of electron transport ability) CYB5 are equally effective in preferentially enhancing lyase activity (Auchus et al 1998). Nonetheless, it is clear that the effect of CYB5 is not selective to the delta 5 pathway in itself, it simply enhances that lyase pathway which is already inherent in the CYP17 protein itself. In this latter regard, recent direct comparisons have shown in fusion proteins (NADPH P450 reductase linked to the terminus of CYP17, so controlling the stoichiometry and raising the potential for interaction) that for pig CYP17 which already has both delta 4 and 5 lyase abilities, both delta 4 and 5 lyase activities were enhanced by CYB5, while for bovine CYP17 which is delta 4 lyase deficient, the CYB5 enhances delta 5 lyase activity alone (Shet 2007).
As the important role of CYB5 emerges, it is possible to speculate on the putative role of CYP17 phosphorylation as a control of lyase activity further. While it has been suggested phosphorylation affects binding of substrate and so changes the rate of reaction (Lohr et al 1997) it has also been proposed that phosphorylation facilitates the interaction of CYP17 with other proteins such as CYB5 (Miller et al 1997, Biason Lauber 2000a, Gupta et al 2001). A multiplicity of putative regulatory mechanisms could certainly exist and this would indeed explain how local markers such as high CYB5 and low HSD3B2 can indicate histologically a tissue’s potential ability to generate DHEA via the delta five pathway, yet other factors may also determine if the cells actually do make C19 steroids, or at least how much steroid.
Much of the above data has been shown in human adrenal or studies of human CYP17, but there is also much still to be learned from comparative studies of nonhuman primates. It is important to consider possible differences in steroid clearance pathways and therefore what steroids should be measured in urine or blood assays when designing such studies, but in general terms, there are many similarities between human and nonhuman primates at the level of both the fetal and adult adrenal cortex, and these have recently been reviewed in detail (Conley et al 2004). Comparative assay of circulating steroids and adrenal histology suggests that many Old World and even New World primates express a fetal zone during development, which regresses after birth (Conley et al 2004). Nonetheless, only chimpanzees (and possibly other Great Apes) have been argued to actually go through a comparably timed, prepubertal adrenarche detectable as a significant event distinct from fetal zone regression (Smail et al 1982, Cutler et al 1978). In contrast it has been argued that species such as rhesus monkeys do not undergo adrenarche at all even though rhesus macaque show a prominent ZR in adulthood, since no separate rise of androgens was detected after fetal regression and prior to puberty (Cutler et al 1978, Smail et al 1982, but see Nguyen and Conley 2008). With regard to New World primates there is very little data. For many years there was debate of even the existence of a ZR in the marmoset, based solely on classical histological stains and measurement of circulating DHEA/DHEAS levels (Levine at al 1982). More recent biochemical and histological examination has shown there is little or no functional ZR in adult male marmosets (Pattison et al 2005). Yet recent studies of both rhesus monkeys and female marmosets, combined with a number of other biochemical considerations of recently isolated CYP17, in each case suggest the verdict may not be out quite yet. These distinctly different primates both have a story to tell which impacts on our understanding of ZR development and function.
The Rhesus Adrenal
Detailed histologic examination of the rhesus adrenal has been undertaken at adulthood as well as throughout most of gestation and it is clear that like the human, the rhesus fetal adrenal displays a distinct fetal zone and neocortex with similar staining for CYP17, CYB5 and HSD3B2 (Mapes et al 1999, 2002) and indeed SULT2A1 (Parker et al 2000). Thus the conditions for a FZ producing large amounts of C19 steroids, together with the neocortex becoming the future mature adrenal cortex, are similar to fetal developmental progression in humans. It has been suggested in the past, based on the similar attributes of the fetal zone to the later developing ZR, that perhaps the ZR derives directly from the fetal zone. Studies in the early (fetal) rhesus adrenal suggest however that distinct bands of CYB5 positive cells form in late gestation in the neocortex that are distinct from those in the fetal zone, and so it seems more likely the ZR forms separately (Mapes et al 2002) and is not derived from the fetal zone. These distinct cells and the later presence of a clearly prominent ZR beg the question of whether the rhesus undergoes adrenarche as we understand it in the human. The lack of a distinct rise in androgens before puberty, but after fetal regression, has been taken to indicate no such event occurs, but what if adrenarche and fetal zone regression overlap, as recently suggested by Nguyen and Conley (2008)? In that case the rise in DHEAS from putative ZR cells already present in the neocortex (adrenarche) may actually occur but could be masked since DHEAS is still being made by the fetal zone which is still present, but regressing. Further studies are clearly necessary to determine if this is indeed the case early in the life of the rhesus monkey.
The endocrine control of CYP17 expression in rhesus monkey adrenocortical cells is similar to that in humans (Pattison et al 2004). From a biochemical perspective it is also important to determine if indeed rhesus monkey CYP17 has the similar properties to human in terms of substrate preference, delta 4 lyase activity deficiency, and regulation of lyase activity through phosphorylation and/or CYB5 stoichiometry. In short, rhesus monkey CYP17 is indeed similar to the human enzyme. Examination of adrenal microsomes (Pattison et al 2005, or of expressed rhesus monkey CYP17 cDNA (Arlt et al 2002, Pattison 2007) shows a preference for pregnenolone over progesterone, and an inability to convert 17-hydroxyprogesterone into androstenedione. A clear relationship exists between changes in CYB5 levels in adrenal microsomes and corresponding lyase activity (Nguyen and Conley (2008)). Enhancement of lyase activity has also been observed for rhesus monkey CYP17 expressed in HEK293 cells with coexpressed CYB5 compared to no coexpressed CYB5 (Pattison 2007). There is little known at present about a possible role for phosphorylation and such studies would be of considerable value in the future.
The Marmoset Adrenal
Studies of the marmoset adrenal over several decades did not agree on the existence of a ZR and there was very little study of the existence of a fetal zone in the fetal adrenal. The study of Levine et al (1982) provided the clearest analysis of this New World primate before the cloning of the adrenal cytochrome P450s and availability of molecular probes for hybridization or histochemistry. By careful histologic examination and assay of circulating steroids it was suggested that the fetal zone was responsible for a high DHEAS production at birth, and regressed within a few months of birth. Thereafter marmosets had little or no detectable circulating DHEAS. One intriguing caveat was stated however, that while males throughout adult life had barely detectable levels of DHEAS, females had at least detectable levels which actually rose with age. Since that time, studies of marmoset adrenal, mainly by Pattison et al., have more completely characterized the adrenal zonation immediately post term and in adulthood. As a result it is now clear that the fetal zone is indeed strongly CYP17 and CYB5 positive with lower levels of HSD3B2 while in the adult male there is no evidence of a distinct ZR (Pattison et al 2005). Further characterization of marmoset CYP17 activity compared to that found in rhesus monkeys, in both microsomal preparations (Pattison et al 2005) and CYP17 expressed in HEK293 cells (Pattison 2007), suggest pregnenolone to also be the preferred substrate of marmoset CYP17 and that while the enzyme indeed shows an ability to undergo the lyase reaction to make DHEA, it is comparatively less than observed in rhesus monkeys (Pattison et al 2005, 2007, Pattison 2007). When provided with 17-hydroxyprogesterone substrate, marmoset CYP17, as in rhesus monkeys, was unable to convert it to androstenedione suggesting relative delta 4 lyase deficiency. While both dexamethasone suppression and ACTH challenge brought about the expected changes in circulating cortisol, little or no parallel change was observed in male DHEAS levels (Pattison et al 2005). So the lack of any CYB5 rich region in the innermost span of the male marmoset adrenal cortex combined with the lack of a HSD3B2 deficient or negative zone in cells otherwise expressing CYP17 is consistent with the lack of a physiologically functional ZR, and the male marmoset in this respect contrasts the findings in both humans and rhesus monkeys. The lack of any ZR in adult male marmosets, combined with low/no detectable DHEA/DHEAS of course precludes the possibility of adrenarche as a postnatal event following fetal zone regression.
While the findings in male marmosets are now enough to give a clearer picture of adrenal function in a New World primate it is intriguing to note the findings in female marmosets are not quite the same. As noted by Levine, females tend to show slightly higher levels of DHEAS in the circulation that continue to rise with age (Levine at al 1982). In addition, there is the interesting feature of female marmosets that they show social subordination that is not induced by fear but by social cooperation (Abbott et al., 1997). In a social group a dominant female will undergo ovarian cycles and breed while subordinate females usually cease to cycle and help raise the dominant’s offspring. On removal from her social group, a subordinate female paired with a male will initiate ovarian cycles and on introduction to a new social group may even become dominant. Thus the process of subordination is fully reversible. Of relevance here, histochemical analysis of the female marmoset adrenal shows that in females undergoing ovarian cycles, there is some evidence of CYB5 staining in the innermost zone of the adrenal (ie at the cortico-medullary junction) and in subordinates this staining actually becomes slightly stronger (Pattison et al 2007). The biggest change, however occurs in the case of ovariectomy, whereby CYB5 staining is intense and indeed almost uniform in the innermost region of the adrenal cortex, suggesting gonadal hormone inhibition of ZR development. This novel gonadal regulation of ZR function is without parallel, and provides an opportunity for increased adrenal androgen production when gonadal inhibition (at times of social subordination, Abbott et al., 1981) or gonadal senescence (in old age, Tardif and Ziegler, 1992) occurs. Whether such adrenal endocrine changes are age-related and whether they provide a source for androgens and estrogenic metabolites important in maintaining organ systems, such as bone, remains to be determined.
A comparison of microsomes from male and female marmosets from all pairs and social groups shows a clear linear regression for increasing lyase activity with increasing CYB5 expression, regardless of gender or social status (Pattison et al 2007). While marmoset adrenal microsomes show relatively low endogenous lyase activity, addition of exogenous CYB5 dramatically enhances the lyase activity (Pattison 2007, Pattison and Conley, personal communication). Further biochemical characterization of factors selectively controlling CYP17 lyase activity have also been performed by expressing marmoset CYP17 in HEK293 cells. While marmoset CYP17 lyase activity is low relative to rhesus monkey CYP17, both marmoset and rhesus monkey CYP17 show a biphasic activation of lyase relative to hydroxylase activity with added co-expressed CYB5 (Pattison 2007). Preliminary studies (JC Pattison) also suggest that while acute forskolin treatment of the host HEK293 cells may have some modest effect on enhancing lyase activity over control treatment, this response was not nearly as effective as coexpression of CYB5. Thus increased expression of CYB5 as observed in vivo in female marmosets may indeed be an effective means of selectively enhancing lyase activity in the newly formed ‘ZR’ of hypoestrogenic ovariectomized or anovulatory subordinate females. Comparative examination of both follicular and luteal phase ovarian expression of these proteins in the marmoset ovary also concur that at times of high C19 steroid biosynthesis in the theca, providing androgen precursors for granulosa cell conversion to estrogen, as indicated by high circulating estradiol levels (Hearn and Lunn, 1975)), there is high coexpression of CYP17 and CYB5, with lower levels of HSD3B2 in the same cell layer (Pattison 2007).
Given the findings in female marmoset adrenal histology, and their difference from that seen in male marmosets, it is relevant to also ask if adrenarche can occur in the female marmoset. As a single acute event of increased circulating DHEA/DHEAS prior to puberty one must say the evidence to date is that it does not occur. But it is also worthy of note that the changes in b5 expression with social status or ovariectomy suggest the marmoset adrenal ZR is dynamic, and the equivalent event can be induced by appropriate interactions that are either social or surgical, and associated with gonadal function. So while classic adrenarche may not be observed in human terms when we look for equivalent events in the marmoset, it is possible that similar events or control mechanisms may be in play in more dynamic terms, responding not so much to age or development as to social status, and further study of the marmoset may provide valuable insights that more traditional Old World models have yet to reveal.
Fetal programming of adrenal androgen excess in female rhesus monkeys
Individual primate species thus possess unique regulatory and physiological attributes related to adrenal steroidogenic biosynthesis that provide otherwise unattainable insight into adrenal steroid hormone regulation. Understanding the diverse patterns of development and function of the adrenal ZR across primate species becomes important not only for understanding normal biosynthesis and regulation of adrenal C19 steroids, but also for understanding adrenal pathophysiology of relevance to humans. In this regard, adrenal androgen excess provides a pathophysiological focus for the remainder this chapter, in particular adrenal androgen excess found in the highly prevalent endocrinopathy of polycystic ovary syndrome (PCOS). PCOS is present in 6–7% of women in their reproductive years (Diamanti-Kandarakis et al., 1999; Ascuncion et al., 2000; Azziz et al., 2004) and 20–30% of these PCOS women have adrenal androgen excess that manifests as elevated circulating levels of DHEA, DHEAS and androstenedione (Wild et al., 1983; Carmina et al., 1992; Kumar et al., 2005), in addition to ACTH-induced adrenal specific hyperandrogenic responses, including excessive increases in DHEA and androstenedione (Azziz et al., 1998). Furthermore, elevated circulating levels of DHEAS are usually found in most women with PCOS, implying a prevalent adrenal ZR contribution to their hyperandrogenic syndrome because of the unique adrenal origins of sulfoconjugated DHEA (Kumar et al., 2005). Some PCOS studies (Yildiz et al., 2007; Kondoh et al., 1999; Lanzone et al., 1995), but not others (Azziz et al., 1998; Pasquali et al, 2007), suggest a generalized hypersecretion of adrenal steroids or ACTH hyper-responsiveness to corticotropin-releasing hormone (CRH). Such heightened hypothalamic-pituitary-adrenal (HPA) axis activity may reflect diminished somatostatin release in a sub-population of PCOS women (Wu et al, 1996), especially as somatostatin replacement therapy reduces CRH-mediated ACTH in these PCOS women (Lanzone et al., 1997).
There are few animal models that replicate the many traits expressed by PCOS women (Abbott et al, 2006). Prenatally androgenized (PA) female rhesus monkeys perhaps recapitulate signs and symptoms of PCOS more comprehensively than any other animal model (Abbott et al, 2005). In terms of adrenal androgen excess, the reason for that is simple: only primates exhibit an androgen synthesizing fetal zone as well as an androgen synthesizing ZR postnatally (as described above; see Conley and Bird, 1997). Thus only primate models will provide opportunities to explore adrenocortical endocrinopathy related to PCOS.
PA female monkeys are produced following exposure to fetal male levels of testosterone during early gestation (Abbott et al., 2008). PA monkeys with PCOS-like traits have also been produced following similar androgen excess exposure during late gestation (Abbott et al., 2005), but adrenal function in such late gestation exposed PA monkeys has not been systematically studied. Fetal androgen excess during early monkey gestation is induced by daily subcutaneous injection of pregnant dams with 10–15 mg testosterone propionate for up to 41 consecutive days, starting on gestation days 40–44. In adulthood, such androgen excess exposed PA female monkeys exhibit irregular or absent ovulatory menstrual cycles, ovarian hyperandrogenism, enlarged polyfollicular ovaries and luteinizing hormone excess, as well as insulin resistance, increased incidence of type 2 diabetes, visceral adiposity and hyperlipidemia (Eisner et al., 2000; Abbott et al., 2005; Dumesic et al, 2005; Bruns et al, 2007; Zhou et al., 2007). This heterogeneous PA monkey reproductive and metabolic pathophysiology is emblematic of PCOS in women (Norman et al, 2007).
Newborn infant PA monkeys demonstrate androgen excess in the form of increased circulating levels of androstenedione (Abbott et al., 2008). Infant adrenals may contribute to this androgen excess since protein expression of adrenal P450 oxidoreductase (the electron-donating redox partner for 17,20 lyase) is increased, in combination with a similar trend for CYB5 (Conley and Abbott, unpublished results). In this respect, it is interesting to note that orchidectomized male infant rhesus monkeys exhibit an adrenal-dependent elevation in circulating testosterone levels up to 6 months of age (Plant and Zorub, 1984), suggesting that early gestation fetal androgen excess (in normal males or prenatally androgenized females) may permanently exaggerate adrenal androgen biosynthesis after birth. At least in infant PA female monkeys, enhanced insulin secretion (Abbott et al., 2007) may contribute to infant hyperandrogenism through insulin-mediated serine phosphorylation of CYP17 (Zhang et al., 1995). Such altered CYP17 function would enhance 17,20 lyase activity by increasing the affinity of CYP17 for its electron donor, P450 oxidoreductase (Pandey et al., 2005).
Adult PA female monkeys clearly demonstrate functional adrenal hyperandrogenism. In comparison to controls, adult PA females exhibit elevated basal circulating levels of DHEA (Zhou et al, 2005) and DHEAS (Eisner et al, 2002), but normal basal levels of cortisol and 17-hydroxyprogesterone (Zhou et al, 2005) probably because circulating cortisol levels are tightly regulated. ACTH-stimulated adrenal steroidogenic function elicits additional hyperandrogenic responses from PA monkeys, including elevated DHEA and androstenedione levels in comparison to controls. ACTH-stimulated cortisol and 17-hydroxyprogesterone levels, however, are normal in PA monkeys (Zhou et al, 2005; Abbott et al, 2008). Not surprisingly, such hyperandrogenic responses to ACTH increase the ratio of serum DHEA to 17-hydroxyprogesterone, while diminishing the ratios of serum DHEAS to DHEA and serum androstenedione to DHEA (Zhou et al, 2005). Taken together, these results suggest a selective enhancement of 17,20 lyase activity in female PA monkeys in the ZR, possibly extending into the zona fasciculata (given the increased androstenedione as well as increased DHEA responses to ACTH). There does not appear to be a concomitant increase in 17-hydroxylase activity, as ACTH-stimulated levels for 17-hydroxyprogesterone are normal (presumably generated in the ZR since 17-hydroxyprogesterone generated in the ZF will undergo rapid onward conversion by 21-hydroxylase).
In terms of adrenal androgen excess, PA monkeys compare favorably with PCOS women. Elevated basal circulating levels of DHEAS, present in PA monkeys, are one of the hallmarks of adrenal androgen excess in women with PCOS (Zhang et al., 1995; Carmina and Lobo, 2007; Yildiz and Azziz, 2007). Elevated ACTH-stimulated DHEA levels often characterize adrenal hyperandrogenic responses in PCOS women (Rosenfield, 1999), and this trait is also emulated by PA monkeys. A putative molecular mechanism to explain such similar adrenal endocrinopathy may involve an isolated increase in 17,20 lyase activity, perhaps due to increased serine phosphorylation of CYP17 (Zhang et al., 1995), but also possibly facilitated by an increase in ZR expression of CYB5 or P450 oxidoreductase. In view of the involvement of insulin action in adrenal androgen excess in both PA monkeys (Abbott et al, 2008) and PCOS women (Lanzone et al., 1992; Moghetti et al., 1996; Arslanian et al., 2002; Azziz et al., 2003), insulin may contribute to the molecular mechanism by stimulating CYP17 perhaps through the PI3-kinase branch of the insulin signaling pathway, synergizing with additional coactivation by cAMP/kinase A (Munir et al., 2004). If similar to its actions in the ovary, insulin’s effect on adrenal steroidogenic function would be independent of its action at the level of glucose metabolism (Rice et al., 2005).
The potential fetal programming origins for such adrenal androgen excess may be found in the actions of both androgen and insulin. Fetal androgen excess may act through fetal adrenocortical androgen receptors (Pelletier, 2000; Stalvey, 2002) to directly alter adrenal steroidogenic function (Nowak et al., 1995; Hines et al., 2001; Stalvey, 2002; Rubinow et al., 2005). Alternatively, insulin may upregulate fetal (and later) androgen biosynthesis in the adrenal cortex since fetal (Abbott et al., 2006b) and infant, (Abbott et al., 2007) PA female monkeys exhibit enhanced insulin secretion. Such potential fetal programming of adrenal hyperandrogenism in women with PCOS, however, remains to be determined.
Final considerations
An understanding of comparative endocrine physiology and morphology of the adrenal cortex across mammals in general, and the Primate Order in particular, is providing an increased appreciation of the regulatory mechanisms operating under physiological and pathophysiological conditions to control C19 steroid biosynthesis. In particular, former studies of zonal expression of steroidogenic enzymes and associated accessory proteins (such as CYB5) combined with a more recent understanding of the roles of cell signaling and post translational modification on selective enhancement of 17,20 lyase activity are leading to a far more complete understanding of the factors which regulate C19 steroid production from the human and nonhuman primate ZR. Clearly there are also considerable parallels in the mechanisms that control C19 steroid biosynthesis in the ovary, and while there are some subtle disparities in the data so far, the overall mechanisms (ratio CYP17:HSD3B2, expression CYB5 and POR, and a possible role for phosphorylation by signaling kinases) operating in these two organs seems be similar. Further study of whether adrenal androgen excess in the PA rhesus monkey model is indeed due to ZF dysfunction as well as ZR dysfunction, and the underlying mechanisms that may apply in each zone are worthy of further investigation in their own right. The findings from such studies, along with an understanding of the mechanisms underlying the fetal origins of this adrenal dysfunction may also be revealing and applicable to ovarian hyperandrogenic dysfunction. Combined with the possible lessons that may be learned from the study of the more socially dynamic ovarian control of adrenal ZR function in the marmoset model, it is clear comparative studies of Old and New World primates still have much to teach us about the origins of androgen excess in humans and may ultimately provide the key to finding a cure.
Acknowledgements
The authors would like to dedicate this manuscript to the memory of Dr J Christina Pattison PhD, who achieved so much during her time in the Endocrinology - Reproductive Physiology Training Program. We would also like to thank the family and friends who supported her during this happy time and to acknowledge NIH training grant T32HD41921 for the support of her studies.
References
- Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11:357–374. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Barnett DK, Bruns CM, Dunaif A, Dumesic DA. Transient hyperglycemia in both mother and fetus from experimental induction of maternal androgen excess in a nonhuman primate model for polycystic ovary syndrome (abstract P3-113). 88th Annual Meeting of the Endocrine Society, Boston; 2006. [Google Scholar]
- Abbott DH, Barnett DK, Levine JE, Padmanabhan V, Dumesic DA, Jacoris S, Tarantal AF. Endocrine Antecedents of Polycystic Ovary Syndrome in Fetal and Infant Prenatally Androgenized Female Rhesus Monkeys. Biol Reprod. 2008a Apr 2; doi: 10.1095/biolreprod.108.067702. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott DH, Dumesic DA, Levine JE, Dunaif A, Padmanabhan V. Animal models and fetal programming of PCOS. In: Azziz R, Nestler JE, Dewailly D, editors. Contemporary Endocrinology: Androgen Excess Disorders in Women: Polycystic Ovary Syndrome and Other Disorders. 2nd Edition. Totowa, NJ: Humana Press Inc.; 2006. pp. 259–272. [Google Scholar]
- Abbott DH, Goodfriend TL, Dunaid A, Muller SJ, Dumesic DA, Tarantal AF. Increased body weight and enhanced insulin sensitivity in infant female rhesus monkeys exposed to androgen excess during early gestation; Abstract P2-348 presented at the 89th Annual Meeting of the Endocrine Society, Toronto, Canada, June 2–5; 2007. [Google Scholar]
- Abbott DH, McNeilly AS, Lunn SF, Hulme MJ, Burden FJ. Inhibition of ovarian function in subordinate female marmoset monkeys (Callithrix jacchus jacchus) J Reprod Fertil. 1981;63:335–345. doi: 10.1530/jrf.0.0630335. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Saltzman W, Schultz-Darken NJ, Smith TE. Specific neuroendocrine mechanisms not involving generalized stress mediate social regulation of female reproduction in cooperatively breeding marmoset monkeys. Ann N Y Acad Sci. 1997;807:219–238. doi: 10.1111/j.1749-6632.1997.tb51923.x. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Zhou R, Bird IM, Dumesic DA, Conley AJ. Fetal programming of adrenal androgen excess: lessons from a nonhuman primate model of polycystic ovary syndrome. In: Flueck CE, Miller WL, editors. Disorders of the Human Adrenal Cortex. vol. 13. Karger, Basel: Endocr. Dev.; 2008b. pp. 145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt W, Martens JW, Song M, Wang JT, Auchus RJ, Miller WL. Molecular evolution of adrenarche: structural and functional analysis of p450c17 from four primate species. Endocrinology. 2002;143(12):4665–4672. doi: 10.1210/en.2002-220456. [DOI] [PubMed] [Google Scholar]
- Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab. 2002;87:1555–1559. doi: 10.1210/jcem.87.4.8398. [DOI] [PubMed] [Google Scholar]
- Asakura H, Zwain IH, Yen SS. Expression of genes encoding corticotropin-releasing factor (CRF), type 1 CRF receptor, and CRF-binding protein and localization of the gene products in the human ovary. J Clin Endocrinol Metab. 1997;82(8):2720–2725. doi: 10.1210/jcem.82.8.4119. [DOI] [PubMed] [Google Scholar]
- Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–2438. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20 lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273:3158–3165. doi: 10.1074/jbc.273.6.3158. [DOI] [PubMed] [Google Scholar]
- Azziz R, Black V, Hines GA, Fox LM, Boots LR. Adrenal androgen excess in the polycystic ovary syndrome: sensitivity and responsivity of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 1998;83:2317–2323. doi: 10.1210/jcem.83.7.4948. [DOI] [PubMed] [Google Scholar]
- Azziz R, Ehrmann DA, Legro RS, Fereshetian AG, O'Keefe M, Ghazzi MN PCOS/Troglitazone Study Group. Troglitazone decreases adrenal androgen levels in women with polycystic ovary syndrome. Fertil Steril. 2003;79:932–937. doi: 10.1016/s0015-0282(02)04914-2. [DOI] [PubMed] [Google Scholar]
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- Biason-Lauber A, Kempken B, Werder E, Forest MG, Einaudi S, Ranke MB, Matsuo N, Brunelli V, Schönle EJ, Zachmann M. 17alpha-hydroxylase/17,20-lyase deficiency as a model to study enzymatic activity regulation: role of phosphorylation. J Clin Endocrinol Metab. 2000a Mar;85(3):1226–1231. doi: 10.1210/jcem.85.3.6475. [DOI] [PubMed] [Google Scholar]
- Biason-Lauber A, Zachmann M, Schoenle EJ. Effect of Leptin on CYP17 Enzymatic Activities in Human Adrenal Cells: New Insight in the Onset of Adrenarche. Endocrinol. 2000b;141:1446–1454. doi: 10.1210/endo.141.4.7402. [DOI] [PubMed] [Google Scholar]
- Bird IM, Zheng J, Corbin CJ, Magness RR, Conley AJ. Immunohistochemical analysis of AT1 receptor versus P450c17 and 3 beta HSD expression in ovine adrenals. Endocr Res. 1996;22(4):349–353. doi: 10.1080/07435809609043717. [DOI] [PubMed] [Google Scholar]
- Brock BJ, Waterman MR. Biochemical differences between rat and human cytochrome P450c17 support the different steroidogenic needs of these two species. Biochemistry. 1999;38(5):1598–1606. doi: 10.1021/bi9821059. [DOI] [PubMed] [Google Scholar]
- Bruns CM, Baum ST, Colman RJ, Dumesic DA, Eisner JR, Jensen MD, Whigham LD, Abbott DH. Prenatal androgen excess negatively impacts body fat distribution in a nonhuman primate model of polycystic ovary syndrome. Int J Obes (Lond) 2007;31:1579–1585. doi: 10.1038/sj.ijo.0803638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmina E, Koyama T, Chang L, Stanczyk FZ, Lobo RA. Does ethnicity influence the prevalence of adrenal hyperandrogenism and insulin resistance in polycystic ovary syndrome? Am J Obstet Gynecol. 1992;167:1807–1812. doi: 10.1016/0002-9378(92)91779-a. [DOI] [PubMed] [Google Scholar]
- Carmina E, Lobo RA. Prevalence and metabolic characteristics of adrenal androgen excess in hyperandrogenic women with different phenotypes. J Endocrinol Invest. 2007;30:111–116. doi: 10.1007/BF03347408. [DOI] [PubMed] [Google Scholar]
- Challis JRG, Manchester EL, Mitchell BF, Patrick JE. Activation of adrenal function in fetal sheep by the infusion of adrenocorticotropin to the fetus in utero. Biology Reproduction. 1982;27:1026–1032. doi: 10.1095/biolreprod27.5.1026. [DOI] [PubMed] [Google Scholar]
- Conley AJ, Bird IM. The role of cytochrome P450 17 alpha-hydroxylase and 3 beta-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the delta 5 and delta 4 pathways of steroidogenesis in mammals. Biol Reprod. 1997;56:789–799. doi: 10.1095/biolreprod56.4.789. [DOI] [PubMed] [Google Scholar]
- Conley AJ, Head JR, Stirling DT, Mason JI. Expression of steroidogenic enzymes in the bovine placenta and fetal adrenal glands throughout gestation. Endocrinology. 1992;130(5):2641–2650. doi: 10.1210/endo.130.5.1374010. [DOI] [PubMed] [Google Scholar]
- Conley AJ, Kaminski MA, Dubowsky SA, Jablonka-Shariff A, Redmer DA, Reynolds LP. Immunohistochemical localization of 3 beta-hydroxysteroid dehydrogenase and P450 17 alpha-hydroxylase during follicular and luteal development in pigs, sheep, and cows. Biol Reprod. 1995;52(5):1081–1094. doi: 10.1095/biolreprod52.5.1081. [DOI] [PubMed] [Google Scholar]
- Conley AJ, Pattison JC, Bird IM. Variations in adrenal androgen production among (nonhuman) primates. Semin Reprod Med. 2004;22:311–326. doi: 10.1055/s-2004-861548. [DOI] [PubMed] [Google Scholar]
- Cutler GB, Jr, Glenn M, Bush M, Hodgen GD, Graham CE, Loriaux DL. Adrenarche: a survey of rodents, domestic animals, and primates. Endocrinology. 1978;103(6):2112–2118. doi: 10.1210/endo-103-6-2112. [DOI] [PubMed] [Google Scholar]
- Dharia S, Slane A, Jian M, Conner M, Conley AJ, Parker CR., Jr Colocalization of P450c17 and cytochrome b5 in androgen-synthesizing tissues of the human. Biol Reprod. 2004;71:83–88. doi: 10.1095/biolreprod.103.026732. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, Zapanti ED, Bartzis MI. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84:4006–4011. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Schramm RD, Abbott DH. Early origins of polycystic ovary syndrome. Reproduction, Fertility and Development. 2005;17:349–360. doi: 10.1071/rd04092. [DOI] [PubMed] [Google Scholar]
- Eisner JR, Barnett MA, Dumesic DA, Abbott DH. Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertil Steril. 2002;77:167–172. doi: 10.1016/s0015-0282(01)02947-8. [DOI] [PubMed] [Google Scholar]
- Eisner JR, Dumesic DA, Kemnitz JW, Abbott DH. Timing of prenatal androgen excess determines differential impairments in insulin secretion and action in adult female rhesus monkeys. J. Clin. Endocrinol. Metab. 2000;85:1206–1210. doi: 10.1210/jcem.85.3.6453. [DOI] [PubMed] [Google Scholar]
- Estabrook RW, Mason JI, Simpson ER, Peterson JA, Waterman MR. The heterologous expression of the cytochrome P450: a new approach for the study of enzyme activities and regulation. Adv Enzyme Regul. 1991;31:365–383. doi: 10.1016/0065-2571(91)90024-g. [DOI] [PubMed] [Google Scholar]
- Gell JS, Atkins B, Margraf L, Mason JI, Sasano H, Rainey WE, Carr BR. Adrenarche is associated with decreased 3 beta-hydroxysteroid dehydrogenase expression in the adrenal reticularis. Endocr Res. 1996;22:723–728. doi: 10.1080/07435809609043768. [DOI] [PubMed] [Google Scholar]
- Gell JS, Carr BR, Sasano H, Atkins B, Margraf L, Mason JI, Rainey WE. Adrenarche results from development of a 3beta-hydroxysteroid dehydrogenase-deficient adrenal reticularis. J Clin Endocrinol Metab. 1998;83(10):3695–3701. doi: 10.1210/jcem.83.10.5070. [DOI] [PubMed] [Google Scholar]
- Gupta MK, Geller DH, Auchus RJ. Pitfalls in Characterizing P450c17 Mutations Associated with Isolated 17,20-Lyase Deficiency. J Clin Endocrinol Metab. 2001;86:4416–4423. doi: 10.1210/jcem.86.9.7812. [DOI] [PubMed] [Google Scholar]
- Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med. 2004;22(4):337–347. doi: 10.1055/s-2004-861550. [DOI] [PubMed] [Google Scholar]
- Hearn JP, Lunn SF. LABORATORY ANIMAL HANDBOOK. Vol. 6. 1975. The reproductive biology of the marmoset monkey, Callithrix jacchus; pp. 191–204. [Google Scholar]
- Hines GA, Smith ER, Azziz R. Influence of insulin and testosterone on adrenocortical steroidogenesis in vitro: preliminary studies. Fertil Steril. 2001;76:730–735. doi: 10.1016/s0015-0282(01)02014-3. [DOI] [PubMed] [Google Scholar]
- Ishimura K, Fujita H. Light and electron microscopic immunohistochemistry of the localization of adrenal steroidogenic enzymes. Microsc Res Tech. 1997;36(6):445–453. doi: 10.1002/(SICI)1097-0029(19970315)36:6<445::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Katagiri M, Kagawa N, Waterman MR. The role of cytochrome b5 in the biosynthesis of androgens by human P450c17. Arch Biochem Biophys. 1995;317(2):343–347. doi: 10.1006/abbi.1995.1173. [DOI] [PubMed] [Google Scholar]
- Kondoh Y, Uemura T, Ishikawa M, Yokoi N, Hirahara F. Classification of polycystic ovary syndrome into three types according to response to human corticotropin-releasing hormone. Fertil Steril. 1999;72:15–20. doi: 10.1016/s0015-0282(99)00195-8. [DOI] [PubMed] [Google Scholar]
- Kumar A, Woods KS, Bartolucci AA, Azziz R. Prevalence of adrenal androgen excess in patients with the polycystic ovary syndrome (PCOS) Clin Endocrinol (Oxf) 2005;62:644–649. doi: 10.1111/j.1365-2265.2005.02256.x. [DOI] [PubMed] [Google Scholar]
- Lanzone A, Fulghesu AM, Guido M, Cucinelli F, Caruso A, Mancuso S. Somatostatin treatment reduces the exaggerated response of adrenocorticotropin hormone and cortisol to corticotropin-releasing hormone in polycystic ovary syndrome. Fertil Steril. 1997;67:34–39. doi: 10.1016/s0015-0282(97)81852-3. [DOI] [PubMed] [Google Scholar]
- Lanzone A, Fulghesu AM, Guido M, Fortini A, Caruso A, Mancuso S. Differential androgen response to adrenocorticotropic hormone stimulation in polycystic ovarian syndrome: relationship with insulin secretion. Fertil Steril. 1992;58:296–301. doi: 10.1016/s0015-0282(16)55220-0. [DOI] [PubMed] [Google Scholar]
- Lanzone A, Petraglia F, Fulghesu AM, Ciampelli M, Caruso A, Mancuso S. Corticotropin-releasing hormone induces an exaggerated response of adrenocorticotropic hormone and cortisol in polycystic ovary syndrome. Fertil Steril. 1995;63:1195–1199. doi: 10.1016/s0015-0282(16)57596-7. [DOI] [PubMed] [Google Scholar]
- Lee-Robichaud P, Wright JN, Akhtar ME, Akhtar M. Modulation of the activity of human 17 alpha-hydroxylase-17,20-lyase (CYP17) by cytochrome b5: endocrinological and mechanistic implications. Biochem J. 1995;308(Pt 3):901–908. doi: 10.1042/bj3080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, Wolfe LG, Schiebinger RJ, Loriaux DL, Cutler GB., Jr Rapid regression of fetal adrenal zone and absence of adrenal reticular zone in the marmoset. Endocrinology. 1982;111(6):1797–1802. doi: 10.1210/endo-111-6-1797. [DOI] [PubMed] [Google Scholar]
- Lohr JB, Velten NK. Protein phosphorylation chnages ligand binding efficiency of cytochrome P450c17 (CYP17) and accelerates its proteolytic degradation: putative relevance for hormonal regulation of CYP17 activity. Biochem Biophys Res Comm. 1997;231:403–408. doi: 10.1006/bbrc.1997.6113. [DOI] [PubMed] [Google Scholar]
- Mapes S, Corbin CJ, Tarantal A, Conley A. The primate adrenal zona reticularis is defined by expression of cytochrome b5, 17alpha-hydroxylase/17,20-lyase cytochrome P450 () and NADPH-cytochrome P450 reductase (reductase) but not 3beta-hydroxysteroid dehydrogenase/delta5-4 isomerase (3beta-HSD) J Clin Endocrinol Metab. 1999;84(9):3382–3385. doi: 10.1210/jcem.84.9.6105. [DOI] [PubMed] [Google Scholar]
- Mapes S, Tarantal AF, Parker CR, Moran FM, Bahr JM, Pyter L, Conley AJ. Adrenocortical cytochrome b5 expression during fetal development of the rhesus macaque. Endocrinology. 2002;143(4):1451–1458. doi: 10.1210/endo.143.4.8718. [DOI] [PubMed] [Google Scholar]
- Marinelli L, Trevisi E, Da Dalt L, Merlo M, Bertoni G, Gabai G. Dehydroepiandrosterone secretion in dairy cattle is episodic and unaffected by ACTH stimulation. J Endocrinol. 2007;194(3):627–635. doi: 10.1677/JOE-07-0226. [DOI] [PubMed] [Google Scholar]
- Miller WL, Auchus RJ, Geller DH. The regulation of 17,20 lyase activity. Steroids. 1997;62:133–142. doi: 10.1016/s0039-128x(96)00172-9. [DOI] [PubMed] [Google Scholar]
- Moghetti P, Castello R, Negri C, Tosi F, Spiazzi GG, Brun E, Balducci R, Toscano V, Muggeo M. Insulin infusion amplifies 17 alpha-hydroxycorticosteroid intermediates response to adrenocorticotropin in hyperandrogenic women: apparent relative impairment of 17,20-lyase activity. J Clin Endocrinol Metab. 1996;81:881–886. doi: 10.1210/jcem.81.3.8772544. [DOI] [PubMed] [Google Scholar]
- Moritz KM, Butkus A, McFarlane AC, Albiston A, Salenmi R, Wintour EM. Regulation and function of steroid production by mid gestation ovine fetal adrenal cortex in vivo. Endocr Res. 1998;24(3–4):937–941. doi: 10.3109/07435809809032711. [DOI] [PubMed] [Google Scholar]
- Munir I, Yen HW, Geller DH, Torbati D, Bierden RM, Weitsman SR, Agarwal SK, Magoffin DA. Insulin augmentation of 17alpha-hydroxylase activity is mediated by phosphatidyl inositol 3-kinase but not extracellular signal-regulated kinase-1/2 in human ovarian theca cells. Endocrinology. 2004;145:175–183. doi: 10.1210/en.2003-0329. [DOI] [PubMed] [Google Scholar]
- Nguyen AD, Conley AJ. Adrenal androgens in humans and nonhuman primates: production, zonation and regulation. Endocr Dev. 2008;13:33–54. doi: 10.1159/000134765. [DOI] [PubMed] [Google Scholar]
- Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- Nowak KW, Neri G, Nussdorfer GG, Malendowicz LK. Effects of sex hormones on the steroidogenic activity of dispersed adrenocortical cells of the rat adrenal cortex. Life Sci. 1995;57:833–837. doi: 10.1016/0024-3205(95)02015-b. [DOI] [PubMed] [Google Scholar]
- Pandey AV, Mellon SH, Miller WL. Protein Phosphatase 2A and Phosphoprotein SET Regulate Androgen Production by P450c17. J Biol Chem. 2003;278:2837–2844. doi: 10.1074/jbc.M209527200. [DOI] [PubMed] [Google Scholar]
- Pandey AV, Miller WL. Regulation of 17,20 lyase activity by cytochrome b5 and by serine phosphorylation of P450c17. J Biol Chem. 2005;280:13265–13271. doi: 10.1074/jbc.M414673200. [DOI] [PubMed] [Google Scholar]
- Parker CR, Jr, Jian M, Conley AJ. The localization of DHEA sulfotransferase in steroidogenic and steroid metabolizing tissues of the adult rhesus macaque monkey. Endocr Res. 2000;26(4):517–522. doi: 10.3109/07435800009048564. [DOI] [PubMed] [Google Scholar]
- Pasquali R, Patton L, Pocognoli P, Cognigni GE, Gambineri A. 17-hydroxyprogesterone responses to gonadotropin-releasing hormone disclose distinct phenotypes of functional ovarian hyperandrogenism and polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:4208–4217. doi: 10.1210/jc.2007-0870. [DOI] [PubMed] [Google Scholar]
- Pattison JC, Abbott DH, Saltzman W, Nguyen AD, Henderson G, Jing H, Pryce CR, Allen AJ, Conley AJ, Bird IM. Male marmoset monkeys express an adrenal fetal zone at birth, but not a zona reticularis in adulthood. Endocrinology. 2005;146(1):365–374. doi: 10.1210/en.2004-0689. [DOI] [PubMed] [Google Scholar]
- Pattison JC, Conley AJ, Bird IM. Isolation of marmoset P450c17 cDNA and gene regulation in vitro. Endocr Res. 2004;30(4):737–743. doi: 10.1081/erc-200044018. [DOI] [PubMed] [Google Scholar]
- Pattison JC, Saltzman W, Abbott DH, Hogan BK, Nguyen AD, Husen B, Einspanier A, Conley AJ, Bird IM. Gender and gonadal status differences in zona reticularis expression in marmoset monkey adrenals: Cytochrome b5 localization with respect to cytochrome P450 17,20-lyase activity. Mol Cell Endocrinol. 2007:265–266. 93–101. doi: 10.1016/j.mce.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison JC. PhD Thesis. UW Madison: University Wisconsin Library; 2007. Dec, Marmoset 17 alpha-Hydroxylase/17,20-Lyase Cytochrome P450: Relationship Between Enzyme Structure and Function to Low Circulating Dehydroepiandrosterone Levels Observed In Vivo. [Google Scholar]
- Pelletier G, Li S, Luu-The V, Tremblay Y, Bélanger A, Labrie F. Immunoelectron microscopic localization of three key steroidogenic enzymes (cytochrome P450(scc), 3 betahydroxysteroid dehydrogenase and cytochrome P450(c17)) in rat adrenal cortex and gonads. J Endocrinol. 2001;171(2):373–383. doi: 10.1677/joe.0.1710373. [DOI] [PubMed] [Google Scholar]
- Pelletier G. Localization of androgen and estrogen receptors in rat and primate tissues. 1: Histol Histopathol. 2000;15:1261–1270. doi: 10.14670/HH-15.1261. [DOI] [PubMed] [Google Scholar]
- Plant TM, Zorub DS. A study of the role of the adrenal glands in the initiation of the hiatus in gonadotropin secretion during prepubertal development in the male rhesus monkey (Macaca mulatta) Endocrinology. 1984;114:560–565. doi: 10.1210/endo-114-2-560. [DOI] [PubMed] [Google Scholar]
- Rice S, Christoforidis N, Gadd C, Nikolaou D, Seyani L, Donaldson A, Margara R, Hardy K, Franks S. Impaired insulin-dependent glucose metabolism in granulosa-lutein cells from anovulatory women with polycystic ovaries. Hum Reprod. 2005;20:373–381. doi: 10.1093/humrep/deh609. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL. Ovarian and adrenal function in polycystic ovary syndrome. Endocrinol Metab Clin North Am. 1999;28:265–293. doi: 10.1016/s0889-8529(05)70070-0. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Roca CA, Schmidt PJ, Danaceau MA, Putnam K, Cizza G, Chrousos G, Nieman L. Testosterone suppression of CRH-stimulated cortisol in men. Neuropsychopharmacology. 2005;30:1906–1912. doi: 10.1038/sj.npp.1300742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasano H, Mori T, Sasano N, Nagura H, Mason JI. Immunolocalization of 3 beta-hydroxysteroid dehydrogenase in human ovary. J Reprod Fertil. 1990 Jul;89(2):743–751. doi: 10.1530/jrf.0.0890743. [DOI] [PubMed] [Google Scholar]
- Shet MS, Fisher CW, Arlotto MP, Shackleton CH, Holmans PL, Martin-Wixtrom CA, Saeki Y, Estabrook RW. Purification and enzymatic properties of a recombinant fusion protein expressed in Escherichia coli containing the domains of bovine P45017A and rat NADPH-P450 reductase. Arch. Biochem. Biophys. 1994;311:402–417. doi: 10.1006/abbi.1994.1255. [DOI] [PubMed] [Google Scholar]
- Shet MS, Fisher CW, Tremblay Y, Belanger A, Conley AJ, Mason JI, Estabrook RW. Comparison of the 17a-hydroxylase/C17,20-lyase activities of porcine, guinea pig and bovine P450c17 using purified recombinant fusion proteins containing P450c17 linked to NADPHP450 reductase. Drug Metab Rev. 2007;39:1–19. doi: 10.1080/03602530701468391. [DOI] [PubMed] [Google Scholar]
- Smail PJ, Faiman C, Hobson WC, Fuller GB, Winter JS. Further studies on adrenarche in nonhuman primates. Endocrinology. 1982;111(3):844. doi: 10.1210/endo-111-3-844. [DOI] [PubMed] [Google Scholar]
- Stalvey JR. Inhibition of 3beta-hydroxysteroid dehydrogenase-isomerase in mouse adrenal cells: a direct effect of testosterone. Steroids. 2002;67:721–731. doi: 10.1016/s0039-128x(02)00023-5. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, Rainey WE. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf) 2000;53:739–747. doi: 10.1046/j.1365-2265.2000.01144.x. [DOI] [PubMed] [Google Scholar]
- Swart P, Lombard N, Swart AC, van der Merwe T, Murry BA, Nicol M, Mason JI. Ovine steroid 17a-hydroxylase cytochrome P450: characteristics of the hydroxylase and lyase activities of the adrenal cortex enzyme. Archives Biochem Biophys. 2003;409:145–152. doi: 10.1016/s0003-9861(02)00547-7. [DOI] [PubMed] [Google Scholar]
- Tangalakis K, Coghlan JP, Connell J, Crawford R, Darling P, Hammond VE, Haralambidis J, Penschow J, Wintour EM. Tissue distribution and levels of gene expression of three steroid hydroxylases in ovine fetal adrenal glands. Acta Endocrinol (Copenh) 1989 Feb;120(2):225–232. doi: 10.1530/acta.0.1200225. [DOI] [PubMed] [Google Scholar]
- Tangalakis K, Coghlan JP, Crawford R, Hammond VE, Wintour EM. Steroid hydroxylase gene expression in the ovine fetal adrenal gland following ACTH infusion. Act Endocrinologica (Copenh) 1990;123:371–377. doi: 10.1530/acta.0.1230371. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Ziegler TE. Features of female reproductive senescence in tamarins (Saguinus spp.), a New World primate. JOURNAL OF REPRODUCTION AND FERTILITY. 1992;94:411–421. doi: 10.1530/jrf.0.0940411. [DOI] [PubMed] [Google Scholar]
- Tee MK, Dong Q, Miller WL. Pathways leading to phosphorylation of P450c17 and the the post-translational regulation of androgen biosynthesis. Endocrinology. 2008 doi: 10.1210/en.2007-1527. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman MR. Heterologous expression of cytochrome P-450 in Escherichia coli. Biochem. Soc. Trans. 1993;21:1081–1085. doi: 10.1042/bst0211081. [DOI] [PubMed] [Google Scholar]
- Wild RA, Umstot ES, Andersen RN, Ranney GB, Givens JR. Androgen parameters and their correlation with body weight in one hundred thirty-eight women thought to have hyperandrogenism. Am J Obstet Gynecol. 1983;146:602–606. doi: 10.1016/0002-9378(83)90998-5. [DOI] [PubMed] [Google Scholar]
- Wintour EM, Brown EH, Denton DA, Hardy KJ, McDougal JG, Oddie CJ, Whipp GT. The ontogeny and regulation of corticosteroid secretion by the ovine foetal adrenal. Acta Endocrinologica. 1975;79:301–315. doi: 10.1530/acta.0.0790301. [DOI] [PubMed] [Google Scholar]
- Wu XK, Wang CH, Su YH. Responses of somatostatin, beta-endorphin and dynorphin A to a glucose load in two groups of women with polycystic ovarian syndrome. Horm Res. 1996;46(2):59–63. doi: 10.1159/000184997. [DOI] [PubMed] [Google Scholar]
- Yildiz BO, Azziz R. The adrenal and polycystic ovary syndrome. Rev Endocr Metab Disord. 2007;8:331–342. doi: 10.1007/s11154-007-9054-0. [DOI] [PubMed] [Google Scholar]
- Zhang LH, Rodriguez H, Oho S, Miller WL. Serine phosphorylation of human P450c17 increases 17,20-lyase activity: Implications for adenarche and the polycystic ovarian syndrome. Proc Natl Acad Sci. 1995;92:10619–10623. doi: 10.1073/pnas.92.23.10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Bruns CM, Bird IM, Kemnitz JW, Goodfriend TL, Dumesic DA, Abbott DH. Pioglitazone improves insulin action and normalizes menstrual cycles in a majority of prenatally androgenized female rhesus monkeys. Reprod Toxicol. 2007;23:438–448. doi: 10.1016/j.reprotox.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Bird IM, Dumesic DA, Abbott DH. Adrenal hyperandrogenism is induced by fetal androgen excess in a rhesus monkey model of polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2005;90:6630–6637. doi: 10.1210/jc.2005-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]