Abstract
Objective:
Perceived impairment and psychomotor performance following acute alcohol administration in older (ages 50-74, n = 42; 22 male) and younger (ages 25-35, n = 26; 12 male) adults were investigated in this study.
Method:
Double-blind, placebo-controlled alcohol administration techniques were designed to produce peak levels of breath alcohol concentration consistent with an episode of social drinking (40 mg/100 ml). Behavioral measures (Trail Making Test, Forms A and B), as well as measures of self-reported perceived intoxication and impairment, were administered on the ascending and descending limbs at common time points after beverage ingestion.
Results:
Results indicated that psychomotor performance differences did not parallel self-reported levels of perceived impairment. Relative to younger adults, older adults exhibited performance deficits on the ascending limb while simultaneously reporting less perceived impairment. Conversely, on the descending limb, older adults who received alcohol reported more perceived impairment than did those who received placebo, although psychomotor performance between these two groups of older drinkers did not differ. For younger participants, a moderate dose of alcohol facilitated performance on the ascending limb; however, these differences were not reflected on the descending limb.
Conclusions:
These results reinforce the common knowledge that self-reported measures may not provide an accurate reflection of performance outcomes and, importantly, that older adults may be impaired even under a moderate dose of alcohol, although they may not be aware (i.e., report) of this impairment.
The u.s. bureau of the census (2008) predicts that by 2030, the percentage of the population older than age 65 will rise dramatically to comprise one in five U.S. residents. A majority of this population (52% of older adults above age 55) continue to consume alcoholic beverages in social settings (Substance Abuse and Mental Health Services Administration, 2008). Thus, established drinking patterns may continue into later years because of longer life expectancies that encourage older adults to remain socially active (Adams et al., 1990; Goodwin et al., 1987; Zucker, 1998).
Although changes in cognition are typically not demonstrated until the seventh or eighth decade of life, alcohol may compound already slight age-related cognitive differences (Gilbertson et al., 2006; Logan et al., 2002). The literature remains sparse with regard to studies of the effects of acute alcohol administration among older social drinkers, but studies of younger adults show that impairment caused by blood alcohol concentrations (BACs) of 40 mg/100 ml may adversely affect a variety of cognitive and behavioral measures (Holloway, 1995; Moskowitz and Robinson, 1988). Performance deficits at lower BACs (≤40 mg/100 ml) are less consistently observed but often include decrements of reaction time, divided attention, focusing on a target, attention to stimuli in the peripheral visual field, and scanning of the visual field (Linnoila et al., 1986; Moskowitz and Robinson, 1988). Given the negative effects of aging on the metabolism of alcohol, such as slower metabolism of alcohol and ineffective clearing (Kinney, 2006; Saitz, 2003), findings obtained from studies of younger adult participants may not reflect performance effects of acute low or moderate alcohol doses in older adults (see Nixon, 1998).
Existing studies of acute alcohol-related effects among older adults typically have focused on pharmacokinetics and end-point performance of behavioral tasks while often ignoring the potentially confounding effects of participants' self-reported responses to alcohol (Jones and Neri, 1994; Quillian et al., 1999; Tupler et al., 1995). This is an important consideration, as the perceived impairment and behavioral effects of alcohol are complex and may be influenced by a variety of factors (Fillmore and Vogel-Sprott, 1997). For instance, self-reports of alcohol intoxication are limited by factors common to all self-reported research methods, including social desirability effects (Bechhofer and Paterson, 2000). At typical “social” levels of alcohol consumption, participants may not be consciously aware of their level of physiological intoxication (Kinney, 2006, p. 58), an effect that may be modulated by age-related changes in both alcohol metabolism and cognitive processes. This potential disconnection between self-reported and behavioral effects of alcohol may have significant implications for public health and safety, particularly among active, older social drinkers. For these reasons, the disentanglement of self-reported and behavioral effects of acute alcohol consumption among non-diseased older adults is becoming an increasingly important focus of current research.
To address these issues, the current study was designed primarily to measure perceived intoxication and impairment, as well as psychomotor performance on the Trail Making Test (Forms A [Trails A] and B [Trails B]) among younger versus older adults in response to a target BAC consistent with an episode of social drinking (Russell et al., 1970). Placebo controls were used to clarify the nature of acute alcohol effects. Given the cognitive and metabolic effects of aging, the authors predicted that older adults would demonstrate greater negative, alcohol-related, psychomotor performance effects compared with younger adults at a similar breath alcohol concentration (BrAC). A secondary aim of the study focused on the potential disconnection between perceived impairment and behavioral performance in older versus younger participants.
Method
Participants
Participants were older (ages 50-74; n = 42) and younger (ages 25-35; n = 26) moderate social drinkers consuming at least one drink per month. Older participants were over-sampled because of greater expected variability, as well as the lack of a guiding literature regarding effect sizes in this age group. Participants were recruited via newspaper ads, flyers, radio ads, and word of mouth and were paid for their participation. Participants listed their ethnicity/race as white (87%), black (10%), Asian (2%), and undeclared (1%). All procedures were approved by the University of Kentucky Medical Institutional Review Board.
Screening
Basic demographic information, health status, medication history, smoking status, alcohol consumption, and illicit drug use for potential participants were assessed by telephone. Eligible participants were then scheduled for a screening visit in which they completed a packet assessing the quantity and frequency of alcohol consumption over the last 6 months (quantity-frequency index [QFI; Cahalan et al., 1969]), current levels of depressive symptomatology (Beck Depression Inventory-II [Beck et al., 1996] for younger participants and the Geriatric Depression Scale [Yesavage et al., 1982] for older participants), and state anxiety (Spielberger State Anxiety Inventory [Spielberger, 1983]). The Shipley Institute of Living Vocabulary and Abstraction Tests were also administered to assess general intellectual ability (Zachary, 1986). The Michigan Alcoholism Screening Test (MAST; Selzer, 1971) and the geriatric version of the MAST were administered to screen for current problematic use of alcohol in younger and older participants, respectively (Blow, 1991). Psychiatric history was also assessed using the computerized National Institute of Mental Health Diagnostic Interview Schedule (Robbins et al., 1995). Thus, participants invited to the laboratory session had no current Axis I disorders, neurological trauma (stroke, seizure disorder, or serious head injury resulting in unconsciousness longer than 10 hours), medical illnesses (heart, lung, liver, or kidney disease; uncontrolled diabetes; or blood pressure), or alcohol/drug abuse or dependence (as defined by the Diagnostic Interview Schedule). All participants were current nonsmokers.
On the laboratory testing day, all participants were screened via urine analysis for tetrahydrocannabinol, cocaine, benzodiazepines, morphine, and methamphetamine. Participants also provided a breath sample for baseline BrAC analysis, the result of which was required to be no greater than zero. Women who were pregnant or breast feeding, as determined by self-report and urine analysis, were not allowed to participate in the study.
Acute alcohol administration
Older and younger adults enrolled in the laboratory portion of the study received either a moderate dose of alcohol or a placebo beverage. Beverages were administered in a randomized, double-blind procedure. Placebo controls were used to distinguish the effects of acute alcohol administration from effects attributable to aging alone. Standard alcohol administration procedures (see Fillmore et al., 2000) were modified to allow participants to achieve a specific BrAC level of 40 mg/100 ml (Watson et al., 1980) during the psychomotor task. This BrAC was chosen because it approximates the BrAC achieved in a social drinking situation for older adults. Further, Holloway (1995) concluded that, in contrast to the general assumption that BACs of 40 mg/100 ml or greater were needed to affect psychomotor skills, such deficits can be observed at levels of approximately 20 mg/100 ml. Koelega (1995) also argues that low to moderate doses may have profound effects, if appropriate measures are applied (i.e., attention and information processing).
Alcohol beverages were mixed as one part 100% medical grade alcohol and three parts vehicle beverage consisting of ice-cold, noncaffeinated lime soda. Placebo beverages contained only the vehicle dose with a negligible amount of alcohol floating on the surface. Both beverage types were misted with alcohol before administration to facilitate an expectancy effect. The beverage was administered in two glasses, and participants were given 2 minutes to drink each beverage. All participants were provided with a booster beverage 30 minutes after the original beverage administration consisting of half the original alcohol and vehicle dose or, for younger women, the full alcohol and vehicle dose—a procedure that allowed both older and younger participants to achieve a peak BrAC of approximately 40 mg/100 ml. All participants were fed a standardized lunch (∼500 kcal) and a snack (∼115 kcal) approximately 4 hours and 1 hour before beverage administration, respectively. Participants were debriefed as to whether they had received alcohol at the conclusion of testing. Participants who were administered alcohol were told their current BrAC and were released once they registered less than 10 mg/100 ml.
Trail making Forms A and B
Participants completed the Trail Making Test (Forms A [Trails A] and B [Trails B]) (Russell et al., 1970) on the ascending limb (25 minutes) and descending limb (75 minutes) following beverage administration. To approximate administration at similar BrACs, the order of Trails A/Trails B was reversed on the descending limb. Trails A and B are subtests of the Halstead Reitan Battery (Russell et al., 1970) and measure psychomotor and set shifting skills. Trails A requires participants to connect numbered dots (e.g., 1-13) with a line and takes approximately 1 minute to complete. Trails B requires participants to connect alternating numbers and letters (e.g., 1 to A to 2 to B) and take a variable amount of time, but generally no more than 3 minutes, to complete. Dependent variables are the time to complete and the number of errors, which are rare in normal participants. Limited data suggest that older participants may not acquire acute tolerance as readily as younger participants (Kalant, 1998). Therefore, it was scientifically and clinically relevant that both limbs be studied. To meet such demands, tests must be quickly administered. Trails A and B were selected to meet these task specifications. Additionally, differences in Trails A and B performance between equivalent BACs on the ascending and descending limb are well documented (Nicholson et al., 1992). The Trails tests are also sensitive to aging effects because time to complete them slows in normal adults who are advanced in age (older than age 70; Wahlin et al., 1996). Normative data for Trails A and B suggest that differences between age groups are slight; however, alcohol could accentuate slight aging differences.
Breath alcohol concentrations and self-reported measurements
Breath samples (Intoxilyzer, Model 400; CMI, Inc., Owensboro, KY) were used to determine BrACs. BrACs were measured 25,45, 55, 65, and 75 minutes following beverage administration.
Self-reported measurements were assessed by asking participants to answer the question regarding perceived intoxication (“how intoxicated do you currently feel”) on a 10-point Likert scale (1 = not intoxicated to 10 = most intoxicated in my life). Participants were also asked about perceived impairment (“rate the degree that you felt your drink impaired your performance on the task”) for the Trail Making Test (1 = no impairment to 10 = extreme impairment). Self-reported measurements of perceived alcohol intoxication and impairment were adapted from Harrison and colleagues (2007) and are commonly used in acute alcohol administration studies (e.g., Fillmore et al., 2002; Harrison and Fillmore, 2005b). Ratings were assessed immediately following Trails A and B task completion on the ascending and descending limbs of the BrAC (25 and 75 minutes following beverage ingestion).
Data analysis
Statistical analyses were completed using SAS Version 9.1 (SAS Institute, Inc., Cary, NC). Demographic data comparing older and younger participants were analyzed with Student's t test assuming equal variances or, where appropriate, Satterthwaite's t test. Other data were analyzed by separate analysis of variance (ANOVAs) for both self-reported (perceived intoxication and impairment) and behavioral (psychomotor performance on the Trails task) measures. The hypothesis that older adults would demonstrate greater alcohol-related behavioral effects on the Trails tasks, compared with younger adults, was assessed by 2 (Age: older vs younger) × 2 (Group: alcohol vs placebo) × 2 (ascending vs descending) repeated measures ANOVAs for Trails A and B. The question of whether objective performance and perceived intoxication or impairment would be dissociated in older as opposed to younger drinkers was assessed by 2 (Age: older vs younger) × 2 (Group: alcohol vs placebo) × 2 (ascending vs descending) repeated measure ANOVAs for each variable. The administration of Trails A and B on the ascending versus descending limb was confirmed for each participant who received alcohol by identifying the peak BrAC. All participants included in data analysis performed in the nonimpaired range for Trails A and B. BrACs for older versus younger participants were analyzed with repeated measures ANOVA. When interactions occurred, post hoc analyses were conducted to determine group significances. Type III sum of squares F statistics are reported for all ANOVAs to account for unequal sample sizes. Degrees of freedom differences indicate missing data.
Results
Demographics
Demographic data for older and younger participants are presented in Table 1. Depression and problematic alcohol use were assessed using age-appropriate measures for older and younger participants; therefore, these data were not compared statistically. However, mean depression scores (the Geriatric Depression Scale and the Beck Depression Inventory-II) and problematic alcohol use (MAST-G, MAST) were below the level of clinical significance for older and younger participants, respectively. Older and younger participants did not differ in their quantity or frequency of alcohol consumption (quantity-frequency index), measurements of anxiety, or body composition (body mass index).
Table 1.
Demographic characteristics of subjects, by age and group
| Variable | Older |
Younger |
||
| Alcohol (n = 17) Mean (SD) | Placebo (n = 25) Mean (SD) | Alcohol (n = 11) Mean (SD) | Placebo (n = 15) Mean (SD) | |
| Age, in years* | 57.24 (6.77) | 57.28 (6.57) | 29.54 (4.18) | 28.53 (3.11) |
| Male, % | 58.82 | 48.00 | 45.45 | 46.67 |
| Education, in years* | 16.06 (3.27) | 15.32 (2.01) | 18.64 (3.88) | 17.20 (1.32) |
| Depressive symptoms | 6.00 (6.15) | 4.46 (4.16) | 5.36 (7.35) | 4.13 (4.41) |
| STAIa | 46.06 (5.49) | 45.64 (10.11) | 46.00 (6.29) | 43.80 (4.44) |
| SILS-Vb,* | 18.75 (2.05) | 18.68 (1.32) | 17.85 (1.46) | 17.71 (1.31) |
| SILS-Ab,* | 16.21 (2.53) | 15.97 (2.88) | 18.90 (1.43) | 17.67 (1.98) |
| QFIc | 0.54 (0.46) | 0.98 (1.36) | 0.70 (0.81) | 0.41 (0.28) |
| MAST/Gd,e | 5.53 (3.69) | 3.75 (3.53) | 2.22 (1.71) | 2.13 (2.03) |
| BMIf | 28.25 (7.77) | 28.45 (5.24) | 27.30 (6.67) | 26.64 (4.57) |
Spielberger State Anxiety Inventory (Spielberger, 1983);
Shipley Institute of Living Scale Vocabulary (V) and Abstraction (A) (Zachary, 1986);
quantity-frequency index (Cahalan et al., 1969);
Michigan Alcoholism Screening Test (Selzer, 1971);
Michigan Alcoholism Screening Test-Geriatric Version (Blow, 1991);
body mass index.
Differences between older and younger participants significant (age: t = 22.99, p < .0001; education: t = -3.32, p = .0015; SILS-V: t = 2.47, p = .0161; SILS-A: t = -3.84, p = .0003).
Trails A and B
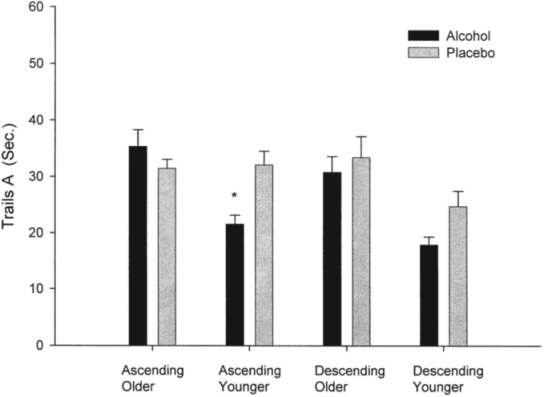
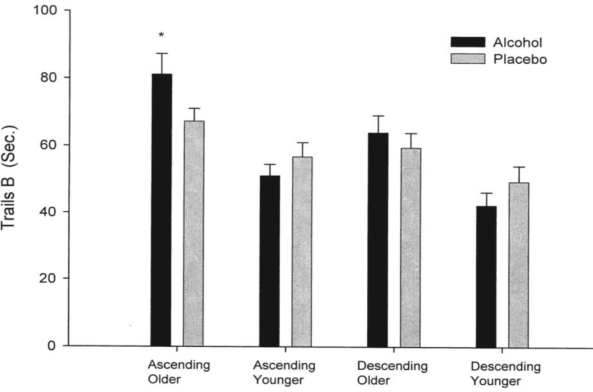
Means for Trails A and B task administration for both the ascending and descending limbs are reported in Table 2. Overall, older participants took longer to complete Trails A (F = 12.61, 1/61 df, p < .001) and Trails B (F = 18.13, 1/62 df, p < .0001). The time to completion (latency) was significantly faster on the descending limb (as opposed to the ascending limb) for Trails A (F = 4.01, 1/61 df, p = .04) and for Trails B (F = 16.60, 1/62 df, p < .0001). Initial analyses showed that the three-way interaction (Age × Group × Ascending vs Descending Limb) for the repeated measures was nonsignificant for both Trails A and B (F = 2.52, 1/61 df, p = .12; F = 0.61, 1/62 df, p = .44). However, visual inspection of psychomotor performance data suggested that the Age × Group interaction was different on the ascending versus descending limbs (see Figures 1a and 1b). Thus, ANOVAs with Age and Group as factors were repeated separately on psychomotor performance data for each limb of the BrAC curve. This data analysis strategy was deemed appropriate because of the obvious differences in the plotted data and the original conception of the project as a pilot study (with a small sample size). These analyses revealed a significant Age × Group interaction on the ascending limb for Trails A (F = 9.63, 1/63 df, p < .005) and approached significance on Trails B (F = 3.94, 1/62 df, p = .051), respectively. Post hoc analyses for these interactions on the ascending limb showed that older participants who received alcohol were slower to complete Trails A and B than were younger participants who also received alcohol (Trails A: F = 12.58, 1/26 df, p < .001; Trails B: F = 16.23, 1/26 df, p < .0005). These age differences were not found in those receiving the placebo beverage. Age-related performance differences remained significant when BrAC was included in the model as a covariate (Trails A: F = 8.49, 1/25 df, p < .001; Trails B: F = 13.40, 1/25 df, p < .001).
Table 2.
Trails A and B: Means and standard errors
| Group | Ascending |
Descending |
||
| Trails A Mean (SE) | Trails B Mean (SE) | Trails A Mean (SE) | Trails B Mean (SE) | |
| Younger | ||||
| Placebo | 32.13 (2.44)a | 56.73 (4.29) | 24.73 (2.74) | 49.40 (4.69) |
| Alcohol | 21.54(1.60)a,‡ | 51.09(3.40)‡ | 17.90(1.42) | 42.27 (3.98) |
| Older | ||||
| Placebo | 31.46(1.61) | 67.26 (3.74)b | 33.43 (3.76) | 59.52 (4.39) |
| Alcohol | 35.35 (2.94)‡ | 81.06 (6.17)b,‡ | 30.81 (2.81) | 64.00 (5.09) |
Differences in Trails A in younger adults who received alcohol versus placebo significant (p = .008);
differences in Trails B in older adults who received alcohol versus placebo significant (p = 0.03).
Differences in Trails A and Trails B in older and younger adults who received alcohol significant (Age × Alcohol interaction; Trails A: p = .0006; Trails B: p = .00004).
Figure 1a.
Performance on Trails A of the Trail Making Test (Russell et al., 1970) in older and younger drinkers. Younger adults who received alcohol performed more quickly than younger adults receiving the placebo beverage on the ascending limb of the breath alcohol concentration curve (p = .008). Ascending = ascending limb; descending = descending limb; sec. = seconds. *p = .008.
Figure 1b.
Performance on Trails B of the Trail Making Test (Russell et al., 1970) in older and younger drinkers. Older adults who received alcohol performed more slowly than older adults who received the placebo beverage on the ascending limb of the breath alcohol concentration curve (p = .03). Ascending = ascending limb; descending = descending limb; sec. = seconds. * P = .03.
Older adults who received alcohol performed more slowly than older adults who received placebo (F = 5.03, 1/38 df, p = .03). However, alcohol facilitated performance on Trails A in younger adults, compared with placebo on the ascending limb (F = 8.43, 1/24 df, p = .008).
Self-reported measurements
Perceived intoxication.
Analyses of perceived intoxication revealed expected effects. Participants receiving alcohol reported feeling more intoxicated than those receiving placebo (F = 11.58, 1/62 df, p < .001). Those receiving alcohol reported more intoxication on the ascending limb than they did on the descending limb (Group × Time [F = 5.95, 1/62 df, p = .02]). As expected, these differences were not apparent in the placebo group.
Perceived impairment.
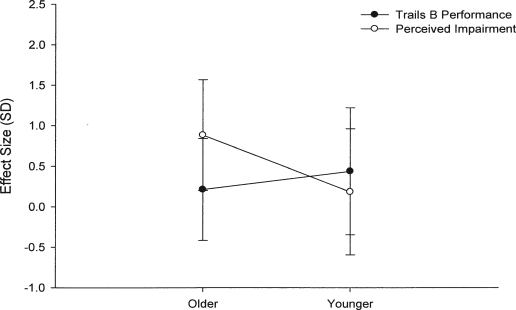
Participants were asked how impaired they felt while completing Trails A and B on both limbs of the BrAC curve (Table 3). Initial analyses revealed a three-way interaction (Age × Group × Time in these self-reported measures [F = 3.95, 1/62 df, p = .051]). Subsequent post hoc analyses showed age differences on both limbs. On the ascending limb, older adults reported less perceived impairment than did younger adults (F = 18.79, 1/64 df, p < .001; Figure 2a), regardless of alcohol group. In contrast, on the descending limb, further analyses revealed an Age × Group interaction (F = 4.48, 1/62 df, p = .04). Pairwise comparisons showed that older adults receiving alcohol reported more perceived impairment on the tasks, compared with older adults receiving the placebo beverage (F = 17.05, 1/38 df, p < .001). There was no difference between younger adults' perceived impairment (Figure 2b).
Table 3.
Perceived intoxication and impairment of subjects, by age and group
| Variable | Older |
Younger |
||
| Alcohol (n = 17) Mean (SD) | Placebo (n = 25) Mean (SD) | Alcohol (n = 11) Mean (SD) | Placebo (n = 15) Mean (SD) | |
| Intoxicationa | ||||
| Ascending | 3.94 (2.25) | 2.12(1.36) | 3.91 (1.76) | 2.47 (1.60) |
| Descending | 2.53(1.54) | 1.34(0.65) | 2.54(1.37) | 2.26(1.58) |
| Impairment | ||||
| Ascendingb | 3.41 (2.03) | 1.80(1.41) | 4.09 (2.07) | 2.13 (0.99) |
| Descendingc | 2.82 (2.19) | 1.43 (0.90) | 2.45(1.21) | 2.20(1.52) |
Differences between ascending, descending limb significant for alcohol group (F = 5.95, 1/62 df, p = .02);
differences between older and younger participants significant (F = 18.79, 1/64 df, p < .001);
differences between alcohol and placebo groups significant for older subjects (F = 17.05, 1/38 df, p < .001).
Figure 2a.
Ascending limb performance on Trails B of the Trail Making Test (Russell et al., 1970) and perceived impairment in older and younger adults. Data are shown as effect sizes (difference between alcohol and placebo [in standard deviations]) with 95% confidence intervals. Data not shown here (but discussed in text) revealed that, although older adults receiving alcohol were more impaired on the psychomotor task—compared with younger adults receiving alcohol—older adults reported less perceived impairment than did younger adults.
Figure 2b.
Descending limb performance on Trails B of the Trail Making Test (Russell et al., 1970) and perceived impairment in older and younger adults. Data are shown as effect sizes (difference between alcohol and placebo [in standard deviations]) with 95% confidence intervals. Data not shown here (but discussed in text) revealed that, although differences in task performance in alcohol and placebo groups were not significant in older adults, older adults receiving alcohol reported more perceived impairment than placebo. These differences in self-reported and performance measures between alcohol and placebo groups were not found in younger adults.
Breath alcohol concentration
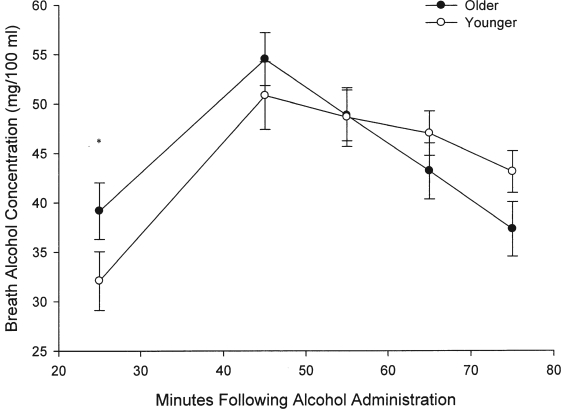
Overall, BrACs averaged across time did not differ between younger and older participants (p = .74). However, a repeated measures ANOVA showed slight differences in BrACs between older and younger participants over the course of testing (Time × Age interaction; F = 3.22, 4/104 df, p = .02). As Trails A and B task administration occurred on both the ascending and descending limbs, post hoc tests were also conducted at these time points. Post hoc tests showed that alcohol levels acquired just before Trails A and Trails B task administration were significantly different between older and younger participants for the ascending limb (F = 6.48, 1/26 df, p = .02) with mean (SD) BrACs for younger participants being 32 mg/100 ml (9 mg/100 ml) versus 39 mg/100 ml (1 1mg/100 ml) for older participants. As noted previously, age-related performance differences in Trails A and B remained significant when covaried for BrAC. Differences in mean BrAC levels were not significant at peak (56 mg/100 ml [12 mg/100 ml] versus 50 mg/100 ml [11 mg/100 ml] for older and younger participants, respectively) or on the descending limb (including the 75-minute time point). As expected, BrAC did change significantly over time (F = 22.94, 4/104 df, p < .0001) (see Figure 3).
Figure 3.
Breath alcohol concentration (BrAC) as a function of age. BrACs taken at 25 minutes following beverage administration were higher in older adults than in younger adults (Time × Age interaction, p = .02; post hoc p = .02). Despite age differences at the first BrAC measurement, groups did not differ at peak levels. The age-related differences in psychomotor performance remained significant when covaried for initial differences in BrAC (see Results). *p = .02.
Discussion
The issue of alcohol's effects among older adults is of particular importance given the impending increase in number of this segment of the U.S. population (Bureau of the Census, 2008; Merrick et al., 2008). Although a number of reports suggest that moderate alcohol consumption may have beneficial effects in aging populations (e.g., cardiovascular function), other research suggests that alcohol consumption among older individuals may lead to increased health risk (e.g., motor vehicle crashes and alcohol misuse) (King et al., 2008).
The current study was designed to investigate perceived impairment and psychomotor performance differences between older and younger social drinkers at BrACs of 40 mg/100 ml (Watson et al., 1980), consistent with an episode of social drinking. To this end, participants completed the Trail Making Test (Forms A and B) and self-reported measures of perceived intoxication and impairment on both the ascending and descending limbs of the alcohol concentration curve at time points most likely to produce equivalent BrACs between the age groups (Oneta et al., 2001). Placebo controls were used to distinguish the effects of acute alcohol administration from effects attributable to aging alone.
Age-related performance differences were shown in participants receiving the alcohol beverage but not in those participants receiving the placebo beverage. The age-related performance differences in Trails A and B were most affected by alcohol on the ascending limb of the BrAC curve. Older participants receiving alcohol demonstrated poorer performance on Trails A and B when moderately intoxicated, compared with younger participants who received alcohol—a difference not found in placebo groups. Self-reported measures also showed age-related differences because older participants reported less perceived impairment and intoxication overall. Younger adults with less alcohol experience demonstrate stronger alcohol expectancies, which may have contributed to these differences (Leigh, 1989; Leigh and Stacy, 2004; Satre and Knight, 2001). However, the most intriguing finding of the current study was related to the disconnection of perceived impairment and behavioral performance. Although older adults exhibited greater alcohol-related performance decrements on the ascending limb, they reported less perceived impairment than younger adults. Conversely, alcohol did not differentially affect performance on the descending limb. Older adults who received alcohol reported significantly more perceived impairment than those who received placebo.
Regarding behavioral performance under the influence of acute alcohol administration, these findings extend those of Vogel-Sprott and Barrett (1984). The study of Vogel-Sprott and Barrett, in which participants ranged in age from 19 to 63, focused on the effect of aging and alcohol administration (0.72 ml/kg) on balance and bead-stringing (psychomotor) tasks. Although balance was not tested in the current study, Trails A and B also have a psychomotor component similar to bead stringing. Interestingly, Vogel-Sprott and Barrett noted that bead stringing was most affected by age on the ascending limb of the blood alcohol curve, congruent to Trails A and B findings in the current study.
Tupler and colleagues (1995) conducted a similar study involving acute alcohol administration (with BACs > 1.0 mg/ml) and subsequent performance on cognitive functioning in “young” (25.0 ± 2.9 years), “middle-age” (41.1 ± 6.6 years), and “young-elderly adults” (60.9 ± 2.6 years). Although overall perceived impairment was not different between the three age groups, possibly because of the high BACs achieved in this study, digit-symbol substitution performance was initially more affected in the “young-elderly” group on the ascending limb, showing some similarity to the current study.
Although the finding that a moderate dose of alcohol facilitated performance in younger participants on the ascending limb is at first counterintuitive, it is possible that the slight intoxication caused younger participants to better focus their attention on the task (e.g., alcohol myopia; Josephs and Steele, 1990; Zeichner et al., 1993). Alternatively, these effects could have been the result of a stimulating effect of alcohol on the ascending limb (Earleywine and Martin, 1993; Martin et al., 1993). Interestingly, the facilitation/stimulatory aspect of alcohol in younger drinkers appeared on the simple task only (Trails A), as opposed to the more complicated task (Trails B). Thus, the cognitive demand of a task may be an important variable in predicting age and alcohol-related performance effects (Craik and Salthouse, 2000).
Although alcohol dosages were designed to equate peak levels consistent with an episode of social drinking, slight differences were noted between younger and older adults across the breath alcohol curve. As anticipated, the peak BrACs did not differ between older and younger drinkers. Older adults did have slightly higher BrACs at first measurement on the ascending limb. Finally, despite older drinkers having slightly higher BrACs on the ascending limb and performing more poorly, self-reported measures were not reflective of greater alcohol-induced perceived impairment. In fact, older drinkers actually reported more perceived impairment on the descending limb of the BrAC curve, a time when their performance measures were relatively unaffected by their mild intoxication. This incongruence between performance and self-reported measures suggests that the ability of older adults to accurately report behavioral effects may be compromised when they are slightly intoxicated.
It is possible that metabolic differences between older and younger adults contributed to performance measure and perceived impairment differences. In general, a swifter rate of rise of BAC on the ascending limb is associated with poorer performance outcomes (Fillmore and Vogel-Sprott, 1998). However, exploratory analyses showed that older and younger adults had similar rates of rise on the ascending limb, although older adults demonstrated poorer performance. On the descending limb, although younger adults demonstrated an attenuated rate of decline, this was not reflected in the perceived impairment measure. Thus, given a moderate dose of alcohol, age-related metabolic differences cannot account for these effects.
Limitations
Although the findings of the current study are intriguing, a number of limitations remain to be addressed in future research. Notably, the current study used only one measure of psychomotor performance (e.g., Trails A and Trails B), which may not have provided adequate power for age effects in the placebo group given the relatively small sample size. A wider variety of cognitive tasks is needed to address possible behavioral differences in tasks assessing other psychological domains (e.g., visual spatial skills, attention, short-term memory). In addition, some participants in both age groups were under the care of a physician and were required to take prescription medications. Slightly more than 40% of the alcohol-receiving groups were taking medication (44% of the older groups and 42% of the younger groups). The types of medications were similar, including blood pressure, antidepressant, and thyroid medications. Although these percentages may be higher than expected, it should be noted that data regarding both over-the-counter and prescription medications were obtained. It is important to note that the participants who reported taking their medications were stabilized on these medications for months before study entry, and all had continued to drink moderately. However, given the high rate of medical morbidity among older individuals, future studies are needed to extend these findings to clinical populations and to determine the potential impact of age-related illnesses on the disconnection between perceived impairment and psychomotor responses to alcohol.
Conclusions
In conclusion, these results indicate that social drinking BrAC levels (e.g., 40 mg/100 ml) may intensify age-related differences on behavioral tasks. Further, and most important, older participants' perceptions of impairment are affected differently at this level. These results have public health and safety implications, because a greater number of older social drinkers continue to engage in complex behaviors such as operating a motor vehicle. These findings warrant further investigation, with a more comprehensive evaluation consisting of a broader array of neurocognitive tasks, a wider range of ages, and a range of drinking levels.
Acknowledgments
The authors thank Christine Stilz, Amanda Ross, M.S., and Andy Shelton, M.S., for their assistance in data collection. We are also grateful to Mark Fillmore, Ph.D., for his assistance in developing the acute alcohol administration protocol used in this study.
Footnotes
This research was supported by National Institute on Alcohol Abuse and Alcoholism grant R03 AA14039 awarded to Sara Jo Nixon. A preliminary abstract of this work was published in Alcoholism: Clinical and Experimental Research, Vol. 29, No. 5, Supplement, p. 84A, 2005.
References
- Adams WL, Garry PJ, Rhyne R, Hunt WC, Goodwin JS. Alcohol intake in the healthy elderly: Changes with age in a cross-sectional and longitudinal study. J. Amer. Geriat. Soc. 1990;38:211–216. doi: 10.1111/j.1532-5415.1990.tb03493.x. [DOI] [PubMed] [Google Scholar]
- Bechhofer F, Paterson L. Principles of Research Design in the Social Sciences. New York: Routledge; 2000. p. 77. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory-Second Edition Manual. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Blow FC. Michigan Alcoholism Screening Test-Geriatric Version. Ann Arbor, MI: Alcohol Research Center, University of Michigan; 1991. [Google Scholar]
- Bureau of the Census. An older and more diverse nation by midcentury (press release) Washington, DC: Department of Commerce; 2008. [August 14, 2008]. (available at: www.census.gov/Press-Release/www/releases/archives/population/012496.html). [Google Scholar]
- Cahalan, D, Cisin, IH, and Crossley, HM American Drinking Practices: A National Study of Drinking Behavior and Attitudes, Rutgers Center of Alcohol Studies Monograph No. 6, New Brunswick, NJ, 1969.
- Craik FIM, Salthouse TA. The Handbook of Aging and Cognition. 2nd Edition. Mahwah, NJ: Lawrence Erlbaum; 2000. [Google Scholar]
- Earleywine M, Martin CS. Anticipated stimulant and sedative effects of alcohol vary with dosage and limb of the blood alcohol curve. Alcsm Clin. Exp. Res. 1993;17:135–139. doi: 10.1111/j.1530-0277.1993.tb00738.x. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Dixon MJ, Schweizer TA. Alcohol affects processing of ignored stimuli in a negative priming paradigm. J. Stud. Alcohol. 2000;61:571–578. doi: 10.15288/jsa.2000.61.571. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Roach EL, Rice JT. Does caffeine counteract alcohol-induced impairment? The ironic effects of expectancy. J. Stud. Alcohol. 2002;63:745–754. doi: 10.15288/jsa.2002.63.745. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Resistance to cognitive impairment under alcohol: The role of environmental consequences. Exp. Clin. Psychopharmacol. 1997;5:251–255. doi: 10.1037//1064-1297.5.3.251. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Behavioral impairment under alcohol: Cognitive and pharmacokinetic factors. Alcsm Clin. Exp. Res. 1998;22:1476–1482. [PubMed] [Google Scholar]
- Gilbertson, R, Prather, R, Tivis, R, and Nixon, S. Relationship between alcohol, age and performance in older social drinkers (abstract). Presented at the ISBRA 2006 World Congress on Alcohol Research, Sydney, Australia, September 10–13, 2006.
- Goodwin JS, Sanchez CJ, Thomas P, Hunt C, Garry PJ, Goodwin JM. Alcohol intake in a healthy elderly population. Amer. J. Publ. Hlth. 1987;77:173–177. doi: 10.2105/ajph.77.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison EL, Fillmore MT. Transfer of learning to compensate for impairment by alcohol and visual degradation. Psychopharmacology. 2005;182:461–467. doi: 10.1007/s00213-005-0130-4. [DOI] [PubMed] [Google Scholar]
- Harrison EL, Marczinski CA, Fillmore MT. Driver training conditions affect sensitivity to the impairing effects of alcohol on a simulated driving test to the impairing effects of alcohol on a simulated driving test. Exp. Clin. Psychopharmacol. 2007;15:588–598. doi: 10.1037/1064-1297.15.6.588. [DOI] [PubMed] [Google Scholar]
- Holloway FA. Low-Dose Alcohol Effects on Human Behavior and Performance: A Review of Post-1984 Research (Final Report). Federal Aviation Administration Office of Aerospace Medicine Technical Report No. DOT/FAA/AM-94/24. Washington: Government Printing Office; 1994
- Jones AW, Neri A. Age-related differences in the effects of ethanol on performance and behaviour in healthy men. Alcohol Alcsm. 1994;29:171–179. [PubMed] [Google Scholar]
- Josephs RA, Steele CM. The two faces of alcohol myopia: Attentional mediation of psychological stress. J. Abnorm. Psychol. 1990;99:115–126. doi: 10.1037//0021-843x.99.2.115. [DOI] [PubMed] [Google Scholar]
- Kalant H. Gomberg ESL, Hegedus AM, Zucker RA, editors. Pharmacological interactions of aging and alcohol. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; Alcohol Problems and Aging. NIAAA Research Monograph No. 33, NIH Publication No. 98-4163. 1998:99–116.
- King DE, Mainous AG, 3rd, Geesey ME. Adopting moderate alcohol consumption in middle age: Subsequent cardiovascular events. Amer. J. Med. 2008;121:201–206. doi: 10.1016/j.amjmed.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney J. Loosening the Grip: A Handbook of Alcohol Information. 8th Edition. New York: McGraw Hill; 2006. [Google Scholar]
- Koelega HS. Alcohol and vigilance performance: A review. Psychopharmacology. 1995;118:233–249. doi: 10.1007/BF02245951. [DOI] [PubMed] [Google Scholar]
- Leigh BC. In search of the seven dwarves: Issues of measurement and meaning in alcohol expectancy research. Psychol. Bull. 1989;105:361–373. doi: 10.1037/0033-2909.105.3.361. [DOI] [PubMed] [Google Scholar]
- Leigh BC, Stacy AW. Alcohol expectancies and drinking in different age groups. Addiction. 2004;99:215–227. doi: 10.1111/j.1360-0443.2003.00641.x. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Stapleton J, Lister R, Guthrie S, Eckhardt M. Effects of alcohol on accident risk. Pathologist. 1986;40:36–41. [Google Scholar]
- Logan JM, Sanders AL, Synder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: Dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcsm Clin. Exp. Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Merrick EL, Horgan CM, Hodgkin D, Garnick DW, Houghton SF, Panas L, Saitz R, Blow FC. Unhealthy drinking patterns in older adults: Prevalence and associated characteristics. J. Amer. Geriat. Soc. 2008;56:214–223. doi: 10.1111/j.1532-5415.2007.01539.x. [DOI] [PubMed] [Google Scholar]
- Moskowitz H, Robinson CD. Washington, DC: Department of Transportation; 1988. Effects of Low Doses of Alcohol on Driving-Related Skills: A Review of the Evidence. National Highway Traffic Safety Administration Report No. DOT HS 807 280. [Google Scholar]
- Nicholson ME, Wang M, Airhihenbuwa CO, Mahoney BS, Christina R, Maney DW. Variability in behavioral impairment involved in the rising and falling BAC curve. J. Stud. Alcohol. 1992;53:349–356. doi: 10.15288/jsa.1992.53.349. [DOI] [PubMed] [Google Scholar]
- Nixon SJ. Gomberg ESL, Hegedus AM, Zucker RA, editors. Alcohol, aging and cognition. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; Alcohol Problems and Aging. NIAAA Research Monograph No. 33, NIH Publication No. 98-4163. 1998:213–227.
- Oneta CM, Pedrosa M, Rüttimann S, Russell RM, Seitz HK. Age and bioavailability of alcohol (German) Z. Gastroenterol. 2001;39:783–788. doi: 10.1055/s-2001-17196. [DOI] [PubMed] [Google Scholar]
- Quillian WC, Cox DJ, Kovatchev BP, Phillips C. The effects of age and alcohol intoxication on simulated driving performance, awareness and self-restraint. Age Ageing. 1999;28:59–66. doi: 10.1093/ageing/28.1.59. [DOI] [PubMed] [Google Scholar]
- Robbins LN, Cottler L, Bucholz K, Compton W. The Diagnostic Interview Schedule, Version, IV. St. Louis, MO: Washington University; 1995. [Google Scholar]
- Russell EW, Neuringer C, Goldstein G. Assessment of Brain Damage: A Neuropsychological Key Approach. Hoboken, NJ: John Wiley & Sons; 1970. [Google Scholar]
- Saitz R. Overview of medical and surgical complications. In: Graham AW, Schultz TK, Mayo-Smith MF, Ries RK, Wilford BB, editors. Principles of Addiction Medicine. 3rd Edition. Chevy Chase, MD: American Society of Addiction Medicine; 2003. pp. 1027–1052. [Google Scholar]
- Satre DD, Knight BG. Alcohol expectancies and their relationship to alcohol use: Age and sex differences. Aging Ment. Hlth. 2001;5:73–83. doi: 10.1080/13607860020020672. [DOI] [PubMed] [Google Scholar]
- Selzer ML. The Michigan Alcoholism Screening Test: The quest for a new diagnostic instrument. Amer. J. Psychiat. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory-Revised. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (Office of Applied Studies) Results From the 2005 National Survey on Drug Use and Health: National Findings, (Chapter 3-Alcohol Use), DHHS Publication No. SMA 06-4194. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2006. (available at: www.oas.samhsa.gov/NSDUH/2k5NSDUH/2k5results.htm#Ch3). [Google Scholar]
- Substance Abuse and Mental Health Services Administration (Office of Applied Studies) Results from the 2007 National Survey on Drug Use and Health: National Findings, DHHS Publication No. SMA 08-4343. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2008. (available at: http://oas.samhsa.gov/NSDUH/2k7NSDUH/2k7Results.pdf). [Google Scholar]
- Tupler LA, Hege S, Ellinwood EH., Jr Alcohol pharmacodynamics in young-elderly adults contrasted with young and middle-aged subjects. Psychopharmacology. 1995;118:460–470. doi: 10.1007/BF02245947. [DOI] [PubMed] [Google Scholar]
- Vogel-Sprott M, Barrett P. Age, drinking habits and the effects of alcohol. J. Stud. Alcohol. 1984;45:517–521. doi: 10.15288/jsa.1984.45.517. [DOI] [PubMed] [Google Scholar]
- Wahlin T-BR, Backman L, Wahlin Å, Winblad B. Trail Making Test performance in a community-based sample of healthy very old adults: Effects of age on completion time, but not on accuracy. Arch. Gerontol. Geriat. 1996;22:87–102. doi: 10.1016/0167-4943(95)00681-8. [DOI] [PubMed] [Google Scholar]
- Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Amer. J. Clin. Nutr. 1980;33:27–39. doi: 10.1093/ajcn/33.1.27. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiat. Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale-Revised. Los Angeles, CA: Western Psychological Services; 1986. [Google Scholar]
- Zeichner A, Allen JD, Petrie CD, Rasmussen PR, Giancola P. Attention allocation: Effects of alcohol and information salience on attentional processes in male social drinkers. Alcsm Clin. Exp. Res. 1993;17:727–732. doi: 10.1111/j.1530-0277.1993.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Zucker RA. Gomberg ESL, Hegedus AM, Zucker RA, editors. Developmental aspects of aging, alcohol involvement, and their interrelationship. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; Alcohol Problems and Aging. NIAAA Research Monograph No. 33, NIH Publication No. 98-4163. 1998:3–23.