Abstract
Signaling by Bone morphogenetic proteins (Bmps) has multiple and diverse roles in patterning and morphogenesis of the kidney, eye, limbs and the neural tube. Here, we employed the Bmp7lacZ strain to perform a detailed analysis of Bmp7 expression and the null phenotype during development of the mouse urogenital system. The urethral compartment originates in mid-embryogenesis from the ventral part of the cloaca, a transient cavity at the caudal end of the hindgut. At mid-gestation, Bmp7 expression was detected within several specific domains in the cloacal epithelium and mesenchyme. In late embryogenesis, Bmp7 expression was present in the urethra, rectum, the urethral glands, corpus cavernosum, and in the male and female genital ducts. Importantly, loss of Bmp7 resulted in arrest in cloacal septation, and severe defects in morphogenesis of the genital urethra and mesenchyme. Together, our analysis of Bmp7 expression and the null phenotype, indicates that Bmp7 may play an important role in re-organization of the epithelium during cloacal septation and morphogenesis of the genital tubercle.
Keywords: Bone morphogenetic protein 7 (Bmp7), urogenital, anorectal, cloaca, rectourethral fistula, genital tubercle, hypospadia
1.Results and discussion
The mammalian urogenital system, the bladder, urethra and external genitalia, develop from the epithelium and mesenchyme of the cloaca, a transient embryonic cavity at the caudal end of the hindgut (Fig. 1A). Urogenital malformations occur at high frequency in humans, and often combine malformations of the genital tubercle (GT), the penis in males and clitoris in females, and defects in separation of the urethral and rectal compartments (Hendren, 1992;1996;1998;Kurzock et al., 1999; Baskin et al., 2001; Mo et al., 2001). Morphogenetic mechanisms that direct development of the urogenital system are not well understood. The caudal homeobox genes, Hoxa13 and Hoxd13, are expressed in the cloacal epithelium and mesenchyme, and are essential for morphogenesis of all cloacal derivatives (Warot et al., 1997; Morgan et al., 2003). Expression of p63 (Cheng et al., 2006) and Sonic hedgehog (Shh) (Haraguchi et al., 2001; 2007; Mo et al., 2001; Seifert et al., 2008) in the cloacal and urethral epithelium is also important both for septation of the cloaca, and development of the bladder and external genitalia. In addition, Fibroblast Growth Factors 8 and 10, Bmp4 and 7, and Wnt5a have been implicated in development of the GT (Yamada et al., 2006), but their role in morphogenesis of the cloaca has not been explored. In this study, we employed the Bmp7lacZ reporter strain (Godin et al., 1998) to perform a detailed analysis of Bmp7 expression and the null phenotype during development of the urogenital system in males and females.
Fig. 1.
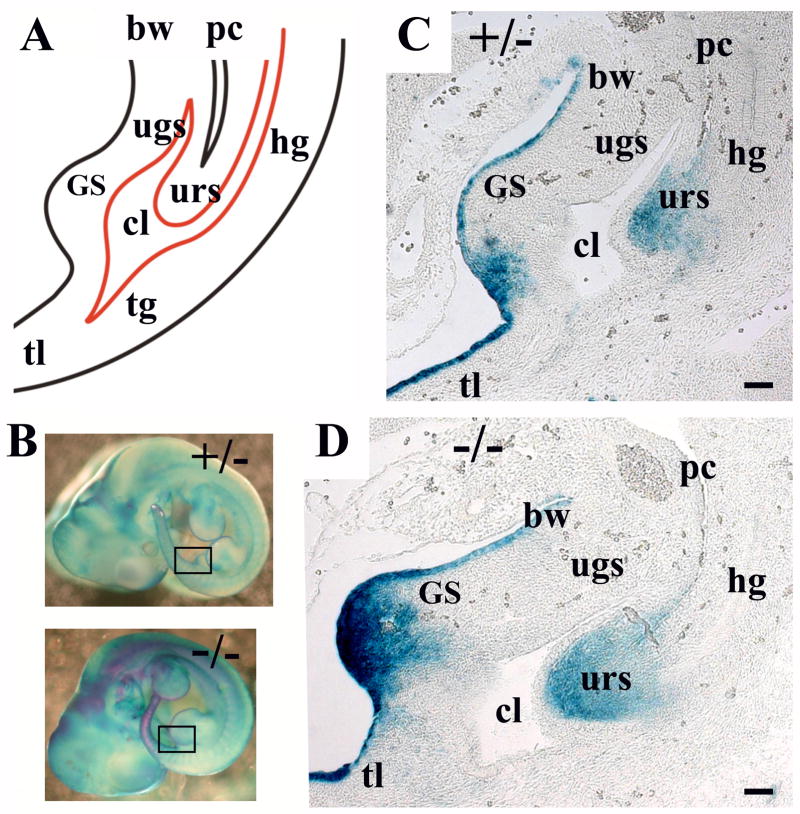
Bmp7 expression in the cloacal area at E11.5. (A) Schematic depiction of the cloacal. The genital swellings (GS), cloaca (cl), urorectal septum (urs), urogenital sinus (ugs), hindgut (hg), tail gut (tg), peritoneal cavity (pc), body wall (bw) and tail (tl) are indicated. (B) Whole mount X-gal stained Bmp7lacZ/+ and Bmp7lacZ/lacZ embryos at E11.5. Areas of sections in (C, D) are indicated with frames. (C, D) Sagittal sections of Bmp7lacZ/+ (C) and Bmp7lacZ/lacZ (D) embryos show strong LacZ-activity in the urorectal septum (urs), the mesenchyme of the genital swellings (GS) and the ventral ectoderm of the body wall and the tail. Scale bar: 50 μm.
1.1 LacZ activity accurately reflects expression of Bmp7 in the urogenital system
Comparative analysis of Bmp7 expression by in situ hybridization with an antisense Bmp7 RNA probe (Lyons et al., 1995), and by X-gal staining of tissues in Bmp7lacZ/+ background has shown that LacZ activity accurately reflects the expression of the wild type Bmp7 allele in many embryonic tissues, including the kidney (Godin et al., 1998; Dudley et al., 1999) and the urethra (Fig. 4C, D). In addition, our analysis of Bmp7lacZ/+ GT (Fig.1–3) showed LacZ activity in the urethral plate and genital mesenchyme in complete agreement with previous reports on Bmp7 expression (Morgan et.al, 2003; Suzuki et al., 2003). Thus, we conclude that LacZ activity in Bmp7lacZ strain is an accurate readout of the activity Bmp7 promoter in the lower urogenital system and the hindgut.
Fig. 4.
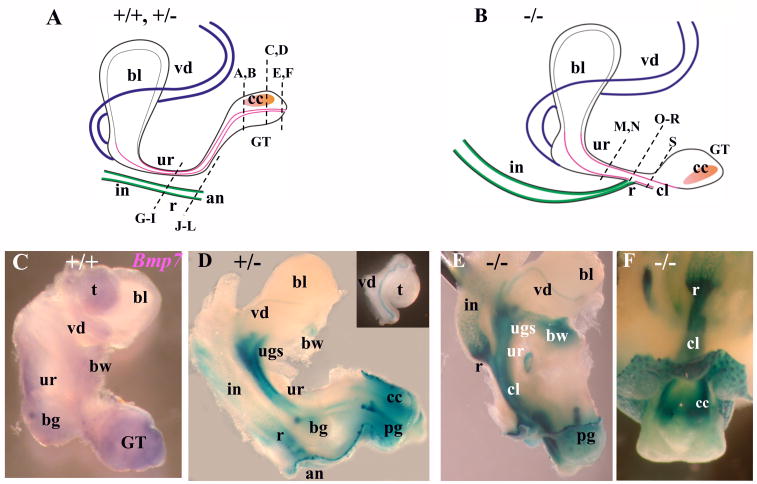
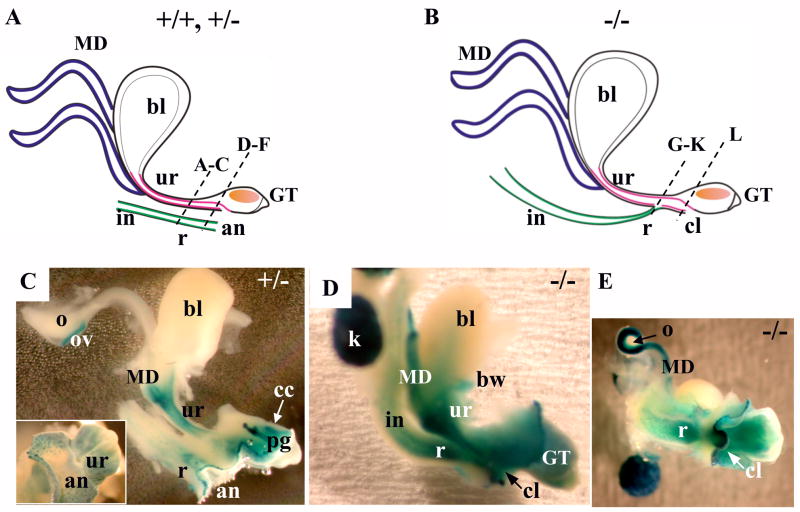
Bmp7 expression and null phenotype in the male urogenital system at E17.5 (I). (A, B) Schematic representation of a normal male urogenital system (A), and in Bmp7 null (B). Bladder (bl), vas deferens (vd), urethra (ur), genital tubercle (GT), corpus cavernosum (cc), intestine (in), rectum (r), anus (an), and the cloaca (cl) in the null (B), are indicated. Dashed lines indicate the planes of sections in Fig. 5. (C) Wild type male urogenital tissues were hybridized in whole mount with digoxigenin labeled antisense Bmp7 RNA probe (Lyons et al., 1995) which detects both Bmp7-1 and Bmp7-2 transcripts (Vega Mouse Database). Bmp7-1 corresponds to the complete Bmp7 mRNA. Bmp7-2 is a short non-translatable RNA specific for the testes (Ross et al., 2007; Steve Munger, personal communication). Bmp7 expression was detected in the urethra, bulbourethral gland (bg), genital tubercle, vas deferens, body wall (bw) and the testes (t). Negative control with sense probe produced no color (not shown). (D) X-gal stained E17.5 Bmp7lacZ/+ male urogenital system, lateral view. LacZ activity was detected in the urethra, urogenital sinus (ugs), vas deferens (insert), bulbourethral and preputial glands (pg), corpus cavernosum, the rectum and and the body wall. LacZ activity was not detected in the bladder and the testes (insert). (E, F) X-gal stained Bmp7lacZ/lacZ null male, lateral view (E), and ventro-caudal view (F). In the null, the rectum adjoins the urethra (E). Bulbourethral glands were not detected (E). Strong LacZ activity was detected in the rectum (E, F), urethra (E), cloaca (E, F), preputial gland (E), body wall (E), and the mesenchyme of the corpus cavernosum at the ventral side of the GT (F).
Fig. 3.
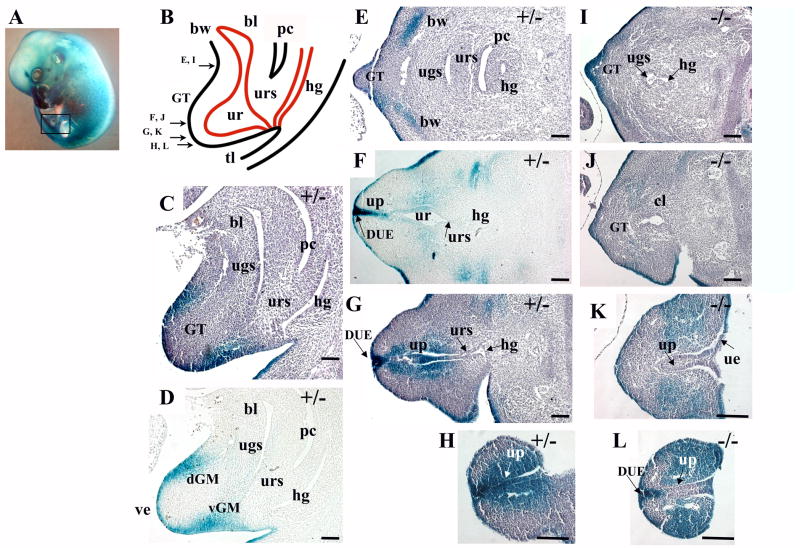
Bmp7 expression and null phenotype in the urogenital system at E13.5. (A) Bmp7lacZ/+ embryo at E13.5 stained with X-gal, left hindlimb is removed, the area of the sections is shown in a frame. (B) Schematic depiction of the urogenital area at E13.5. The genital tubercle (GT), cloaca (cl), urorectal septum (urs), bladder (bl), urethra (ur), hindgut (hg), peritoneal cavity (pc), body wall (bw) and tail (tl) are indicated. Arrows indicate planes of transverse sections in (E-L). (C, D) Sagittal sections of a Bmp7lacZ/+ embryo show Bmp7 expression in the ventral (vGM) and dorsal (dGM) genital mesenchyme. (E-H) Transverse sections of Bmp7lacZ/+ embryo show Bmp7 expression in the lateral mesenchyme of the body wall (E), in the epithelium of the urethra (ur) and the urethral plate (up) (F-H), and the adjacent genital mesenchyme (F-H). Strong LacZ activity was observed in the distal urethral epithelium (DUE) at the tip of the GT (F, G). (I-L) In the null, LacZ activity was lost in the medial (L) and proximal (K) parts of the urethral plate, but maintained in the DUE (L and G). In the null, LacZ-positive genital mesenchyme occupied a broad lateral domain (L). Scale bar: 100 μm.
1.2 Bmp7 expression and null phenotype in the cloacal area at E11.5-E13.5
The cloacal cavity is defined as the caudal end of the hindgut when, at embryonic day 9.5 (E9.5) in mice, it comes in contact with the ectoderm just anterior to the tail at the site of the future anal opening (Perriton et al., 2002; Hynes and Fraher, 2004a,b,c; Sasaki et al., 2004). Starting at E10, the ventral end of the cloaca grows anteriorly giving rise to the embryonic urogenital sinus (UGS), the primordium of the pelvic urethra and the bladder (Fig. 1). Studies in mouse models indicate that septation of the cloaca is driven by the caudal growth of the lateral mesenchyme known as the urorectal septum at E10.5 – E13.5, and is completed with disintegration of the cloacal membrane to uncover the anal and the caudal urethral orifices at E13.5 – E14.0 (Kluth et al., 1995; Hynes and Fraher, 2004a,b,c; Sasaki et al., 2004; Seifert et al., 2008). Interestingly, analysis of Bmp7lacZ/+ heterozygous embryos showed that Bmp7 is expressed specifically in the urorectal septum (URS) at E11.5 (Fig. 1C) and E12.5 (Fig. 2F), and expression is dramatically reduced at E13.5 (Fig. 3C, D). We further asked whether Bmp7 function may be required for cloacal septation. From E11.5 to E13.5, heterozygous embryos showed normal descent of the URS (Fig. 1C; 2E; 3C; Hynes and Fraher, 2004a-c; Sasaki et al., 2004), and progressive separation of the urethral and hindgut compartments from anterior to caudal (Fig. 2G–I; 3E–G; Hynes and Fraher, 2004a-c; Sasaki et al., 2004). Bmp7 null embryos showed normal invagination of the URS into dorsal cloaca at E11.5 (Fig. 1D). However, further septation was delayed at E12.5 (Fig. 2K, L compare to G, H) and E13.5 (Fig. 3I, J compare to E, F). At E12.5 and E13.5, transverse sections of Bmp7 null GT showed persistent cloaca, whereas corresponding sections of heterozygous animals showed separate urethral and hindgut compartments (Fig. 2, 3).
Fig. 2.
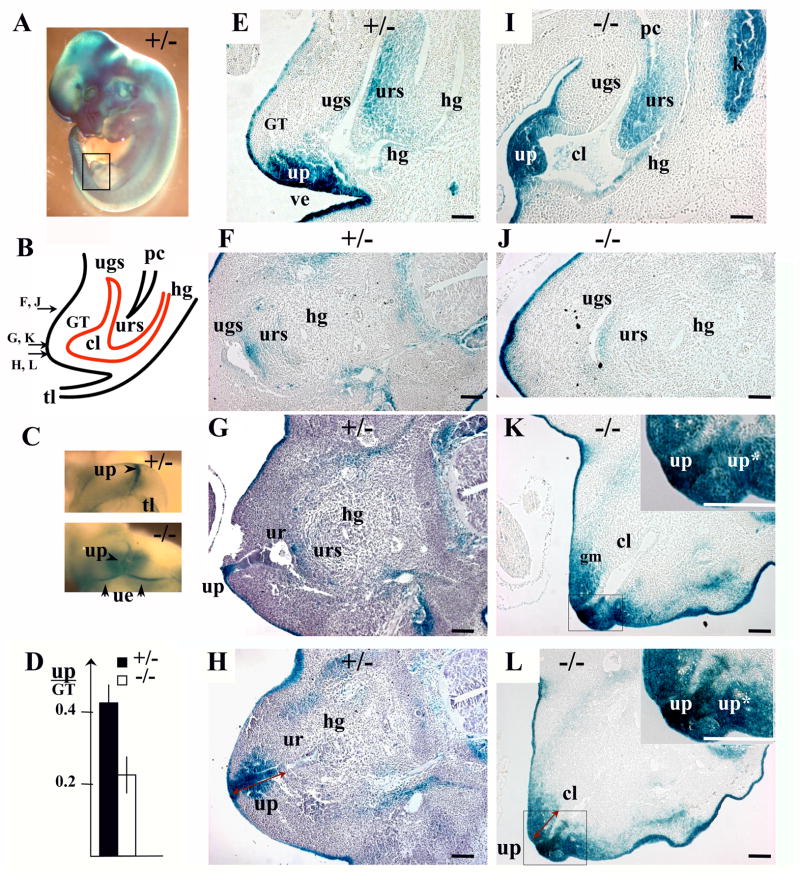
Bmp7 expression and null phenotype in the urogenital system at E12.5. (A) Bmp7lacZ/+ embryo stained with X-gal, left hindlimb is removed, area of sections is shown in a frame. (B) Schematic depiction of the cloacal area at E12.5 showing the genital tubercle (GT), cloaca (cl), urorectal septum (urs), urogenital sinus (ugs), hindgut (hg), peritoneal cavity (pc), and the tail (tl). Arrows indicate planes of transverse sections in (F-H, J-L). (C) Ventral view of X-gal stained Bmp7lacZ/+ and Bmp7lacZ/lacZ GT. In heterozygous, Bmp7 is expressed in the urethral plate (up) and adjacent mesenchyme. In the null, the urethral plate unzips ventrally to form a large groove. LacZ activity marks the urethral epithelium (ue) at the surface of the groove. (D) Ventral extension of the urethral plate was measured on transverse sections (red arrows in H, L) in heterozygous (n=8) and null (n=6), and presented relative to the length of the GT. Standard deviation values are indicated as error bars. P< 0.01. (E, I) Sagittal sections of heterozygous (E) and null (I) embryos. LacZ activity was detected in the urorectal septum, the urethral plate and adjacent mesenchyme, ventral ectoderm (ve) and the kidney (k). Transverse sections of heterozygous (F-H) and null (J-L) embryos. Bmp7 is detected in urorectal septum (F, G), the urethral plate (G, H) and adjacent genital mesenchyme (H). In the null, LacZ-positive genital mesenchyme (gm) spreads laterally from the urethral plate (K, L), which makes two (*) or more contacts with the ventral ectoderm (K, L, inserts). Scale bar: 100 μm.
1.3 Bmp7 expression and null phenotype in the GT at E11.5-E13.5
The initial stages of development of the GT at E11 – E15 are gender-independent, and begin with the appearance of genital swellings laterally to the cloaca at E10.5 (Perriton et al., 2002). At E11.5, Bmp7 expression was detected in the mesenchyme of the genital swellings, and the ectoderm of the ventral body wall and the tail (Fig. 1C). Distal outgrowth of the genital swellings is accompanied by the extension of the urethral epithelium and lumen (Kuzrock et al., 1999; Yamada et al., 2003; 2006; Seifert et al., 2008). At E12, the distal urethral walls adhere to form a solid urethral plate (Fig. 2H, I; Kuzrock et al., 1999; Yamada et al., 2003; 2006; Seifert et al., 2008). Analysis at E12.5 showed Bmp7 expression in the urethral plate (Fig. 2G, H), the adjacent mesenchyme (Fig. 2H), and the ventral ectoderm (Fig. 2E). In the null, LacZ activity marked the urethral plate, and was present in a broad domain in the GT mesenchyme (Fig. 2K, L). Distal extension of the urethral plate appeared 50% shorter in the null (Fig. 2D, H, L), most likely due to unzipping of the urethral folds at the ventral side of the GT (Fig. 2C; and K, L, inserts) and formation of a large urethral groove (Fig. 2C).
At E13.5, Bmp7 expression appeared in the dorsal GT mesenchyme (Fig. 3C, D) which may contribute to the corpus cavernosum (Yamada et. al., 2003). Bmp7 expression was maintained in the ventral mesenchyme, but decreased in the URS (Fig. 3C, D). In the genital urethra, Bmp7 was expressed in a gradient with the strongest expression in the distal urethral epithelium (DUE) at the tip of the GT, which also expresses Fgf8 (Haraguchi et al., 2000), and the weakest at the urorectal septum (Fig. 3F, G). In the null, LacZ activity was lost in the medial and proximal parts of the urethral plate, but maintained in the DUE (Fig. 3K, L). In the ventral GT mesenchyme, Bmp7 expression was restricted to the urethral plate in heterozygous (Fig. 2H; 3G, H). In contrast, in the null, LacZ-positive mesenchyme spread laterally (Fig. 2K, L; 3K, L).
1.4 Bmp7 expression and null phenotype in the male urogenital system at E17.5 and P0
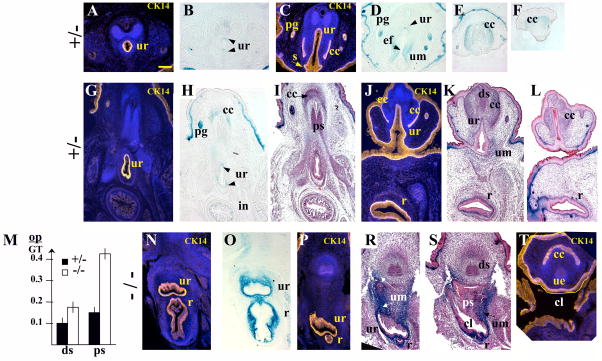
Starting at E15.5 in mice, the external genitalia undergo sex-specific differentiation largely controlled by androgens in the male (Drews et al., 2002; Kim et al., 2002; Yucel et al., 2003, 2004; Buckley et al, 2006). In E17.5 male embryos, Bmp7 expression was detected in the urethra (Fig. 4D, 5B,D, H), the bulbourethral and preputial glands (Fig. 4D, 5H), the vas deferens (Fig. 4D, insert) and the corpus cavernosum (Fig. 4D, 5E, F, H). LacZ activity was not detected in the bladder, nor in the testes (Fig. 4D, and insert). To precisely localize LacZ activity to the epithelial and mesenchymal compartments of the GT, we embedded X-gal stained tissues in paraffin and carried out analysis of histological sections (Fig. 5). Some of the sections were co-immunostained for cytokeratin 14 (CK14) which marked the squamous epithelium in the urethra and the rectum (Fig. 5). Bmp7 expression was detected in the dorsal and ventral epithelium of the urethra (Fig. 5B, H), in the preputial glands (Fig. 5D, H), and in the crypts of the intestine (Fig. 5H). Bmp7 was also present in the mesenchyme of the urethra (Fig. 5D, K), the rectum (Fig. 5K), and in the corpus cavernosum (Fig. 4D; 5E, F, H). Normal morphogenesis of the penile urethra involves closure of the original caudal orifice (Perriton et al., 2002; Yamada et al., 2003), fusion of the ventral urethral groove (Fig. 5C; D; Yamada et al., 2003), and displacement of the ventral urethral seam by the genital mesenchyme (Fig. 5A;Kuzrock et al., 1999; Yamada et al., 2003; Seifert et al., 2008). In heterozygous GT, Bmp7 was expressed in the mesenchyme surrounding the ventral epithelial fusion (Fig. 5D). LacZ-positive mesenchyme also localized to the abnormal genital urethra in Bmp7 null (Fig. 5P-T, bellow). Further analysis showed that Bmp7 null males developed complex rectourethral malformations, including rectourethral fistula and severe hypospadia (Fig. 4E, F; 5P-T; 8B). In the null, the hindgut lacked a separate anal opening (Fig. 4E, F; 8B), and a narrowed rectum opened directly into the urethra (Fig. 4E, F; 5P-S), consistent with the arrest in cloacal septation in mid-embryogenesis (Fig. 2, 3). Strong LacZ activity was detected in the mesenchyme of the fistula and the cloaca (Fig. 4E, F; 5O, R, S). LacZ activity was also detected in the basal epithelium of the urethra and hindgut (Fig. 5 N, O). In Bmp7 null males, the caudal urethral opening was maintained (Fig. 4E; 8B), and the tubular penile urethra did not form (Fig. 5T compare to 5A, C). Instead, the urethral epithelium lined the ventral surface of the GT adjacent to the cloacal opening (Fig. 5T). Strong LacZ-activity was present in the mesenchyme adjacent to the urethral epithelium (Fig. 4E, F; 5R, S). Other urogenital abnormalities in Bmp7 null males included loss of bulbourethral glands (Fig. 4E compare to D) and enlarged os penis (Fig. 5M, and S compare to I, K).
Fig. 5.
Bmp7 expression and null phenotype in the male urogenital system at E17.5 (II). (A-F) Coronal sections of Bmp7lacZ/+ penis, dorsal side of the penis is up: (A, B) proximal area, (C, D) mid-penis, (E, F) distal glans. (G-L, N-T) Transverse sections of Bmp7lacZ/+ (G-L) and Bmp7lacZ/lacZ (N-T) embryos, ventral side of the trunk is up. Planes of sections are shown in Figs. 4A, B. (A, C, G, J, N, P, T) Immunolabeling for cytokeratin 14 (CK14, yellow) marks the squamous epithelium in the urethra (ur), the ventral urethral seam (s) (C), the rectum (r) (J, N, P), the preputial glands (pg) (C), the mesothelial membrane of the corpus cavernosum (cc) (C, J, T), and the ectoderm (ec) (J). In the male, Bmp7 is expressed in the urethral epithelium (ur) (B, D, H) and mesenchyme (um) (D, K). During formation of the penile urethra, LacZ-positive mesenchyme is concentrated to the secondary epithelial fusion (ef) (D). Bmp7 is also expressed in the mesenchyme of rectum (r) (K) and corpus cavernosum (cc) (E, F, H), and in the epithelium of the preputial glands (pg) (D, H) and the intestinal crypts (in). (N-T) In Bmp7lacZ/lacZ null, LacZ activity was detected in the mesenchyme of the urethra, cloaca (cl) and the rectum (N, P, R). In E17.5 null males, the urethral epithelium (ue) (T) and LacZ-positive mesenchyme (um) (R) localized to the ventral surface of the GT (T). (M) Bmp7 null males showed wider cartilage condensations in the proximal and distal segments of the os penis (M, and S compare to I, K). The width of the proximal (ps) and distal (ds) segments of the os penis (op) was measured on sections of heterozygous (n=10) and null (n=8) males and presented in relation to the width of the GT. Standard deviation values are indicated as error bars. P<0.01. Scale bar 100 μm in (A) is same for all panels.
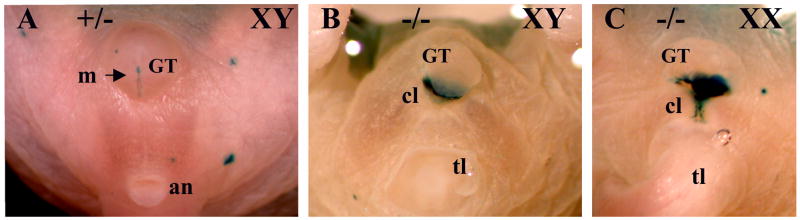
Fig. 8.
External genitalia in a normal Bmp7lacZ/+ male (A), and in a Bmp7lacZ/lacZ null male (B) and female (C) at P0. (A) In a normal newborn male, the genital tubercle (GT) is located ventrally to the anus (an). LacZ activity is present in the urethral meatus (m). (B, C) In the null male (B) and female (C), strong LacZ activity is detected in the cloacal area. Tail (tl) is removed in (B).
1.6 Bmp7 expression and null phenotype in the female urogenital system
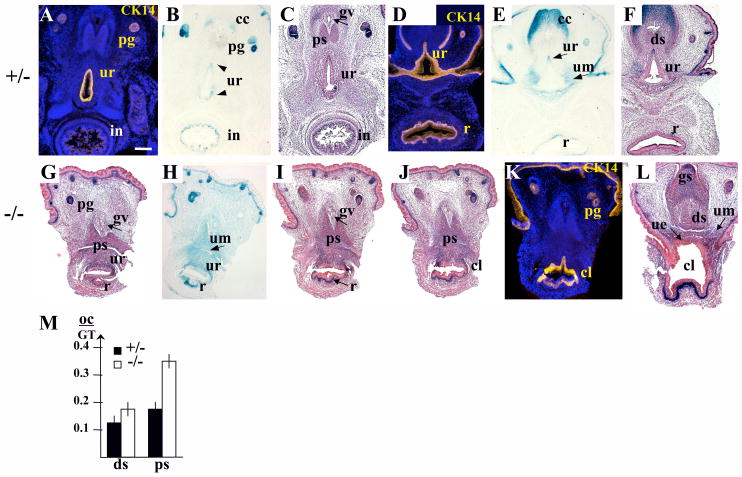
In the female urogenital system (Fig. 6–8), the pattern of Bmp7 expression and the Bmp7 null phenotype were very similar to what was observed in the male (Fig. 4, 5 and 8). In females, Bmp7 was expressed in the urethra (Fig. 6C; 7B, E), the rectum (Fig. 6C; 7E), the intestinal crypts (Fig. 7B), the preputial glands (Fig. 6C; 7B), and the corpus cavernosum (Fig. 7E). In addition, LacZ activity was detected in all Mullerian duct derivatives, the oviducts, uterus and developing vagina (Fig. 6C–E). LacZ activity was not present in the ovaries, nor in the bladder (Fig. 6C, D). Analysis of Bmp7 null females showed rectourethral fistula (Fig. 7I, J), enlarged caudal urethral/cloacal opening (Fig. 7L, 8C), and abnormalities in the topology of the genital epithelium (Fig. 7L) and mesenchyme very similar to those observed in the null males (Fig. 4, 5, 8B). Strong LacZ activity was detected in the mesenchyme of the fistula and the ventral mesenchyme of the GT (Fig. 6E; 7G–L). Bmp7 null females also showed enlarged os clitoris (Fig. 7M, and I, L compare to C, F), and excessive branching of the genital vein (Fig. 7I and C) similar to what was reported in the Hoxa13−/− GT (Morgan et al., 2003).
Fig. 6.
Bmp7 expression and null phenotype in the female urogenital system at E17.5. (I) Whole mount. (A, B) Schematic depiction of normal (A) and Bmp7 null (B) female urogenital systems. The bladder (bl), Mullerian ducts (MD), urethra (ur), genital tubercle (GT), intestine (in), rectum (r), anus (an), and the cloaca (cl) in the null, are indicated. Dashed lines indicate planes of sections in Fig. 7. (C) X-gal stained Bmp7lacZ/+ female urogenital system, lateral view, and caudal view (insert). Bmp7 expression was present in the Mullerian ducts, the urethra, corpus cavernosum (cc), preputial gland (pg), and the rectum. Bmp7 expression was detected in the oviducts (ov), but not in the ovaries (o). (D, E) Bmp7lacZ/LacZ null female, lateral view (D), caudal view (E). In the null, the rectum adjoins the urethra (E). Cloacal opening is visible beneath the genital tubercle (D, E). LacZ activity was detected in the urethra, rectum, Mullerian ducts, body wall (bw), the mesenchyme of the genital tubercle, and the kidney (k).
Fig. 7.
Bmp7 expression and null phenotype in the female urogenital system at E17.5. (II) Transverse sections of Bmp7lacZ/+ (A-F) and Bmp7lacZ/lacZ (G-L) females. Planes of sections are indicated in Fig. 6A, B. (A, D, K) Immunolabeling for CK14 marks the squamous epithelium of the urethra (ur) (A, D), rectum (r) (D), and the cloaca (cl) in the null (K). Heterozygous female shows Bmp7 expression in the epithelium and mesenchyme (um) of the urethra (B, E), in the intestine (in) (B), the mesenchyme of the rectum (E, F), in the preputial glands (pg) (B), and the corpus cavernosum (cc) (E, F). (G-L) Bmp7 null female shows rectourethral fistula (G-J) and enlarged urethral/cloacal opening (L). In Bmp7 null, LacZ activity was detected in the epithelium and mesenchyme of the urethra (G-I) and the cloaca (J), and in the mesenchyme of the rectum (G-H), corpus cavernosum (cc) and glans clitoris (gs) (L). Bmp7 null female shows increased branching of the genital vein (I compare to C), and a wider proximal (ps) segment of the os clitoris (M, and I compare to C). (M) The width of the proximal (ps) and distal (ds) segments of the os clitoris (oc) was measured on sections of heterozygous (n=8) and null (n=8) females and presented in relation to the width of the GT. Standard deviation values are indicated as error bars. Pps <0.01, Pds<0.05. Scale bar in (A) same for all sections: 250 μm.
Bmp signaling mediated by Smad1/5/8 transcriptional mediators has multiple and diverse roles in organogenesis, including regulation of programmed cell death (Morgan et al., 2003), cell survival (Dudley et al., 1999), neural and limb patterning (Chesnutt et al., 2004; Pizette et al., 2001) and cell adhesion (Linecum et al., 1998). Although many details in morphogenesis of the anus and external genitalia are still unclear, our analysis of Bmp7 expression and the null phenotype indicates that Bmp7 signaling may play an important role in reorganization of the urethral epithelium and mesenchyme during cloacal septation and formation of the penile urethra. Bmp7 targets in the GT include the homeobox genes Msx1,2 (Morgan et al., 2003) which can regulate cadherin-dependent cell adhesion (Linecum et al., 1998). Thus, further studies are needed to determine whether Bmp7 may regulate cell adhesion in the urethral epithelium, and contribute to the migration and differentiation of the genital mesenchyme.
2. Experimental procedures
Animal work was conducted under NYUSM IACUC approved animal protocol. Bmp7lacZ strain has been described previously (Godin et al., 1998; Dudley et al., 1999). Genotyping, whole mount X-gal staining, and preparation of histological sections have been performed as described previously (Grishina et al., 2005). Some of the sections were counterstained with hematoxylin and eosin. At each stage from E11.5 to E13.5, we analyzed six to ten animals of the same genotype. At E17.5 and P0, we analyzed six to ten animals of the same sex and genotype. Measurements of the genital structures was performed on transverse sections of the null and heterozygous embryos, and Student’s t-test was used to determine the significance of differences. For immunofluorescent analysis, paraffin sections were incubated with antibodies for cytokeratin 14 (CK14, Covance, PRB-155P) at 1:100 dilution over night at 4°C, followed by 546 Alexa Fluor secondary antibodies (Invitrogen) diluted at 1:1000 for 1 hour at room temperature. In situ hybridization analysis was performed in whole mount as previously described (Grishina et al., 2005) using 0.9 kb digoxigenin-labeled antisense RNA probe for Bmp7 (Lyons et al., 1995).
Acknowledgments
We thank Lee Niswander, Tung-Tien Sun, Cathy Mendelson, Gen Yamada, Ashley Seifert, Steve Munger, Xue-Ru Wu and Susan Logan for critical reading of the manuscript and/or useful discussions, and Elizabeth Robertson for the gift of Bmp7lacZ strain and Bmp7 cDNA. This work was supported by the Department of Urology, NYU Langone Medical Center; National Institute of Diabetes, Kidney and Digestive Diseases grant DK-068007 to IG; and the Kidney and Urology Foundation of America Award to IG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baskin LS, Himes K, Colborn T. Hypospadias and endocrine disruption: is there a connection? Environ Health Perspective. 2001;109:1175–1183. doi: 10.1289/ehp.011091175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley J, Willingham E, Agras K, Baskin LS. Embryonic exposure to the fungicide vinclozolin causes virilization of females and alteration of progesterone receptor expression in vivo: an experimental study in mice. Environ Health. 2006;5:4. doi: 10.1186/1476-069X-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnutt C, Burrus LW, Brown AM, Niswander L. Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev Biol. 2004;274:334–347. doi: 10.1016/j.ydbio.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Cheng W, Jacobs WB, Zhang JJ, Moro A, Park JH, Kushida M, Qiu W, Mills AA, Kim PC. DeltaNp63 plays an anti-apoptotic role in ventral bladder development. Development. 2006;133:4783–4792. doi: 10.1242/dev.02621. [DOI] [PubMed] [Google Scholar]
- Drews U, Sulak O, Schenck PA. Androgens and the development of the vagina. Biol Reprod. 2002;67:1353–1359. doi: 10.1095/biolreprod67.4.1353. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Godin RE, Robertson EJ. Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev. 1999;13:1601–1613. doi: 10.1101/gad.13.12.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin RE, Takaesu NT, Robertson EJ, Dudley AT. Regulation of BMP7 expression during kidney development. Development. 1998;125:3473–3482. doi: 10.1242/dev.125.17.3473. [DOI] [PubMed] [Google Scholar]
- Grishina IB, Kim SY, Ferrara C, Makarenkova HP, Walden PD. BMP7 inhibits branching morphogenesis in the prostate gland and interferes with Notch signaling. Dev Biol. 2005;288:334–347. doi: 10.1016/j.ydbio.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi R, Suzuki K, Murakami R, Sakai M, Kamikawa M, Kengaku M, Sekine K, Kawano H, Kato S, Ueno N, Yamada G. Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development. 2000;127:2471–2479. doi: 10.1242/dev.127.11.2471. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, Shiroishi T, Gaffield W, Yamada G. Unique functions of Sonic hedgehog signaling during external genitalia development. Development. 2001;128:4241–4250. doi: 10.1242/dev.128.21.4241. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Motoyama J, Sasaki H, Satoh Y, Miyagawa S, Nakagata N, Moon A, Yamada G. Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development. 2007;134:525–533. doi: 10.1242/dev.02736. [DOI] [PubMed] [Google Scholar]
- Hendren WH. Cloacal malformations: experience with 105 cases. J Pediatr Surg. 1992;27:890–901. doi: 10.1016/0022-3468(92)90393-l. [DOI] [PubMed] [Google Scholar]
- Hendren WH. Urogenital sinus and cloacal malformations. Semin Pediatr Surg. 1996;5:72–79. [PubMed] [Google Scholar]
- Hendren WH. Cloaca, the most severe degree of imperforate anus: experience with 195 cases. Ann Surg. 1998;228:331–346. doi: 10.1097/00000658-199809000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes PJ, Fraher JP. The development of the male genitourinary system. I. The origin of the urorectal septum and the formation of the perineum. Review. Br J Plast Surg. 2004a;57:27–36. doi: 10.1016/j.bjps.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Hynes PJ, Fraher JP. The development of the male genitourinary system. II. The origin and formation of the urethral plate. Br J Plast Surg. 2004b;57:112–121. doi: 10.1016/j.bjps.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Hynes PJ, Fraher JP. The development of the male genitourinary system. III. The formation of the spongiose and glandar urethra. Br J Plast Surg. 2004c;57:203–214. doi: 10.1016/j.bjps.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Kim KS, Liu W, Cunha GR, Russell DW, Huang H, Shapiro E, Baskin LS. Expression of the androgen receptor and 5 alpha-reductase type 2 in the developing human fetal penis and urethra. Cell Tissue Res. 2002;307:145–153. doi: 10.1007/s004410100464. [DOI] [PubMed] [Google Scholar]
- Kluth D, Hillen M, Lambrecht W. The principles of normal and abnormal hindgut development. J Pediatr Surg. 1995;30:1143–1147. doi: 10.1016/0022-3468(95)90007-1. [DOI] [PubMed] [Google Scholar]
- Kurzrock EA, Baskin LS, Cunha GR. Ontogeny of the male urethra: theory of endodermal differentiation. Differentiation. 1999;64:115–122. doi: 10.1046/j.1432-0436.1999.6420115.x. [DOI] [PubMed] [Google Scholar]
- Levitt MA, Pena A. Pitfalls in the management of newborn cloacas. Pediatr Surg Int. 2005;21:264–269. doi: 10.1007/s00383-005-1380-2. [DOI] [PubMed] [Google Scholar]
- Lincecum JM, Fannon A, Song K, Wang Y, Sassoon DA. Msh homeobox genes regulate cadherin-mediated cell adhesion and cell-cell sorting. J Cell Biochem. 1998;70:22–28. doi: 10.1002/(sici)1097-4644(19980701)70:1<22::aid-jcb3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Hogan BL, Robertson EJ. Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech Dev. 1995;50:71–83. doi: 10.1016/0925-4773(94)00326-i. [DOI] [PubMed] [Google Scholar]
- Mo R, Kim JH, Zhang J, Chiang C, Hui CC, Kim PC. Anorectal malformations caused by defects in sonic hedgehog signaling. Am J Pathol. 2001;159:765–774. doi: 10.1016/S0002-9440(10)61747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EA, Nguyen SB, Scott V, Stadler HS. Loss of Bmp7 and Fgf8 signaling in Hoxa13-mutant mice causes hypospadia. Development. 2003;130:3095–3109. doi: 10.1242/dev.00530. [DOI] [PubMed] [Google Scholar]
- Mortlock DP, Innis JW. Mutation of HOXA13 in hand-foot-genital syndrome. Nat Genet. 1997;15:179–180. doi: 10.1038/ng0297-179. [DOI] [PubMed] [Google Scholar]
- Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol. 2002;247:26–46. doi: 10.1006/dbio.2002.0668. [DOI] [PubMed] [Google Scholar]
- Petiot A, Perriton CL, Dickson C, Cohn MJ. Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development. 2005;132:2441–2450. doi: 10.1242/dev.01778. [DOI] [PubMed] [Google Scholar]
- Pizette S, Abate-Shen C, Niswander L. BMP controls proximodistal outgrowth, via induction of the apical ectodermal ridge, and dorsoventral patterning in the vertebrate limb. Development. 2001;128:4463–4474. doi: 10.1242/dev.128.22.4463. [DOI] [PubMed] [Google Scholar]
- Ross A, Munger S, Capel B. Bmp7 regulates germ cell proliferation in mouse fetal gonads. Sex Dev. 2007;1:127–137. doi: 10.1159/000100034. [DOI] [PubMed] [Google Scholar]
- Sasaki C, Yamaguchi K, Akita K. Spatiotemporal distribution of apoptosis during normal cloacal development in mice. Anat Rec A. 2004;279:761–767. doi: 10.1002/ar.a.20062. [DOI] [PubMed] [Google Scholar]
- Seifert AW, Harfe BD, Cohn MJ. Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev Biol. 2008;318:143–152. doi: 10.1016/j.ydbio.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Bachiller D, Chen YP, Kamikawa M, Ogi H, Haraguchi R, Ogino Y, Minami Y, Mishina Y, Ahn K, Crenshaw EB, 3rd, Yamada G. Regulation of outgrowth and apoptosis for the terminal appendage: external genitalia development by concerted actions of BMP signaling. Development. 2003;130:6209–6220. doi: 10.1242/dev.00846. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Haraguchi R, Ogata T, Barbieri O, Alegria O, Vieux-Rochas M, Nakagata N, Ito M, Mills AA, Kurita T, Levi G, Yamada G. Abnormal urethra formation in mouse models of Split-hand/split-foot malformation type 1 and type 4. Eur J Hum Genet. 2008;16:36–44. doi: 10.1038/sj.ejhg.5201925. [DOI] [PubMed] [Google Scholar]
- Yamada G, Satoh Y, Baskin LS, Cunha GR. Cellular and molecular mechanisms of development of the external genitalia. Differentiation. 2003;71:445–460. doi: 10.1046/j.1432-0436.2003.7108001.x. [DOI] [PubMed] [Google Scholar]
- Yamada G, Suzuki K, Haraguchi R, Miyagawa S, Satoh Y, Kamimura M, Nakagata N, Kataoka H, Kuroiwa A, Chen Y. Molecular genetic cascades for external genitalia formation: an emerging organogenesis program. Dev Dyn. 2006;235:1738–1752. doi: 10.1002/dvdy.20807. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 1999. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yucel S, Cavalcanti AG, Desouza A, Wang Z, Baskin LS. The effect of oestrogen and testosterone on the urethral seam of the developing male mouse genital tubercle. Br J Urol. 2003;92:1016–1021. doi: 10.1111/j.1464-410x.2003.04511.x. [DOI] [PubMed] [Google Scholar]
- Yucel S, Desouza A, Baskin LS. In utero prednisone exposure affects genital development. J Urol. 2004;172:1725–1730. doi: 10.1097/01.ju.0000139911.56346.1b. [DOI] [PubMed] [Google Scholar]
- Warot X, Fromental-Ramain C, Fraulob V, Chambon P, Dolle P. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development. 1997;124:4781–4791. doi: 10.1242/dev.124.23.4781. [DOI] [PubMed] [Google Scholar]