Abstract
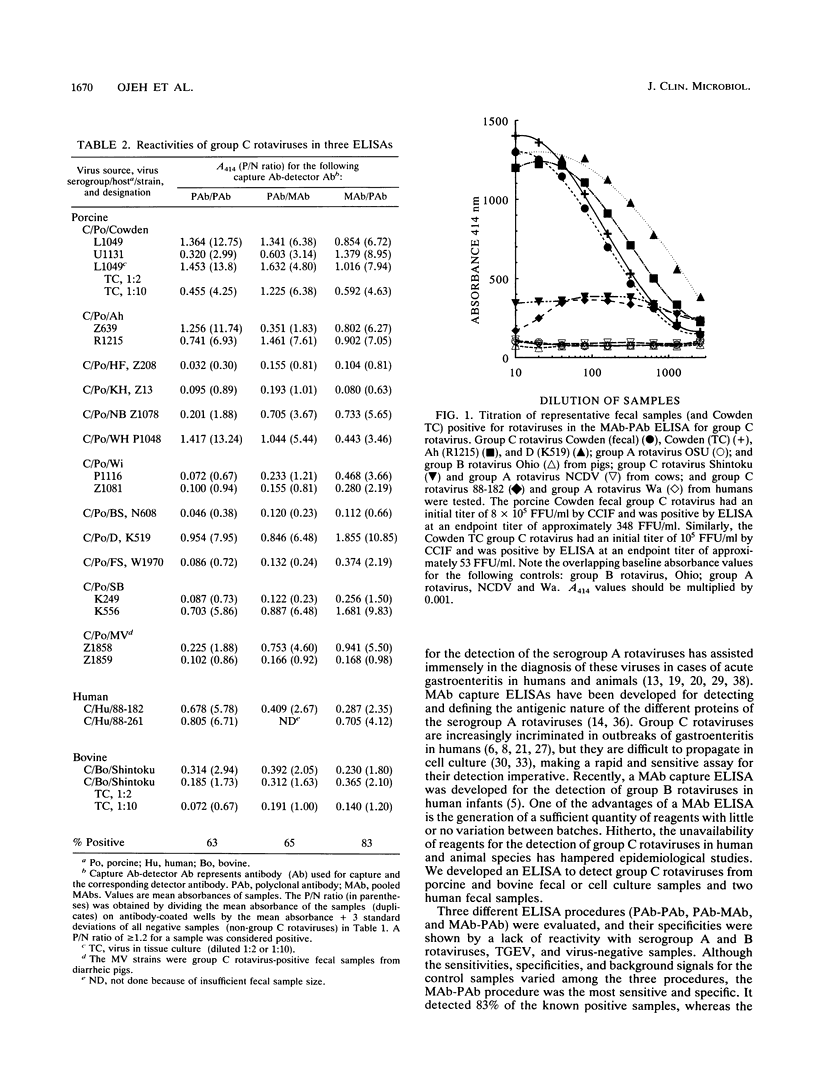
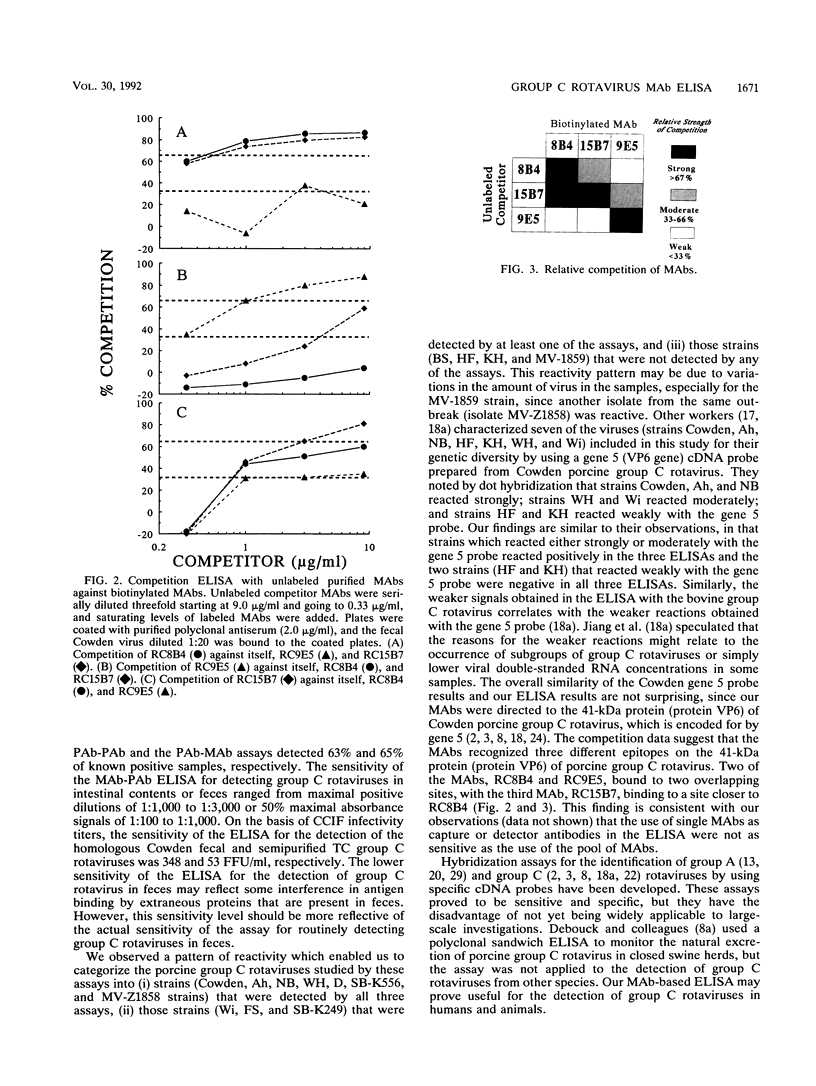
A biotin-streptavidin-enhanced enzyme-linked immunosorbent assay (ELISA) which uses monoclonal antibodies (MAbs) for the detection of group C rotaviruses was developed. An assay in which plates were coated with three pooled MAbs and biotinylated polyclonal immunoglobulin G (IgG) (polyclonal antibody [PAb]) was used as the detector (MAb capture-PAb detector) was found to be the most sensitive and specific of the assays when it was compared with assays in which plates were coated with polyclonal antiserum and detection was done with either biotinylated polyclonal antiserum (PAb capture-PAb detector) or biotinylated pooled MAbs (PAb capture-MAb detector). The MAb capture-PAb detector ELISA detected 83% of samples confirmed to be positive for group C rotaviruses, whereas the PAb capture-PAb detector assay detected 63% of positive samples and the PAb capture-MAb detector assay detected 65% of positive samples. All three procedures detected both of the bovine and the two human group C rotaviruses, but none of the three procedures detected fecal samples containing group A and B rotaviruses or fecal samples negative for group C rotaviruses used in this study. The sensitivity of the MAb capture-PAb detector ELISA was determined by serially diluting fecal group C rotaviruses; antigens were detected in maximal positive dilution ranges of 1:1,000 to 1:3,000 for the samples tested. On the basis of the cell culture immunofluorescence assay infectivity titer of semipurified cell culture-passaged Cowden group C rotavirus, the sensitivity of the MAb capture-PAb detection ELISA for detection of homologous group C rotavirus was 53 fluorescent focus units per ml. Epitope mapping by use of the biotinylated MAbs in competition assay suggested that our MAbs may bind to three different but overlapping epitopes. These results suggest that the MAb capture-PAb detector ELISA can be used to study the epidemiology of group C rotaviruses in humans and animals.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohl E. H., Saif L. J., Theil K. W., Agnes A. G., Cross R. F. Porcine pararotavirus: detection, differentiation from rotavirus, and pathogenesis in gnotobiotic pigs. J Clin Microbiol. 1982 Feb;15(2):312–319. doi: 10.1128/jcm.15.2.312-319.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremont M., Chabanne-Vautherot D., Vannier P., McCrae M. A., Cohen J. Sequence analysis of the gene (6) encoding the major capsid protein (VP6) of group C rotavirus: higher than expected homology to the corresponding protein from group A virus. Virology. 1990 Oct;178(2):579–583. doi: 10.1016/0042-6822(90)90357-w. [DOI] [PubMed] [Google Scholar]
- Bremont M., Cohen J., McCrae M. A. Analysis of the structural polypeptides of a porcine group C rotavirus. J Virol. 1988 Jun;62(6):2183–2185. doi: 10.1128/jvi.62.6.2183-2185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger J. C. Detection by electron microscopy of caliciviruses, astroviruses and rotavirus-like particles in the faeces of piglets with diarrhoea. Vet Rec. 1980 Dec 6;107(23):532–533. [PubMed] [Google Scholar]
- Burns J. W., Welch S. K., Nakata S., Estes M. K. Characterization of monoclonal antibodies to human group B rotavirus and their use in an antigen detection enzyme-linked immunosorbent assay. J Clin Microbiol. 1989 Feb;27(2):245–250. doi: 10.1128/jcm.27.2.245-250.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caul E. O., Ashley C. R., Darville J. M., Bridger J. C. Group C rotavirus associated with fatal enteritis in a family outbreak. J Med Virol. 1990 Mar;30(3):201–205. doi: 10.1002/jmv.1890300311. [DOI] [PubMed] [Google Scholar]
- Chasey D., Davies P. Atypical rotaviruses in pigs and cattle. Vet Rec. 1984 Jan 7;114(1):16–17. doi: 10.1136/vr.114.1.16. [DOI] [PubMed] [Google Scholar]
- Cooke S. J., Lambden P. R., Caul E. O., Clarke I. N. Molecular cloning, sequence analysis and coding assignment of the major inner capsid protein gene of human group C rotavirus. Virology. 1991 Oct;184(2):781–785. doi: 10.1016/0042-6822(91)90452-h. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. H., Estes M. K., Rangelova S. M., Shindarov L. M., Melnick J. L., Graham D. Y. Detection of antigenically distinct rotaviruses from infants. Infect Immun. 1983 Aug;41(2):523–526. doi: 10.1128/iai.41.2.523-526.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Puerto F., Soler C., González N. Characterization of a human pararotavirus. Infect Immun. 1984 Apr;44(1):112–116. doi: 10.1128/iai.44.1.112-116.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Flores J., Boeggeman E., Purcell R. H., Sereno M., Perez I., White L., Wyatt R. G., Chanock R. M., Kapikian A. Z. A dot hybridisation assay for detection of rotavirus. Lancet. 1983 Mar 12;1(8324):555–558. doi: 10.1016/s0140-6736(83)92811-8. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Jiang B. M., Qian Y., Tsunemitsu H., Green K. Y., Saif L. J. Analysis of the gene encoding the outer capsid glycoprotein (VP7) of group C rotaviruses by northern and dot blot hybridization. Virology. 1991 Sep;184(1):433–436. doi: 10.1016/0042-6822(91)90864-8. [DOI] [PubMed] [Google Scholar]
- Jiang B. M., Saif L. J., Kang S. Y., Kim J. H. Biochemical characterization of the structural and nonstructural polypeptides of a porcine group C rotavirus. J Virol. 1990 Jul;64(7):3171–3178. doi: 10.1128/jvi.64.7.3171-3178.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Imai M., Bellamy A. R., Ikegami N., Furuichi Y., Summers D., Nuss D. L., Deibel R. Diagnosis of rotavirus infection with cloned cDNA copies of viral genome segments. J Virol. 1985 Aug;55(2):509–512. doi: 10.1128/jvi.55.2.509-512.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Hatano M., Kobayashi K., Hasegawa A., Yamazaki S., Nakata S., Chiba S., Kimura Y. An outbreak of gastroenteritis associated with acute rotaviral infection in schoolchildren. J Infect Dis. 1989 Oct;160(4):611–615. doi: 10.1093/infdis/160.4.611. [DOI] [PubMed] [Google Scholar]
- Nagesha H. S., Hum C. P., Bridger J. C., Holmes I. H. Atypical rotaviruses in Australian pigs. Arch Virol. 1988;102(1-2):91–98. doi: 10.1007/BF01315565. [DOI] [PubMed] [Google Scholar]
- Nicolas J. C., Cohen J., Fortier B., Lourenco M. H., Bricout F. Isolation of a human pararotavirus. Virology. 1983 Jan 15;124(1):181–184. doi: 10.1016/0042-6822(83)90302-1. [DOI] [PubMed] [Google Scholar]
- Ojeh C. K., Jiang B. M., Tsunemitsu H., Kang S. Y., Weilnau P. A., Saif L. J. Reactivity of monoclonal antibodies to the 41-kilodalton protein of porcine group C rotavirus with homologous and heterologous rotavirus serogroups in immunofluorescence tests. J Clin Microbiol. 1991 Sep;29(9):2051–2055. doi: 10.1128/jcm.29.9.2051-2055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedley S., Bridger J. C., Brown J. F., McCrae M. A. Molecular characterization of rotaviruses with distinct group antigens. J Gen Virol. 1983 Oct;64(Pt 10):2093–2101. doi: 10.1099/0022-1317-64-10-2093. [DOI] [PubMed] [Google Scholar]
- Pedley S., Bridger J. C., Chasey D., McCrae M. A. Definition of two new groups of atypical rotaviruses. J Gen Virol. 1986 Jan;67(Pt 1):131–137. doi: 10.1099/0022-1317-67-1-131. [DOI] [PubMed] [Google Scholar]
- Peñaranda M. E., Cubitt W. D., Sinarachatanant P., Taylor D. N., Likanonsakul S., Saif L., Glass R. I. Group C rotavirus infections in patients with diarrhea in Thailand, Nepal, and England. J Infect Dis. 1989 Sep;160(3):392–397. doi: 10.1093/infdis/160.3.392. [DOI] [PubMed] [Google Scholar]
- Qian Y., Saif L. J., Kapikian A. Z., Kang S. Y., Jiang B., Ishimaru Y., Yamashita Y., Oseto M., Green K. Y. Comparison of human and porcine group C rotaviruses by northern blot hybridization analysis. Arch Virol. 1991;118(3-4):269–277. doi: 10.1007/BF01314037. [DOI] [PubMed] [Google Scholar]
- Rosen B. I., Saif L. J., Jackwood D. J., Gorziglia M. Hybridization probes for the detection and differentiation of two serotypes of porcine rotavirus. Vet Microbiol. 1990 Sep;24(3-4):327–339. doi: 10.1016/0378-1135(90)90181-t. [DOI] [PubMed] [Google Scholar]
- Saif L. J., Bohl E. H., Theil K. W., Cross R. F., House J. A. Rotavirus-like, calicivirus-like, and 23-nm virus-like particles associated with diarrhea in young pigs. J Clin Microbiol. 1980 Jul;12(1):105–111. doi: 10.1128/jcm.12.1.105-111.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppälä I., Sarvas H., Péterfy F., Mäkelä O. The four subclasses of IgG can be isolated from mouse serum by using Protein A-Sepharose. Scand J Immunol. 1981 Oct;14(4):335–342. doi: 10.1111/j.1365-3083.1981.tb00573.x. [DOI] [PubMed] [Google Scholar]
- Simkins R. A., Saif L. J., Weilnau P. A. Epitope mapping and the detection of transmissible gastroenteritis viral proteins in cell culture using biotinylated monoclonal antibodies in a fixed-cell ELISA. Arch Virol. 1989;107(3-4):179–190. doi: 10.1007/BF01317915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Morita Y., Greenberg H. B., Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987 Jun;155(6):1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- Terrett L. A., Saif L. J. Serial propagation of porcine group C rotavirus (pararotavirus) in primary porcine kidney cell cultures. J Clin Microbiol. 1987 Jul;25(7):1316–1319. doi: 10.1128/jcm.25.7.1316-1319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemitsu H., Saif L. J., Jiang B. M., Shimizu M., Hiro M., Yamaguchi H., Ishiyama T., Hirai T. Isolation, characterization, and serial propagation of a bovine group C rotavirus in a monkey kidney cell line (MA104). J Clin Microbiol. 1991 Nov;29(11):2609–2613. doi: 10.1128/jcm.29.11.2609-2613.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]



