Abstract
Affective startle eyeblink modulation by unipolar depressed and nondepressed participants was assessed during the anticipation and viewing of emotional pictures. Anticipatory startle probes were presented at 2,000 ms and 750 ms before picture onset. Startle probes during picture viewing were presented at 300 ms and 3,500–4,500 ms after picture onset. Although nondepressed participants demonstrated the predicted quadratic and linear patterns of responding in the 2,000-ms anticipatory and 3,500–4,500-ms viewing conditions, respectively, depressed participants were not significantly responsive to differences among picture valence categories at these probe conditions. There were no between-groups differences in startle modulation at the other two probe intervals, in picture ratings, or in behavioral responses to pictures. There was also little evidence of hyperresponsivity to negatively valenced stimuli in the depressed group. These results indicate that depression-related affective hyporesponsivity extended to startle modulation but that the nature and magnitude of the differences between depressed and nondepressed individuals were conditional on the specific cognitive and motivational processes recruited at different points in time.
Keywords: depression, startle modulation, anticipation, emotional responding
In recent years, there has been a growing interest in applying models of emotion and motivation emanating from basic research to the study of psychopathology. Several of the most influential models posit higher order dimensions that reflect the operation of biologically based systems that organize and activate responses to rewarding, appetitive, or otherwise positive hedonic stimuli and to aversive, threatening, or otherwise negative hedonic stimuli (e.g., Carver & White, 1994; Higgins, 1997; Tomarken, Shelton, & Hollon, 2007; Watson, Wiese, Vaidya, & Tellegen, 1999). Such models are particularly relevant to unipolar depression in light of the proposal that this disorder is characterized by a combination of a hypoactivation in a biologically based approach system that mediates responses to appetitive stimuli and hyperactivation of a protective–defensive withdrawal system that mediates responses to aversive, threatening, or otherwise stressful stimuli (e.g., Depue & Iacono, 1989; Fowles, 1988).
Whereas this is a promising perspective on depression, there are a number of unanswered issues. The present experiment addresses one overarching question: Is it the case that depressed, relative to nondepressed, individuals are both hyporesponsive to appetitive stimuli and hyperresponsive to aversive stimuli? As Rottenberg, Gross, and Gotlib (2005) have pointed out, when assessing relations between depression and positive and negative affect, it is important to distinguish between disturbances in mood and in emotional reactivity. In this context, moods are defined as diffuse, slow-moving states that are only weakly linked to emotion elicitors and may have a long duration. In contrast, emotional reactions are typically short-term, coordinated responses to specific elicitors.
On self-report measures that assess mood states and traits, unipolar depressed, relative to nondepressed, individuals consistently report (a) lower positive affect, lower behavioral activation, and increased anhedonia and (b) heightened negative affect, behavioral inhibition, and generalized distress (e.g., Kasch, Rottenberg, Arnow, & Gotlib, 2002; Watson et al., 1995). On measures of reactivity to emotional stimuli, however, the story is less clear and often quite different. Depressed individuals consistently demonstrate deficits in the processing of and responses to positive hedonic stimuli on self-report (e.g., Sloan, Strauss, & Wisner, 2001), behavioral (e.g., Henriques & Davidson, 2000) and psychophysiological (e.g., Dichter, Tomarken, Shelton, & Sutton, 2004) measures. However, the evidence concerning reactions to negative affective stimuli is more equivocal. Whereas a few studies have found exaggerated responses to aversive or other negative stimuli (e.g., Sigmon & Nelson-Gray, 1992), others have not and/or have found inconsistent results across dependent measures (e.g., Yee & Miller, 1988; Rottenberg, Kasch, Gross, & Gotlib, 2002). Further, within-subject comparisons often indicate that depressed individuals are less responsive to variations in the affective valence of eliciting stimuli than are nondepressed individuals (for a review, see Rottenberg et al., 2005). On the basis of these data, Rottenberg et al. (2005; Rottenberg, 2007) have argued that depression is associated with a broad emotion-context insensitivity. They have argued that this view is consistent with evolutionary accounts of depression that conceptualize this syndrome as characterized by disengagement and a bias against action (e.g., Nesse, 2000).
The purpose of the present study is to test the emotion-context insensitivity model of depression and, more generally, compare reactivity to affective stimuli among depressed and nondepressed participants in a broader array of contexts than has been used in previous studies. The emotion reactivity literature in depression notably omits an assessment of the time course (i.e., chronometry) of affective responses, because most studies have not sampled reactions at various points in time before, during, and after exposure to stimuli. This is a significant omission given (a) theories of depression that highlight potential dissociations among affective or motivational processes that occur at different points in time (e.g., Klein, 1974, 1987), (b) a wealth of both infrahuman and human studies demonstrating that neural systems mediating anticipatory or preparatory responses to appetitive or aversive stimuli can be dissociated from those systems mediating responses to the actual stimuli themselves (e.g., Berridge & Robinson, 1998; Gray, 1982; Knutson, Adams, Fong, & Hommer, 2001), (c) evidence that depression is associated with self-regulatory deficits linked to the prefrontal cortex that may compromise preparatory adjustments in anticipation of significant stimuli (Tomarken & Keener, 1998), and (d) psychosocial theories of depression that posit the centrality of hopelessness about future events (Abramson, Metalsky, & Alloy, 1989).
Despite evidence that the anticipation of motivational stimuli and actual exposure to such stimuli elicit discriminable patterns of reactions, most studies have not assessed depressed individuals’ responses during these differing phases (for an exception, see, e.g., Yee & Miller, 1988). To address this issue, an experimental paradigm is needed that is reliably linked to affective response systems and that has sufficient sensitivity to resolve different components of responding over time. The affective startle blink paradigm meets these criteria.
Startle Modulation During Anticipation and Viewing of Affective Stimuli
It is well established that affective or motivational foreground context can modulate the amplitude of the startle eyeblink reflex during both anticipation of significant stimuli and actual exposure to such stimuli. Anticipatory startle modulation paradigms involve the elicitation of startle responses during an anticipatory interval between the onsets of an informational cue and an emotional stimulus. We have found that when the latency between the onsets of the startle probe and an affective picture is relatively long (i.e., 2,000 ms), blink magnitudes to both unpleasant and pleasant stimuli are potentiated, relative to responses during the anticipation of neutral stimuli (Dichter, Tomarken, & Baucom, 2002). This pattern of anticipatory startle modulation has also been reported in at least three other studies: Nitschke et al. (2002) presented startle probes during iconic cues, with probes preceding the onset of affective pictures by 1,000 ms; Sabatinelli, Bradley, and Lang (2001) presented light cues to participants who were highly fearful of snakes and administered startle probes 2,000 or 500 ms before affective picture onset; finally, Skolnick and Davidson (2002) compared startle amplitudes 1,000 ms prior to feedback about reward or threat outcome (see also Rich et al., 2005). In all cases, participants demonstrated augmented startle amplitudes during anticipation of pleasant and unpleasant relative to neutral outcomes.
Although the pattern of larger blink responses during anticipation of arousing unpleasant and pleasant stimuli, relative to neutral stimuli, appears to be replicable, the precise mechanisms for this effect are presently unclear. Sabatinelli et al. (2001) have suggested that this anticipatory startle effect reflects a generalized mobilization for action that is consistent with animal data indicating that preparatory activation facilitates motor reflexes (Duffy, 1962; Malmo, 1959). Another perspective is suggested by the observation that the pattern of startle modulation that we and others have observed is isomorphic with a pattern that has been found in several studies assessing startle modulation during emotional imagery: higher amplitudes during imagery of pleasant and unpleasant events, relative to neutral events (Miller, Patrick, & Levenston, 2002; Witvliet & Vrana, 1995, 2000). Perhaps the pattern of startle modulation observed during anticipation of affective stimuli is due to the fact that during the anticipation of positive and negative hedonic stimuli, individuals are likely to engage in spontaneous imagery of affectively congruent stimulus features or response properties (cf. Lang, 1979).
Affective startle modulation during picture viewing is also well established. When nondepressed individuals view affective pictures and the latency between picture and startle probe onsets is relatively long (e.g., 3,500 ms), response magnitude is modulated by the valence of the picture. Unpleasant pictures potentiate and pleasant pictures attenuate the magnitude of the startle blink relative to neutral pictures (e.g., Bradley, Cuthbert, & Lang, 1993). This linear pattern of startle modulation is thought to reflect the priming of neurobiologically based defensive and appetitive systems by unpleasant and pleasant foreground stimuli, respectively (e.g., Lang, Bradley, & Cuthbert, 1998). However, when the latency between picture and startle probe onsets is relatively short (e.g., 300 ms), the magnitude of the eyeblink reflex is quadratic (i.e., both unpleasant and pleasant foreground stimuli attenuate blink magnitudes relative to neutral stimuli), possibly reflecting heightened allocation of attentional resources to the affective stimuli (Bradley, Lang, & Cuthbert, 1993; Dichter et al., 2004).
Affective Startle Modulation: Comparisons Between Depressed and Nondepressed Individuals
Because the startle reflex can be probed at various points before, during, or after exposure to an affective stimulus, it is well suited to assess differences between depressed and nondepressed individuals during the full chronometry of affective processing. Additionally, because the startle blink is a protective–defensive reflex, the affective startle modulation paradigm appears ideal for assessing whether depressed individuals do, in fact, demonstrate exaggerated responsivity to aversive stimuli. No study to date has assessed startle modulation during the anticipation of affective stimuli in depressed individuals. A small number of studies have, however, examined startle modulation in depressed individuals during exposure to affective pictures. Allen, Trinder, and Brennan (1999) and Dichter et al. (2004) employed late-onset probes, and neither group of researchers found the linear pattern commonly observed among nondepressed participants. Other evidence corroborates that anomalous late-probe startle responding is characteristic of unipolar but not bipolar depression (Forbes, Miller, Cohn, Fox, & Kovacs, 2005) and severe but not mild depression (Kaviani et al., 2004). In these studies, late-probe startle responses of depressed participants have been characterized by relatively weaker affective startle modulation, a pattern that is consistent with a generalized emotion-context insensitivity model of depression. However, one study employing an early (i.e., 300 ms) viewing probe condition revealed no startle modulation differences between depressed and nondepressed groups, suggesting that emotion-context insensitivity in depression may not be present during all phases of an affective response (Dichter et al., 2004).
Overview and Predictions
The objective of the present investigation is to test whether unipolar major depressive disorder is characterized by generalized emotion-context insensitivity—that is, decreased reactivity to both positively and negatively valenced stimuli (Rottenberg, 2005, 2007)—during the anticipation and viewing of affective pictures. Primary hypotheses concerned startle responses to probes presented 2,000 ms before and 3,500–4,500 ms after picture presentation for two reasons: (a) Replicable and discriminable patterns of startle modulation have been observed in nonclinical samples at these probe intervals, and (b) the latter condition has been shown to differentiate depressed and nondepressed samples (Allen et al., 1999; Dichter et al., 2004).
Primary predictions were guided by the emotion-context insensitivity model of depression (Rottenberg, 2005, 2007) and theoretical accounts of the mechanisms underlying the startle modulation effects observed among nonclinical samples. First, we predicted that the depressed group would demonstrate blunted responses to probes presented 2,000 ms before the onset of pleasant and unpleasant pictures, relative to neutral pictures. That is, we predicted that depressed individuals would be less likely to demonstrate the pattern of greater startle amplitude to positive and negative, relative to neutral, stimuli shown by nonclinical samples in previous studies. As noted above, two explanations that might be offered to account for the quadratic effect among nonclinical samples focus on the anticipatory startle reflex as an index of mobilization for action (Sabatinelli et al., 2001) and on linkages between startle responding and processes engaged during emotional imagery (Miller et al., 2002; Witvliet & Vrana, 1995, 2000). From these perspectives, a flatter profile of responding among depressed individuals during the anticipatory phase would be broadly consistent with the argument derived from the context insensitivity model that depressed individuals are characterized by a generalized disengagement from action. Such disengagement might also encompass deficits in the spontaneous generation of imagery or other forms of elaborative processing in anticipation of significant events.
Consistent with our prior findings (Dichter et al., 2004), we also hypothesized that depressed participants would demonstrate blunted affective startle modulation when probes were delivered between 3,500 ms and 4,500 ms after picture presentation. This pattern is also consistent with generalized emotion-context insensitivity and might reflect one logical consequence of a generalized disengagement from affective stimuli: the failure of such stimuli to recruit the appetitive and aversive response sets that appear to be critical mediators of the startle modulation effect commonly observed in nonclinical samples (e.g., Lang, Bradley, & Cuthbert, 1990).
Two additional probe conditions were included to assess the boundary conditions of generalized emotion-context insensitivity in depression. A probe presented 300 ms after picture onset was included because, as noted above, a pattern of startle modulation that has been observed among nonclinical samples at this interval (heightened responses to neutral relative to pleasant and unpleasant stimuli) appears to reflect important attentional processes. We sought to assess whether depressed, relative to nondepressed, individuals also demonstrated a relative context insensitivity at this probe interval. However, our expectations for a between-groups difference were much weaker, because a prior study with a relatively small sample indicated no differences between depressed and nondepressed samples at this interval (Dichter et al., 2004).
For exploratory purposes, an anticipatory condition was also included that employed a probe presented 750 ms before picture onset. No published studies have assessed anticipatory startle modulation at this precise probe interval. However, in an unpublished study employing 750-ms anticipatory probes, Erickson (1996) reported a pattern of linear modulation similar to that observed during picture exposure (i.e., highest amplitude responses to negative pictures and lowest amplitude responses to positive pictures). If replicable, this effect might indicate a progressive shift toward appetitive and aversive response sets as the onset of affective stimuli approaches. One might further speculate that the spontaneous imagery and other forms of elaborative processing engaged at earlier points during the anticipatory phase serve to promote this shift. Consistent with our predictions for both the earlier anticipatory condition and the late viewing conditions, we hypothesized that the depressed group would show blunted affective startle modulation at this probe interval as well. Predicted patterns of responding in the nondepressed group, planned statistical tests, and predicted group differences are summarized in Table 1. Consistent with an emotion-context insensitivity model of depression (Rottenberg, 2005, 2007), patterns of startle responding in the depressed group were predicted to be attenuated in all probe conditions relative to the nondepressed control group; however, the specific patterns of responses in the depressed group are not indicated in Table 1 because two probe conditions (750-ms anticipatory and 300-ms viewing) are considered exploratory.
Table 1.
Predicted Affective Startle Modulation Patterns in the Nondepressed Group, Planned Interaction Tests, and Predicted Group Effects for Each Probe Condition
| Probe condition | Predicted startle patterns in the nondepressed group | Planned trend test | Predictions |
|---|---|---|---|
| 2,000-ms anticipatorya |
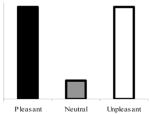
|
Group × Quadratic Trend | Significant group interaction; significant quadratic trend in the nondepressed group only |
| 750-ms anticipatoryb (exploratory) |
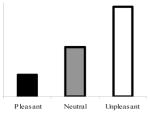
|
Group × Linear Trend | Significant group interaction; significant linear trend in the nondepressed group only |
| 300-ms viewingc |
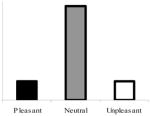
|
Group × Quadratic Trend | No group interaction; significant quadratic trends in both groups |
| 3,500–4,500-ms viewingd |
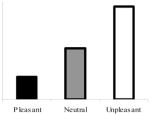
|
Group × Linear Trend | Significant group interaction; significant linear trend in the nondepressed group only |
Note. Bar graphs are idealized and meant for illustrative purposes; they do not reflect possible amplitude differences between probe categories. Consistent with an emotion-context insensitivity model of depression (Rottenberg, 2005, 2007), patterns of startle responding in the depressed group were predicted to be attenuated, relative to the nondepressed control group, across the three levels of the valence factor in all probe conditions.
Method
Participants
Depressed participants qualified for the study if they (a) were at least 18 years of age; (b) met criteria for a current Diagnostic and Statistical Manual of Mental Disorders (4th ed.; American Psychiatric Association, 1994) major depressive episode, assessed with the Structured Clinical Interview for DSM–IV Axis I Disorders (Research Edition; First, Spitzer, Gibbon, & Williams, 1996), performed at Vanderbilt University Medical Center Department of Adult Psychiatry by experienced interviewers; (c) had scores on the 17-item version of the Hamilton Rating Scale for Depression (HRSD-17; Hamilton, 1960) that were greater than 18; (d) did not meet criteria for current or past bipolar disorder or any psychotic disorder; (e) did not meet criteria for a current diagnosis of delirium or dementia; (f) did not have current psychotic symptoms; and (g) did not meet criteria for alcohol or drug abuse or dependence in the previous 6 months. Interrater reliability assessments for the larger study from which patients were drawn (Hollon et al., 2005) indicated an intraclass correlation coefficient of .96 for the 17-item total HRSD-17 score and a kappa coefficient of .80 for the assessment of a major depressive episode based on clinical interview.
Participants provided written informed consent after all procedures were explained. Twenty-seven depressed outpatient adults participated in the startle session. Depressed participants (14 women; age range: 22.9–59.0 years, M = 41.8, SD = 10.6) were recruited from depression treatment studies conducted in the Vanderbilt University Medical Center Department of Adult Psychiatry. Twenty-two depressed participants completed the startle session before they had begun antidepressant medication. Five patients were taking venlafaxine at the time of their startle session (4 of them for 7 or fewer days, 1 for 28 days). We included these patients in the present sample for the following reasons: (a) Beck Depression Inventory (BDI; Beck, Steer, & Brown, 1996) and HRSD-17 scores assessed at the startle session did not differ significantly between these 5 participants (BDI: M = 31.6, SD = 4.2; HRSD-17: M = 23.0, SD = 5.7) and the depressed participants who were medication free (BDI: M = 34.5, SD = 11.5; HRSD-17: M = 21.5, SD = 3.4, ps > .40). (b) The patterns of startle modulation of these 5 participants were nearly isomorphic to those of the other depressed participants (within the depressed group, Medication Status × Valence tests at each probe condition yielded no effects or even trends for medication status, ps > .84). (c) In addition, in a prior study, we found no significant changes (or even trends) in patterns of affective startle modulation before and after 12 weeks of antidepressant medication treatment (Dichter et al., 2004).
Nondepressed participants were recruited by announcements placed in local newspapers and by telephone contacts based on a list of interested study participants maintained through Vanderbilt University. Nondepressed participants were lifetime free of Diagnostic and Statistical Manual of Mental Disorders major depressive episodes and dysthymia and did not meet criteria for any current Axis I disorder, as assessed by a Structured Clinical Interview for DSM–IV interview (First et al., 1996) performed at Vanderbilt University by a trained rater. One hundred one adults were contacted to participate. Seven declined the diagnostic interview. Of those interviewed, 6 were excluded because they were taking antidepressant medication, and 24 were excluded because of past or current psychiatric exclusion criteria. Of the individuals who met inclusion criteria for the current study, 4 declined to participate in the startle session. The final nondepressed sample consisted of 60 participants (31 women; age range: 30.0–69.1 years, M = 46.5, SD = 8.3). The depressed (M = 33.96, SD = 10.55) and nondepressed (M = 1.73, SD = 2.81) groups differed significantly with respect to BDI scores, Welch’s (1938) t(27.7) = 15.36, p< .0001, but did not differ with respect to age, t(85) =1.11, p> .25, or gender distribution, χ2(1, N = 87) = 0.0003, p> .98.
Experimental Design
In addition to the between-subjects variable of depression status (depressed/nondepressed), there were two within-subject variables: picture valence (unpleasant/pleasant/neutral) and probe time (2,000-ms anticipatory/750-ms anticipatory/300-ms viewing/3,500–4,500-ms viewing). Primary analyses were performed separately for each probe condition for the following reasons: a desire to maintain consistency with the approach used in prior studies of affective startle modulation, the fact that different probe conditions assessed discriminably different processes, and the fact that both the foci of the present study and our predictions were stronger for some probe intervals (i.e., 2,000-ms anticipatory and 3,500–4,500-ms viewing) than others. Because of its relevance to an assessment of the overall chronometry of group differences, we also report the results of an omnibus Group × Valence × Probe set of analyses that combined data across probe conditions.
Stimulus Materials
Each participant viewed two sets of color pictures (one habituation set and one experimental set) chosen from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2005) on the basis of their published affective valence and arousal ratings. Pleasant and unpleasant pictures were selected on the basis of extreme normative ratings of valence separately for male and female participants. Average normative arousal ratings of the selected IAPS images were as follows: unpleasant (M = 6.74, SD = 0.33), neutral (M = 2.46, SD = 0.25), pleasant (M = 6.36, SD = 0.34; 1 = extremely unarousing, 9 = extremely arousing). Average normative valence ratings of the selected IAPS images were as follows: unpleasant (M = 1.86, SD = 0.26), neutral (M = 4.83, SD = 0.19), pleasant (M = 7.37, SD = 0.30; 1 = extremely unpleasant, 9 = extremely pleasant; see the Appendix for images used), reflecting a valence rating difference between unpleasant and neutral pictures of 2.80 and between neutral and pleasant pictures of 2.56. Although pictures were classified categorically as unpleasant, neutral, or pleasant for analysis purposes, the fact that the normative valence ratings of neutral pictures were thus almost precisely midway between unpleasant and pleasant pictures appears to justify the use of linear and quadratic trend analyses to test predictions.
The habituation set consisted of three neutral pictures and was included to allow the startle blink reflex to habituate. The experimental set consisted of 54 pictures (18 pleasant, 18 neutral, and 18 unpleasant) presented in nine blocks of six pictures. Each block contained 2 pictures of each of the three picture categories. Pictures were displayed on a 17-in. (43-cm) color monitor approximately 1.3 m in front of the participant.
Procedure
Nondepressed participants first took part in the diagnostic interview session performed at Vanderbilt University Medical Center. If the inclusion criteria for the present study were met, non-depressed participants were then scheduled for the startle session, which took place within 1 week of the diagnostic interview. Depressed participants were recruited by telephone on the day they entered their treatment study and were scheduled for the startle session within 1 week of their enrollment in their treatment study. Control participants received $10 for completing the diagnostic interview, and all participants received $25 for completing the startle session.
During the startle session, participants first completed the BDI. Next, they viewed the habituation pictures and then the experimental pictures while electromyography (EMG) was recorded. Each trial consisted of a 2-s cue arrow indicating the valence of the forthcoming picture, 4 s of no visual stimulation (anticipatory phase), and 6 s of picture presentation (viewing phase). The intertrial interval (i.e., the interval between picture offset and the onset of the subsequent trial’s warning cue) was 7–9 s (M = 8 s).1 The cue was either an upward-pointing arrow indicating a pleasant picture, a downward pointing arrow indicating an unpleasant picture, or a sideways-pointing arrow indicating a neutral picture. Arrows were large, black block figures on a white background. Acoustic startle probes of 50-ms, 100-dB white-noise bursts with instantaneous rise times were binaurally presented. During each of the 48 probed trials, startle probes were presented at one of four intervals: 2,000 ms or 750 ms before the onset of the picture (anticipatory probes) or 300 ms or 3,500–4,500 ms (M = 4,000 ms) after the onset of the picture (viewing probes). Probe latencies in the 3,500–4,500-ms viewing condition were varied to reduce the predictability of the timing of the probes. A pseudo-random procedure was used such that latencies did not differ between picture category conditions or across groups. Picture and probe presentation were controlled by the STIM software package from the James Long Co. (1998).
To diminish the predictability of the procedure, we presented three probes, equally spaced throughout the session, during inter-trial intervals, and 6 of the 54 trials did not contain startle probes. Of the 48 probed trials, 16 trials contained pictures of each of the three valences. Within each valence category, 4 trials contained probes of each of the four probe conditions. In other words, there were 4 trials in each of the 12 cells produced by the crossing of the valence (three levels) and probe (four levels) categories. We included only 4 trials per condition to minimize participant fatigue. Although the complexity of our design resulted in relatively fewer probe trials per condition than are typically employed (although as few as three probes per condition have been shown to be adequate; Bradley, Cuthbert, & Lang, 1993), replication of published affective modulation in the nondepressed group to the late viewing probes (see the Results section) suggests that 4 trials per condition were sufficient. Pictures were presented in one of two counterbalanced orders to diminish the probability that startle modulation would be affected by the particular order of picture presentation. Separate male and female picture sets were used that were matched, picture by picture, with respect to published gender-specific IAPS ratings of valence and arousal. Male and female sets were invariant with respect to order of valence category and probe interval.
The picture stimuli were presented twice to each participant. During the first presentation, startle blinks were recorded. After the startle modulation trials were complete, pictures were presented a second time (without startle probes), and participants rated each picture with respect to pleasure and arousal using 9-point Likert scales. During the rating procedures, participants controlled the duration of picture exposures.
Physiological Recording and Data Reduction
The eyeblink component of the startle reflex was measured by bipolar recording of the EMG activity below the left eye with two miniature silver–silver chloride electrodes filled with electrolyte paste. A ground electrode was placed on the participant’s forehead. All electrode impedances were below 20 kΩ. Custom-built isolated bioelectric amplifiers (James Long Co., Caroga Lake, NY) were used for recording. The input impedance of the amplifiers was greater than 1 GΩ. The raw EMG signal was bandpass filtered (high-pass filter set at 10 Hz, low-pass filter set at 500 Hz), amplified (gain = 5 K), and sampled at 1024 Hz by an Analog Devices (Norwood, MA) RTI-815A analogue to digital converter interfaced to Snapstream (HEM Data Corp., 1991), a commercially available signal acquisition program.
The digitized EMG was digitally filtered offline to highlight signals between 80 and 240 Hz. A truncated convolution filter was used, with weights yielded by an inverse Fourier transformation of a rectangle function with values of unity in the 80–240 Hz range and values of zero outside this range. This filter provided a very sharp cutoff (i.e., in excess of 40 dB down immediately outside the band of interest). We then digitally rectified and low-pass filtered the data by integrating values in 32-ms time windows. Consistent with other research groups (e.g., Bradley, Cuthbert, & Lang, 1993), we computed peak EMG magnitudes in search windows between 10 and 250 ms after the onset of the startle probes. Trials with artifact were detected by EMGART software (James Long Co., 2002). This software computes the mean and standard deviation of all baselines (defined as the 50 ms prior to probe onset) for each participant and rejects trials with baselines that exceed at any time either the baseline mean plus two standard deviations of that mean or four times the mean.
After deletion of trials with artifact (fewer than 1.0% of the trials), magnitude measures were averaged across each of the 12 levels formed by the crossing of the valence (unpleasant/pleasant/neutral) and probe time (2,000-ms anticipatory/750-ms anticipatory/300-ms viewing/3,500–4,500-ms viewing) within-subject factors for each participant. Although the results presented below are based on raw startle magnitudes, analyses using z-transformed startle magnitudes did not in any way alter the primary findings or conclusions. Other researchers have noted highly similar results obtained with both raw and standardized scores (e.g., Grillon & Ameli, 2001). We report raw startle magnitudes to be consistent with studies previously cited (Allen et al., 1999; Bradley, Cuthbert, & Lang, 1993; Dichter et al., 2004).
Data Analysis
Each probe condition was analyzed separately, and the habituation trials were not included in analyses. To assess whether diagnostic groups differed with respect to startle modulation, the omnibus analyses of interest were Group (depressed, control) × Valence (pleasant, neutral, unpleasant) repeated-measures analyses of variance (ANOVAs) performed separately on startle magnitude values during each of the four probe conditions. We also evaluated whether groups differed on planned contrasts that reflected our a priori hypotheses and questions of primary interest. The planned contrasts that we conducted were as follows: (a) In the 2,000-ms anticipatory and 300-ms viewing conditions, Group × Quadratic Trend interaction contrasts tested whether groups differed in a quadratic pattern of startle modulation across the unpleasant, neutral, and pleasant pictures (trend coefficients = 1, −2, and 1, respectively, for the pleasant, neutral, and unpleasant marginal means for the 2,000-ms anticipatory condition and −1, 2, and −1, respectively, for the pleasant, neutral, and unpleasant marginal means for the 300-ms viewing condition). (b) In the 750-ms anticipatory and 3,500–4,500-ms viewing conditions, a Group × Linear Trend interaction contrast tested whether groups differed in a linear pattern of startle modulation across the unpleasant, neutral, and pleasant pictures (trend coefficients = −1, 0, and 1, respectively, for the pleasant, neutral, and unpleasant marginal means). When significant Group × Planned Contrast effects were detected, follow-up analyses tested for group differences in startle modulation to pleasant and unpleasant pictures separately, relative to neutral pictures.
For each probe condition, we tested whether the within-group covariance matrices consisting of the variances and covariances among the unpleasant, neutral, and pleasant amplitude measures were equivalent across the depressed and nondepressed groups (e.g., Morrison, 1976, p. 252). These tests indicated that the covariance matrices were heterogeneous for the 2,000-ms anticipatory, χ2(6, N = 87) = 42.08, p < .0001; 300-ms viewing, χ2(6, N = 87) = 32.32, p < .001; and 3,500–4,500-ms viewing, χ2(6, N = 87) = 13.92, p < .05, conditions. Similarly, Fmax tests indicated between-groups heterogeneity on the variances of the planned contrasts noted above for each of these three probe conditions (all ps < .01). Such indications of variance and covariance heterogeneity are especially important because of the unequal sample sizes used in the present study (i.e., sample sizes for the depressed and nondepressed groups were 27 and 60, respectively). When group sizes are unequal, neither of the two approaches conventionally used to perform repeated-measures omnibus tests and/or contrasts (i.e., epsilon-adjusted univariate tests and multivariate repeated-measures tests) are robust to violations of the assumption of equality of the covariance matrices at each level of the grouping factor (for reviews, see, e.g., J. C. Keselman & Keselman, 1990; Lix & Keselman, 1995). In contrast, a multivariate extension of the Welch–James approximate degrees of freedom (ADF) test originally developed for one-way between-groups designs (e.g., Welch, 1951) is insensitive to heterogeneity of covariance matrices across groups and, thus, will generally provide a more valid test of repeated-measures hypotheses (H. J. Keselman, 1998; H. J. Keselman, Carriere, & Lix, 1993). Accordingly, we used a modified version of the program originally presented by Lix and Keselman (1995) in order to conduct the Welch–James ADF procedure when the analytic design included the depressed versus nondepressed between-groups factor. Our program was written with SAS/IML software Version 9.1 of the SAS System for Windows. Because no tests conducted on startle data from the 750-ms anticipatory probe condition rejected the null hypothesis of homogeneity of variances and covariances across groups (all ps > .10), we used a standard multivariate repeated-measures approach for analyses performed during this condition. Similarly, the multivariate repeated-measures approach was used on analyses conducted within each of the two groups to test the effects of the valence factor.
The differing sample sizes in the depressed and nondepressed groups raise questions concerning the degree to which any differences in levels of statistical significance yielded by within-group analyses could be due to differential power to detect effects. To address this issue, for within-group analyses, we report estimates of effect size in addition to the results of significance tests. In the case of the omnibus analyses, we computed omega-squared estimates of the proportion of partial variance (denoted below) accounted for by the valence factor (e.g., Olejnik & Algina, 2000). In this context, is an estimate of the proportion of the within-subject variability that is due to the main effect of valence. In the case of the planned contrasts that we conducted (e.g., the linear trend within the depressed group in the later viewing condition), we report standardized mean differences (denoted as δ̂ below) as an estimate of effect size (Olejnik & Algina, 2000). When a contrast only involves two means, this measure reduces to Cohen’s d (e.g., Cohen, 1988). Consistent with Cohen’s (1988) recommendations, δ̂ values of .20, .50, and .80 were taken to indicate small, medium, and large effect sizes, respectively.2 Although we report effect size indexes for within-group analyses, we omit such indexes for the omnibus Group × Valence ANOVAs that combined data from both groups. The between-groups heterogeneity of variance on most of the primary dependent variables would render conventional effect size measures difficult to calculate and to interpret.
Results
Startle Modulation
When gender, age, and picture presentation order were entered into analyses of affective startle modulation as covariates, there were no significant main effects or interactions involving these factors on the primary dependent measures. Thus, they were excluded from all the analyses reported below. Diagnostic groups did not differ with respect to the magnitude of startle responses to probes presented during intertrial intervals (depressed, M = 29.3, SD = 24.3; nondepressed, M = 31.7, SD = 34.4), t(85) = 0.33, p > .70.
Startle Probes 2,000 ms Before Picture Onset
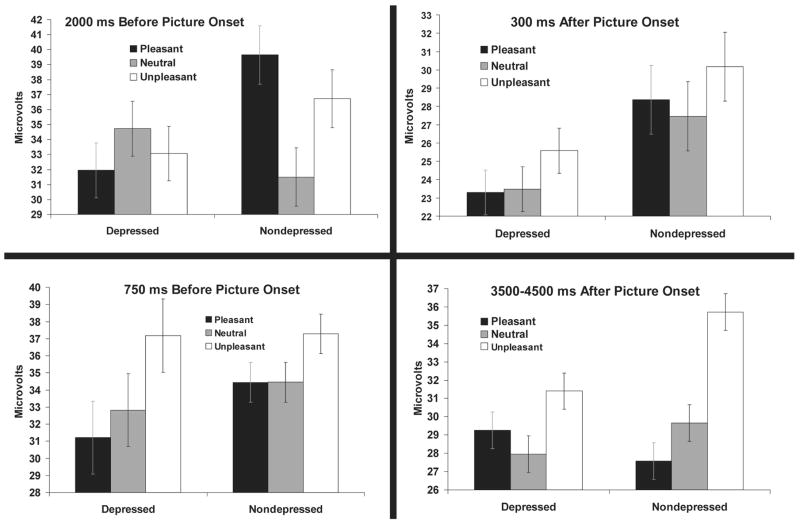
The top left panel of Figure 1 shows mean blink magnitudes in response to probes presented 2,000 ms before picture onset for the depressed and nondepressed groups. A Valence × Group omnibus ANOVA using the Welch ADF procedure revealed a significant Group × Valence interaction, F(2, 59.79) = 4.11, p< .03, but no main effect of valence, F(2, 59.79) = 0.99, p> .30, or group, F(1, 73.41) = 0.14, p> .70. A Group × Quadratic Trend planned comparison using the Welch ADF procedure revealed a Group × Quadratic Trend interaction, F(1, 84.99) = 5.97, p< .02, but no significant overall quadratic trend, F(1, 84.99) = 1.45, p> .23. These results are reflected in the pattern displayed in Figure 1, which reveals a quadratic pattern of startle modulation in the nondepressed group, with larger startle responses during the anticipation of pleasant and unpleasant pictures relative to neutral pictures. In contrast, the depressed group did not demonstrate this pattern and, indeed, if any trend was present, appeared to demonstrate the opposite pattern.
Figure 1.
Startle responding to affective pictures by both diagnostic groups to probes presented 2,000 ms before (top left), 750 ms before (bottom left), 300 ms after (top right), and 3,500–4,500 ms after (bottom right) picture onset. The range of the error bars is one standard error above and below the observed group mean. Because valence was manipulated on a within-subject basis, the standard error bars reflect only the within-subject component of variability (i.e., the Subject × Valence interaction; e.g., Loftus & Masson, 1994).
Corroborating these observations were the results of follow-up one-way ANOVAs and planned comparisons conducted within each diagnostic group. These revealed a significant main effect of valence, F(2, 58) = 3.80, p < .05, , and a significant quadratic trend, F(1, 59) = 4.76, p< .05, δ̂ = .28, within the nondepressed group and no significant effects of valence, F(2, 25) < 1, p > .40, , or quadratic trend, F(1, 26) = 1.28, p > .25, δ̂=.21, within the depressed group. Further comparisons revealed that, although the groups did not differ with respect to startle potentiation to unpleasant, relative to neutral, pictures, F(1, 78.77) = 2.55, p > .10, they did differ with respect to startle potentiation to pleasant, relative to neutral, pictures, F(1, 84.99) = 8.24, p < .006. This difference reflects the fact that nondepressed participants demonstrated startle potentiation in anticipation of pleasant pictures, F(1, 58) = 2.58, p < .02, δ̂ = .33, whereas depressed participants did not, F(1, 25) = −1.31, p > .20, δ̂ = .25. The direction of the pleasant–neutral difference in the depressed group was opposite to that demonstrated by the nondepressed group.
Startle Probes 750 ms Before Picture Onset
The bottom left panel of Figure 1 shows mean blink amplitudes in responses to probes presented 750 ms before picture onset. A Valence × Group ANOVA revealed a main effect of valence, F(2, 84) = 4.04, p < .05, but no main effect, F(1, 85) = 0.04, p > .80, or interactions involving group, F(2, 84) = 0.50, p > .60. Similarly, the linear trend planned comparison revealed a significant overall linear trend, F(1, 85) = 7.81, p < .006, but no Group × Linear Trend interaction, F(1, 85) = 1.00, p > .30. These findings indicate that, overall, startle responses during the anticipation of unpleasant pictures were potentiated relative to startle responses during the anticipation of pleasant pictures.
Startle Probes 300 ms After Picture Onset
The top right panel of Figure 1 shows mean blink magnitudes in response to probes presented 300 ms after picture onset for the depressed and nondepressed groups. Consistent with the pattern of weak startle modulation depicted in this figure, no significant effects were yielded by either the omnibus Group × Valence ANOVA (all ps > .50) or the quadratic trend analysis, F(1, 82.65) = 1.05, p > .30.
Startle Probes 3,500–4,500 ms After Picture Onset
The bottom right panel of Figure 1 shows mean blink magnitudes in response to probes presented 3,500–4,500 ms after picture onset for the depressed and nondepressed groups. A Valence × Group ANOVA using the Welch ADF procedure revealed a main effect of valence, F(2, 61.89) = 9.46, p < .0003; no main effect of group, F(1, 65.15) < 1, p >.80; and a trend toward a Group × Valence interaction, F(2, 61.89) = 2.95, p = .06. More important, a Group × Linear Trend planned comparison using the Welch ADF procedure revealed a significant linear trend, F(1, 76.53) = 16.91, p < .0001, and a significant Group × Linear Trend interaction, F(1, 76.5) = 5.78, p < .02. These results reflect the pattern displayed in Figure 1, which illustrates a linear effect among nondepressed but not depressed participants. Among the former, startle responses to unpleasant and pleasant pictures appeared potentiated and attenuated, respectively, relative to neutral pictures. Consistent with these observations were the results of ANOVAs and planned comparisons conducted within each diagnostic group. These revealed a highly significant valence effect, F(2, 58) = 10.48, p < .001, , and linear trend, F(1, 59) = 19.62, p < .001, δ̂ = .57, within the nondepressed group. Conversely, the main effect of valence, F(2, 25) = 1.39, p > .25, , and the effect for linear trend, F(1, 26) = 1.59, p > .20, δ̂ = .24, were nonsignificant within the depressed group. Follow-up analyses revealed that the diagnostic groups did not differ with respect to startle magnitudes to unpleasant and pleasant pictures, relative to neutral pictures (ps > .17).
Group Differences in Startle Amplitude Across Probe Conditions
In addition to the Group × Valence analyses and planned contrasts conducted separately within each probe condition, we assessed whether the depressed and nondepressed groups displayed overall differences in the pattern of startle responses across probe conditions. An omnibus Group × Valence × Probe Welch ADF analysis yielded significant effects for valence, F(2, 64.01) = 4.97, p < .01, and probe, F(3, 46.17) = 21.75, p < .0001. Bonferroni-corrected pairwise comparisons following up these two main effects indicated that (a) startle amplitudes were greater in response to both the early and the late anticipatory probes relative to both the early and the late viewing probes (all ps < .005), (b) startle amplitudes were larger to the late viewing probes relative to early viewing probes (p < .007), and (c) startle amplitudes across all probe types were greater to unpleasant pictures relative to both neutral (p < .01) and pleasant (p < .005) pictures. Of primary interest in the present context, the ADF test also indicated a nearly significant Group × Valence × Probe three-way interaction, F(6, 45.76) = 2.24, p < .06. This finding indicates that the depressed and nondepressed groups tended to differ in the degree to which the pattern of differential responding to pleasant, neutral, and unpleasant pictures varied with probe condition. Follow-up analyses tested the Valence × Probe interaction separately within the depressed and nondepressed groups. These revealed a highly significant Valence × Probe interaction within the nondepressed group, F(6, 54) = 5.03, p < .001, , but a nonsignificant Valence × Probe interaction and notably smaller effect size within the depressed group, F(6, 21) = 0.38, p > .80, . These results indicate that the pattern of startle modulation to pleasant, neutral, and unpleasant pictures varied depending on probe type among nondepressed participants. Conversely, depressed participants not only demonstrated weaker startle modulation on the analyses conducted within specific probe intervals reported above but also tended to demonstrate a relatively invariant pattern across probe intervals.
We also conducted a more focused examination of whether groups differed in the pattern of startle modulation across probe intervals that we originally hypothesized. We created a repeated-measures contrast across the 12 measures of startle amplitude (3 valence × 4 probe conditions) that coded for the pattern of linear and quadratic effects that we predicted at each probe interval. Specifically, we coded for (a) a quadratic effect during the early anticipatory phase according to which startle amplitude was greater to pleasant and unpleasant, relative to neutral, pictures; (b) a linear effect during the late anticipatory phase according to which startle amplitude was highest to unpleasant pictures and lowest to pleasant pictures; (c) a quadratic effect during the early viewing phase that was the reverse of that hypothesized during the early anticipatory phase (i.e., greater startle amplitude to neutral relative to pleasant and unpleasant pictures), and (d) a linear effect during the late viewing phase that paralleled the pattern hypothesized during the late anticipatory phase and that reflected the well-known “valence” effect during picture viewing found in a number of previous studies with predominantly typical participants. A Welch ADF analysis indicated that the mean value of the contrast pooled across groups was significantly greater than the null value of zero, F(1, 78.91) = 11.38, p < .005, and that groups differed significantly on the contrast, F(1, 78.91) = 8.05, p < .01. Subsequent within-group analyses indicated a highly significant effect for this contrast among the nondepressed group, Hotelling T2 F(1, 59) = 17.04, p < .001, , but a nonsignificant effect within the depressed group, Hotelling T2 F(1, 26) = 0.17, p > .60, (see Olejnik & Algina, 2000, for the formula for ). The larger multivariate effect size estimate within the nondepressed group indicates that these between-groups differences were not simply secondary to the differing sample sizes of the two groups. Considered as a whole, these results indicate that, across time, the nondepressed group, relative to the depressed group, demonstrated greater overall sensitivity to variations in the affective content of pictures in the manner that we originally hypothesized.
Self-Report Responses to Pictures
Because preliminary tests indicated that the within-group covariance matrices of the depressed and nondepressed groups were heterogeneous (all ps < .025), Welch ADF tests were used for analyses of self-report responses to pictures that incorporated a between-groups factor. Ratings data were not available for 1 nondepressed female participant, and viewing time data were not available for 1 nondepressed male participant.
Table 2 shows the mean picture ratings for each diagnostic group. The Group (depressed, nondepressed) × Valence (pleasant, neutral, unpleasant) ANOVA on pleasure ratings revealed a significant main effect of valence, F(2, 34.99) = 329.11, p < .0001, and a significant linear valence contrast, F(1, 39.97) = 613.52, p < .0001. There were no significant main effects or interactions involving the group factor (ps > .25). For both groups, pleasure ratings followed the pattern of the a priori valence categories (i.e., pleasant and unpleasant pictures rated more and less pleasant, respectively, than neutral pictures; see Table 2).
Table 2.
Mean Ratings and Voluntary Viewing Time of Pictures for Both Diagnostic Groups
| Nondepressed |
Depressed |
|||
|---|---|---|---|---|
| Variable | M | SD | M | SD |
| Pleasure rating | ||||
| Pleasant | 1.90 | 0.60 | 1.74 | 0.94 |
| Neutral | 0.09 | 0.25 | 0.12 | 0.35 |
| Unpleasant | −2.83 | 0.80 | −2.58 | 0.95 |
| Arousal rating | ||||
| Pleasant | 4.32 | 1.39 | 4.19 | 1.84 |
| Neutral | 0.78 | 0.84 | 1.20 | 1.44 |
| Unpleasant | 4.93 | 1.69 | 4.44 | 1.92 |
| Viewing time (s) | ||||
| Pleasant | 9.80 | 2.63 | 10.38 | 4.14 |
| Neutral | 7.58 | 2.11 | 8.79 | 3.66 |
| Unpleasant | 10.27 | 3.81 | 11.83 | 5.31 |
Note. The range and direction of the ratings are as follows: pleasure = −4 (extremely unpleasant) to 4 (extremely pleasant), arousal = 0 (not at all aroused) to 8 (extremely aroused).
Analyses of arousal ratings indicated a significant main effect for valence, F(2, 44.22) = 164.20, p < .0001, and a significant quadratic trend, F(1, 39.63) = 327.62, p < .0001. As illustrated in Table 2, both groups judged pleasant and unpleasant pictures to be more arousing than neutral pictures. There were no significant main effects or interactions involving group (ps > .05).
The Group (depressed, nondepressed) × Valence (pleasant, neutral, unpleasant) ANOVA on the time spent rating the pictures revealed a main effect of valence, F(2, 32.74) = 23.33, p < .0001, and a significant quadratic trend, F(1, 34.94) = 9.04, p < .0001, but no significant effects involving the group factor (ps > .15). As shown in Table 2, both groups spent more time rating pleasant and unpleasant pictures than neutral pictures.
Discussion
The present study assesses affective startle modulation by emotional pictures during anticipation and exposure to test whether depression is characterized by broad emotion-context insensitivity across the time course of an affective response. Evidence for such insensitivity would be particularly inconsistent with the notion that depressed individuals demonstrate hyperresponsiveness to negative stimuli. Because the startle response is a defensive reflex(Lang et al., 1990), it is an ideal metric to address whether depression is characterized by hyper- or hyporesponsiveness to negative affective stimuli in particular (Rottenberg et al., 2002, 2005; Sigmon & Nelson-Gray, 1992; Yee & Miller, 1988).
Responses to Anticipatory Probes
Consistent with prior findings (Dichter et al., 2002; Nitschke et al., 2002; Sabatinelli et al., 2001; Skolnick & Davidson, 2002), at the 2,000-ms anticipatory interval, nondepressed participants demonstrated a curvilinear pattern of startle modulation characterized by potentiated startle responses in anticipation of both pleasant and unpleasant pictures relative to neutral pictures (i.e., essentially the inverse of what is typically observed in response to startle probes presented 300 ms after picture onset). Depressed participants did not follow this pattern. Indeed, if anything, the pattern of means across the valence conditions was opposite in shape to that of nondepressed participants.
As discussed earlier, startle modulation in the nondepressed group at this interval may reflect preparatory mobilization for action while participants were anticipating emotionally salient events, and there are strong parallels between startle responses during anticipation of emotional stimuli and startle responses during imagery of highly arousing scenes (Miller et al., 2002; Witvliet & Vrana, 1995, 2000). In both contexts, startle potentiation is greater during unpleasant and high-arousal pleasant conditions, relative to neutral conditions. When the present results are considered in light of the imagery findings, one might speculate that the null findings for the depressed group at the 2,000-ms anticipatory time point reflect a decreased capacity to engage in spontaneous imagery or other forms of mental elaboration in anticipation of unpleasant and pleasant stimuli. This interpretation is broadly consistent with evolutionary accounts of depression that view this disorder as characterized by disengagement (e.g., Nesse, 2000). It also may be consistent with rumination theories of depression, according to which depressive symptoms are maintained by self-focused rumination that limits attentional and working-memory capacity available for the processing of other stimuli (e.g., Davis & Nolen-Hoeksema, 2000; Nolen-Hoeksema, 2000).
Diagnostic group differences were not, however, evident at the exploratory 750-ms anticipatory probe condition; rather, both groups demonstrated a linear pattern at this interval, which suggests that the earlier interval was a more sensitive index of anticipatory deficits in depression. It may be that anticipatory deficits in processing emotionally arousing events are most evident in depression when anticipatory stimuli are relatively more distal; however, as the onset of an emotionally salient stimulus approaches, individuals with depression may display anticipatory responses that are similar to their nondepressed counterparts. Though speculative, such an interpretation is consistent with animal models of depression indicating the greatest deficits in paradigms that require responses for more distal outcomes (see Dunlop & Nemeroff, 2007, for a review).
Responses to Viewing Probes
During the 3,500–4,500-ms viewing probe condition, nondepressed participants exhibited the well-established linear increase in startle magnitude across the pleasant, neutral, and unpleasant picture categories. Conversely, depressed participants failed to show evidence of valence modulation at this probe interval. These results replicate with a larger sample prior findings that depression was associated with anomalies in later-onset processes that subserve responses to the appetitive and aversive properties of affective stimuli (Dichter et al., 2004). Our results, in conjunction with other similar findings (Allen et al., 1999; Forbes et al., 2005; Kaviani et al., 2004), suggest that this pattern of startle responding in depression is a robust effect. Future studies should investigate the relation between blunted startle modulation in depression and other demonstrations of emotion-context insensitivity, its possible correlates (comorbidity, depression subtype, treatment response; e.g., Allen et al., 1999), and its status as a marker of vulnerability to depression. Concerning the latter issues, we note that our previous study found that depressed and nondepressed differences observed before treatment were maintained after successful treatment with antidepressants (Dichter et al., 2004). While this finding is not conclusive, it suggests that blunted affective startle modulation might be a marker of vulnerability.
In contrast, startle responses of nondepressed participants to 300-ms viewing probes did not replicate the startle findings of Bradley, Cuthbert, and Lang (1993) and Dichter et al. (2004). Furthermore, diagnostic groups did not differ on this measure. Bradley, Cuthbert, and Lang (1993) suggested that the 300-ms probe effect may be an example of prepulse inhibition, characterized in this context by the protection of processing of arousing foreground content from disruption by the startle probe. Our failure to replicate this effect may have been due to the influence of the anticipatory cues on orienting and allocation of attention to the prepulse pictures. The strength of the orienting response depends on the novelty of an imperative stimulus (Barry & Sokolov, 1993; Sokolov, Nezlina, Polyanskii, & Evtikhin, 2002; Voronin & Sokolov, 1960), and because picture valence had been established by the informational cue, the affective properties of the pictures may have been less novel than in the absence of such a cue, resulting in attenuated prepulse inhibition. Indeed, because the 300-ms viewing interval probes were presented 6,300 ms after the onset of the informational cue, this probe might have been processed similarly to probes in the later viewing condition. This speculation is consistent with the pattern of modulation observed in the 300-ms condition (e.g., the direction of the difference in amplitudes to unpleasant and pleasant pictures) and with prior evidence that the time courses of attentional modulation of the startle reflex can vary depending on task demands (e.g., Vanman, Boehmelt, Dawson, & Schell, 1996).
Self-Report and Behavioral Responses to Pictures
The absence of between-groups differences in self-report ratings of valence corroborates findings from our research group (Dichter et al., 2004) and contrasts with evidence from other studies (Allen et al., 1999; Rottenberg et al., 2005; Sloan, Strauss, Quirk, & Sajatovic, 1997; Sloan et al., 2001). One contributory factor may be that the current investigation did not employ the Self Assessment Manikin (Bradley & Lang, 1994). Perhaps the pictorial displays used for Self Assessment Manikin ratings make respondents’ actual affective reactions more salient, while the rating scales used in the present study (using verbal endpoints, e.g., pleasant and unpleasant) make more salient to respondents the intended affective content of the pictures or other stimulus features. Additionally, instructions given to participants (i.e., to rate the pictures themselves rather than their reactions to the pictures) may have been less sensitive to diagnostic group differences (although we note that similar instructions have been employed by others; Allen et al., 1999; Sloan et al., 1997, 2001). Despite these disparities, there is a notable contrast between self-report and startle modulation, suggesting that variations in startle modulation may be a more sensitive index of the affective response deficits linked to depression. In this regard, the present findings are different from those of Rottenberg et al. (2005), who found that self-report provided stronger evidence of emotion-context insensitivity than several psychophysiological measures (i.e., corrugator and zygomatic EMG and several measures of autonomic physiology). They did not, however, assess startle modulation. Clearly, more research is needed to understand the discrepancies between self-report and psychophysiological responses to affective stimuli in depression in both this and other studies.
Synthesis of Findings
In summary, (a) two of the startle measures (2,000-ms anticipatory, 3,500–4,500-ms viewing) indicated emotion-context insensitivity among depressed participants. (b) Several other measures indicated that depressed individuals were as responsive to variations in the affective content of pictures as nondepressed individuals (i.e., 750-ms anticipatory, self-report, and behavioral responses to pictures). (c) One measure (300-ms viewing) indicated that both groups were relatively insensitive to variations in affective content. These results suggest that the nature of the differences between depressed and nondepressed individuals is conditional on both the specific measures of emotional reactivity used and the specific time points assessed (cf. Yee & Miller, 1988). Although the data are thus not completely consistent across all measures, we note that our three-way Group × Valence × Probe omnibus analysis and planned contrast yielded a three-way interaction, which is consistent with the observation that, overall, depressed individuals displayed less variation in patterns of response to the different valence categories across probe intervals. Thus, overall, depressed individuals displayed less emotion-context sensitivity at specific time points and less variability in patterns of sensitivity across time points.
Our results also shed light on whether depressed participants exhibit hyperreactivity to negative stimuli and/or hyporeactivity to positive stimuli. Consistent with prior findings (Dichter et al., 2004), we failed to find evidence for hyperresponsivity to negative stimuli among depressed, relative to nondepressed, participants with respect to both startle and self-report responses to affective stimuli. The present study extends these prior findings to include startle reactions to unpleasant stimuli during an anticipatory phase and corroborates previous findings that depression is associated with a broad emotion-context insensitivity (e.g., Rottenberg, 2005, 2007). Concerning reactivity to positive stimuli, the depressed group demonstrated relative attenuation of startle modulation by positive stimuli at the 2,000-ms anticipatory interval but not during any other startle interval or on any other measures.
Cautions and Limitations
Several cautions and limitations are necessary. Our between-groups effects reflect differences between currently depressed and never-depressed individuals. Strict exclusionary criteria for the nondepressed group were employed to increase power to detect diagnostic group differences; nevertheless, a cost of this design feature is reduced generalizability to control groups with a history of depression. In addition, the inclusion of other measures of emotional reactivity might have indicated stronger between-groups differences and/or greater sensitivity to variations in affective content. In particular, recent evidence for a linkage between startle modulation of the postauricular reflex and the activation of appetitive motivation (Benning, Patrick, & Lang, 2004) suggests the possible utility of this measure for revealing depression-related deficits in responding to positive stimuli. However, the construct validity of this measure and the psychobiological mechanisms for its observed effects have yet to be established.
In several respects, we also caution against overgeneralizing the lack of evidence in our study for exaggerated responses to negative stimuli among depressed participants. First, we have argued that at least some of the group differences that we observed may reflect depressed participants’ reduced mobilization for action and cognitive elaboration during the anticipatory phase and reduced recruitment of appetitive versus aversive motivation during the viewing phase. These accounts raise the question of whether the inclusion of less symbolic, more personally evocative affective stimuli would have yielded a pattern of responding among depressed participants that more closely paralleled the pattern observed among nondepressed participants. While this is certainly a question that merits additional study, we note that Rottenberg et al. (2005) found essentially equivalent emotion-context insensitivity effects to both normative stimuli and more personally meaningful idiographic stimuli.
Second, one possible explanation for our failure to find hyper-responsivity to unpleasant stimuli among depressed individuals is that startle responses to unpleasant stimuli may reflect primarily fear responses rather than general anxiety. Fear is related to phobic responses and panic attacks, whereas depression is characterized by high negative affect but not by high autonomic arousal (Barlow, 1991; D. A. Clark, Steer, & Beck, 1994; L. A. Clark & Watson, 1991; Gray, 1982; Mineka, Watson, & Clark, 1998), and there is evidence that stronger startle potentiation is observed in phobic relative to anxious participants (Cuthbert et al., 2003). Therefore, startle potentiation may reflect fear and collateral emotional responses (e.g., disgust) that are not specifically relevant to theories of depression.
Finally, as Tomarken et al. (2007) have pointed out, there is evidence that (a) depressed individuals have particular difficulty regulating long-term responses to stressors or other negative stimuli and (b) such deficits are not well captured by laboratory studies that typically assess short-term responses to affective stimuli. Indeed, one of the notable commonalities between psychological (e.g., Nolen-Hoeksema, 2000) and biological (e.g., Siever & Davis, 1985) perspectives on depression is the view that a hallmark feature is deficits in counterregulatory processes designed to “turn off” long-term responses to stressors and promote a return to prestress levels. Evidence for such exaggerated negative affective responding may require naturalistic paradigms that track emotional responding across time (Goplerud & Depue, 1985).
Conclusions
Our findings indicate that unipolar depression may be associated with a relative insensitivity to variations in affective content but that such effects are dependent on the particular measures and time points assessed. Additionally, our results underscore the importance of assessing the temporal chronometry of emotional responding (Davidson, 1998). Overall, these data add to the growing body of literature documenting startle modulation anomalies linked to depression (Allen et al., 1999; Dichter et al., 2004; Forbes et al., 2005; Kaviani et al., 2004) and other manifestations of psychopathology (e.g., Grillon & Morgan, 1999; Patrick, Bradley, & Lang, 1993; Sabatinelli, Bradley, Cuthbert, & Lang, 1996) and highlight that inclusion of anticipatory conditions in studies of psychopathology may yield unique and novel insights into the pathophysiology of psychiatric disorders.
Acknowledgments
This study was conducted as the dissertation research of Gabriel S. Dichter under the supervision of Andrew J. Tomarken. Gabriel S. Dichter was supported by National Institute of Mental Health Predoctoral Training Grant T32-MH18921, National Institute of Child Health and Human Development Postdoctoral Training Grant T32-HD40127, a positive psychology microgrant from the Martin Seligman Research Alliance, and an American Psychological Association Science Directorate dissertation research award. We thank Richard Shelton and Steven D. Hollon for their valuable assistance and feedback and Margaret Lovett and Marijean S. Stewart for their assistance with participant recruitment.
Appendix
International Affective Picture System Pictures Used in This Study
Pictures are presented grouped by valence condition (pleasant, neutral, unpleasant) and probe condition (2,000-ms anticipatory, 750-ms anticipatory, 300-ms viewing, 3,500–4,500-ms viewing).
Male Picture Set
Pleasant, 2,000-ms anticipatory: 5629, 8170, 4660, 4240.
Pleasant, 750-ms anticipatory: 4689, 4320, 8380, 4653.
Pleasant, 300-ms viewing: 8501, 8470, 1650, 8080.
Pleasant, 3,500–4,500-ms viewing: 8180, 8260, 7501, 8300.
Neutral, 2,000-ms anticipatory: 2630, 5130, 5534, 7950.
Neutral, 750-ms anticipatory: 2580, 7006, 7025, 7009.
Neutral, 300-ms viewing: 7491, 7217, 7030, 7150.
Neutral, 3,500–4,500-ms viewing: 7224, 2570, 7187, 7175.
Unpleasant, 2,000-ms anticipatory: 3150, 9810, 3110, 3130.
Unpleasant, 750-ms anticipatory: 6570, 9570, 3053, 9410.
Unpleasant, 300-ms viewing: 3530, 3080, 6313, 3015.
Unpleasant, 3,500–4,500-ms viewing: 6260, 3060, 3071, 3170.
After 1 control and 3 depressed male participants had completed the startle session, three pleasant pictures from the set presented to men were replaced: 4660 replaced 4210, 4689 replaced 4659, and 4653 replaced 4652.
Female Picture Set
Pleasant, 2,000-ms anticipatory: 4572, 4690, 8370, 7270.
Pleasant, 750-ms anticipatory: 8200, 4660, 8034, 7502.
Pleasant, 300-ms viewing: 8080, 5910, 5460, 8210.
Pleasant, 3,500–4,500-ms viewing: 8180, 8400, 8030, 8185.
Neutral, 2,000-ms anticipatory: 7150, 5130, 7110, 7187.
Neutral, 750-ms anticipatory: 7010, 7491, 7185, 6150.
Neutral, 300-ms viewing: 5740, 2840, 7040, 9360.
Neutral, 3,500–4,500-ms viewing: 5530, 7035, 7031, 7004.
Unpleasant, 2,000-ms anticipatory: 3500, 3030, 9600, 6350.
Unpleasant, 750-ms anticipatory: 9433, 9050, 3100, 3010.
Unpleasant, 300-ms viewing: 3120, 3015, 9571, 2730.
Unpleasant, 3,500–4,500-ms viewing: 3053, 6312, 9921, 3060.
Footnotes
An intertrial interval of 7–9 s is briefer than is generally employed in other affective startle investigations (Dichter et al., 2004; Vrana, Spence, & Lang, 1988). However, affective startle modulation is not evident at 2,000 ms (Dichter et al., 2002) or 3,800 ms (Bradley, Cuthbert, & Lang, 1993) after picture offset. Thus, the affective effects of the IAPS images appear to subside well before the end of the 7–9-s intertrial interval used in the current study. This shorter intertrial interval was used to minimize participant fatigue.
Although several methodologists have appropriately recommended that between-subjects components of variance be included in effect size estimates derived from within-subject designs (e.g., Dunlap, Cortina, Vaslow, & Burke, 1996; Olejnik & Algina, 2000), both the proportion of partial variance and the standardized mean difference measures used here excluded from computations components of variance because of individual differences between participants in overall startle magnitude. We believe that the indexes that we computed are more meaningful in the present context for the following reasons: (a) Because almost all affect-modulated startle studies use within-subject designs and test statistics, the issue of comparability of effect size measures across studies that use between-versus within-subject designs is not particularly salient, and (b) there are substantial individual (i.e., between-subjects) differences in startle magnitude that have, in general, not been linked to psychologically meaningful variables.
References
- Abramson LY, Metalsky GI, Alloy LB. Hopelessness depression: A theory-based subtype of depression. Psychological Review. 1989;96:358–372. [Google Scholar]
- Allen NB, Trinder J, Brennan C. Affective startle modulation in clinical depression: Preliminary findings. Biological Psychiatry. 1999;46:542–550. doi: 10.1016/s0006-3223(99)00025-6. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Barlow DH. The nature of anxiety: Anxiety, depression, and emotional disorders. In: Rapee RM, Barlow DH, editors. Chronic anxiety: Generalized anxiety disorder and mixed anxiety-depression. New York: Guilford Press; 1991. pp. 1–28. [Google Scholar]
- Barry RJ, Sokolov EN. Habituation of phasic and tonic components of the orienting reflex. International Journal of Psychophysiology. 1993;15(1):39–42. doi: 10.1016/0167-8760(93)90093-5. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory—II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Benning SD, Patrick CJ, Lang AR. Emotional modulation of the post-auricular reflex. Psychophysiology. 2004;41:426–432. doi: 10.1111/j.1469-8986.00160.x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Pictures as prepulse: Attention and emotion in startle modification. Psychophysiology. 1993;30:541–545. doi: 10.1111/j.1469-8986.1993.tb02079.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The Self-Assessment Manikin and the Semantic Differential. Journal of Behavioral Therapy and Experimental Psychiatry. 1994;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ, Cuthbert BN. Emotion, novelty, and the startle reflex: Habituation in humans. Behavioral Neuroscience. 1993;107:970–980. doi: 10.1037//0735-7044.107.6.970. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Clark DA, Steer RA, Beck AT. Common and specific dimensions of self-reported anxiety and depression: Implications for the cognitive and tripartite models. Journal of Abnormal Psychology. 1994;103:645–654. [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Cuthbert BN, Lang PJ, Strauss C, Drobes D, Patrick CJ, Bradley MM. The psychophysiology of anxiety disorder: Fear memory imagery. Psychophysiology. 2003;40:407–422. doi: 10.1111/1469-8986.00043. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition & Emotion. 1998;12:307–330. [Google Scholar]
- Davis RN, Nolen-Hoeksema S. Cognitive inflexibility among ruminators and nonruminators. Cognitive Therapy and Research. 2000;24:699–711. [Google Scholar]
- Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. Annual Review of Psychology. 1989;40:457–492. doi: 10.1146/annurev.ps.40.020189.002325. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ, Baucom B. Startle modulation before, during, and after exposure to emotional stimuli. International Journal of Psychophysiology. 2002;43:191–196. doi: 10.1016/s0167-8760(01)00170-2. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ, Shelton RC, Sutton SK. Early- and late-onset startle modulation in unipolar depression. Psychophysiology. 2004;41:433–440. doi: 10.1111/j.1469-8986.00162.x. [DOI] [PubMed] [Google Scholar]
- Duffy E. Activation and behavior. New York: Wiley; 1962. [Google Scholar]
- Dunlap WP, Cortina JM, Vaslow JB, Burke MJ. Meta-analysis of experiments with matched groups or repeated measures designs. Psychological Methods. 1996;1:170–177. [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Archives of General Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Erickson LM. Interplay of emotion and attention: An analysis of startle reflex modulation during anticipation and perception of affective slides. Florida State University; 1996. Unpublished doctoral dissertation. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I Disorders (SCID) Clinician Version administration booklet. Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- Forbes EE, Miller A, Cohn JF, Fox NA, Kovacs M. Affect-modulated startle in adults with childhood-onset depression: Relations to bipolar course and number of lifetime depressive episodes. Psychiatry Research. 2005;134(1):11–25. doi: 10.1016/j.psychres.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Fowles DC. Psychophysiology and psychopathology: A motivational approach. Psychophysiology. 1988;25:373–391. doi: 10.1111/j.1469-8986.1988.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Goplerud E, Depue RA. Behavioral response to naturally occurring stress in cyclothymia and dysthymia. Journal of Abnormal Psychology. 1985;94:128–139. doi: 10.1037//0021-843x.94.2.128. [DOI] [PubMed] [Google Scholar]
- Gray JA. The neuropsychology of anxiety: An inquiry into the functions of the septo-hippocampal system. Oxford, England: Oxford University Press; 1982. [Google Scholar]
- Grillon C, Ameli R. Conditioned inhibition of fear-potentiated startle and skin conductance in humans. Psychophysiology. 2001;38:807–815. [PubMed] [Google Scholar]
- Grillon C, Morgan CA., III Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1999;108:134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Hamilton MA. A rating scale for depression. Journal of Neurology and Neurosurgery in Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEM Data Corp. Snapstream (Revision B) [computer software] Springfield, MI: Author; 1991. [Google Scholar]
- Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cognition & Emotion. 2000;14:711–724. [Google Scholar]
- Higgins ET. Beyond pleasure and pain. American Psychologist. 1997;52:1280–1300. doi: 10.1037//0003-066x.52.12.1280. [DOI] [PubMed] [Google Scholar]
- Hollon SD, DeRubeis RJ, Shelton RC, Amsterdam JD, Salomon RM, O’Reardon JP, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Archives of General Psychiatry. 2005;62:417–422. doi: 10.1001/archpsyc.62.4.417. [DOI] [PubMed] [Google Scholar]
- James Long Co. STIM Auditory/Visual Stimulus Presentation and Laboratory Control System (Version 7.286) [computer software] Caroga Lake, NY: Author; 1998. [Google Scholar]
- James Long Co. EMGART (Version 7.519) [computer software] Caroga Lake, NY: Author; 2002. [Google Scholar]
- Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. Journal of Abnormal Psychology. 2002;111:589–597. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]
- Kaviani H, Gray JA, Checkley SA, Raven PW, Wilson GD, Kumari V. Affective modulation of the startle response in depression: Influence of the severity of depression, anhedonia, and anxiety. Journal of Affective Disorders. 2004;83(1):21–31. doi: 10.1016/j.jad.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Keselman HJ. Testing treatment effects in repeated measures designs: An update for psychophysiological researchers. Psychophysiology. 1998;35:470–478. [PubMed] [Google Scholar]
- Keselman HJ, Carriere K, Lix L. Testing repeated measures hypotheses when covariances are heterogeneous. Journal of Educational Statistics. 1993;18:305–319. [Google Scholar]
- Keselman JC, Keselman HJ. Analysing unbalanced repeated measures designs. British Journal of Mathematical and Statistical Psychology. 1990;43:265–282. [Google Scholar]
- Klein DF. Endogenomorphic depression. A conceptual and terminological revision. Archives of General Psychiatry. 1974;31:447–454. doi: 10.1001/archpsyc.1974.01760160005001. [DOI] [PubMed] [Google Scholar]
- Klein DF. Depression and anhedonia. In: Clark DC, Fawcett J, editors. Anhedonia and deficit states. New York: PMA Publishing; 1987. pp. 1–34. [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ. A bio-informational theory of emotional imagery. Psychophysiology. 1979;16:495–512. doi: 10.1111/j.1469-8986.1979.tb01511.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–395. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biological Psychiatry. 1998;44:1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Digitized photographs, instruction manual and affective ratings. Gainesville: University of Florida; 2005. (Tech. Rep. A-6) [Google Scholar]
- Lix LM, Keselman HJ. Approximate degrees of freedom tests: A unified perspective on testing for mean equality. Psychological Bulletin. 1995;117:547–560. [Google Scholar]
- Loftus GR, Masson MJ. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994;1:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Malmo RB. Activation: A neuropsychological dimension. Psychological Review. 1959;66:367–386. doi: 10.1037/h0047858. [DOI] [PubMed] [Google Scholar]
- Miller MW, Patrick CJ, Levenston GK. Affective imagery and the startle response: Probing mechanisms of modulation during pleasant scenes, personal experiences, and discrete negative emotions. Psychophysiology. 2002;39:519–529. doi: 10.1017/s0048577202394095. [DOI] [PubMed] [Google Scholar]
- Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annual Review of Psychology. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- Morrison DF. Multivariate statistical methods. 2. New York: McGraw-Hill; 1976. [Google Scholar]
- Nesse RM. Is depression an adaptation? Archives of General Psychiatry. 2000;57:14–20. doi: 10.1001/archpsyc.57.1.14. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Larson CL, Smoller MJ, Navin SD, Pederson AJ, Ruffalo D, et al. Startle potentiation in aversive anticipation: Evidence for state but not trait effects. Psychophysiology. 2002;39:254–258. doi: 10.1017/S0048577202010156. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109:504–511. [PubMed] [Google Scholar]
- Olejnik S, Algina J. Measures of effect size for comparative studies: Applications, interpretations, and limitations. Contemporary Educational Psychology. 2000;25:241–286. doi: 10.1006/ceps.2000.1040. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bradley MM, Lang PJ. Emotion in the criminal psychopath: Startle reflex modulation. Journal of Abnormal Psychology. 1993;102:82–92. doi: 10.1037//0021-843x.102.1.82. [DOI] [PubMed] [Google Scholar]
- Rich BA, Bhangoo RK, Vinton DT, Berghorst LH, Dickstein DP, Grillon C, et al. Using affect-modulated startle to study phenotypes of pediatric bipolar disorder. Bipolar Disorders. 2005;7:536–545. doi: 10.1111/j.1399-5618.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg J. Mood and emotion in major depression. Current Directions in Psychological Science. 2005;14:167–170. [Google Scholar]
- Rottenberg J. Major depressive disorder: Emerging evidence for emotion context insensitivity. In: Rottenberg J, Johnson SL, editors. Emotion and psychopathology: Bridging affective and clinical science. Washington, DC: American Psychological Association; 2007. p. 1. [Google Scholar]
- Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. Journal of Abnormal Psychology. 2005;114:627–639. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Kasch KL, Gross JJ, Gotlib IH. Sadness and amusement reactivity differentially predict concurrent and prospective functioning in major depressive disorder. Emotion. 2002;2:135–146. doi: 10.1037/1528-3542.2.2.135. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Cuthbert BN, Lang PJ. Wait and see: Aversion and activation in anticipation and perception. Psychophysiology. 1996;33(Suppl 1):S72. [Google Scholar]
- Sabatinelli D, Bradley MM, Lang PJ. Affective startle modulation in anticipation and perception. Psychophysiology. 2001;38:719–722. [PubMed] [Google Scholar]
- Siever LJ, Davis KL. Overview: Toward a dysregulation hypothesis of depression. American Journal of Psychiatry. 1985;142:1017–1031. doi: 10.1176/ajp.142.9.1017. [DOI] [PubMed] [Google Scholar]
- Sigmon ST, Nelson-Gray RO. Sensitivity to aversive events in depression: Antecedent, concomitant, or consequent. Journal of Psychopathology and Behavioral Assessment. 1992;14:225–246. [Google Scholar]
- Skolnick AI, Davidson RI. Affective modulation of eyeblink startle with reward and threat. Psychophysiology. 2002;39:835–850. doi: 10.1111/1469-8986.3960835. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Strauss ME, Quirk SW, Sajatovic M. Subjective and expressive emotional responses in depression. Journal of Affective Disorders. 1997;46:135–141. doi: 10.1016/s0165-0327(97)00097-9. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Strauss ME, Wisner KL. Diminished response to pleasant stimuli by depressed women. Journal of Abnormal Psychology. 2001;110:488–493. doi: 10.1037//0021-843x.110.3.488. [DOI] [PubMed] [Google Scholar]
- Sokolov EN, Nezlina NI, Polyanskii VB, Evtikhin DV. The orientating reflex: The “targeting reaction” and “searchlight of attention. Neuroscience and Behavioral Physiology. 2002;32:347–362. doi: 10.1023/a:1015820025297. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Keener AD. Frontal brain asymmetry and depression: A self-regulatory perspective. Cognition & Emotion. 1998;12:387–420. [Google Scholar]
- Tomarken AJ, Shelton RC, Hollon SD. Affective science as a framework for understanding the mechanisms and effects of anti-depressant medication. In: Rottenberg J, Johnson SL, editors. Emotion and psychopathology: Bridging affective and clinical science. Washington, DC: American Psychological Association; 2007. pp. 263–283. [Google Scholar]
- Vanman EJ, Boehmelt AH, Dawson ME, Schell AM. The varying time courses of attentional and affective modulation of the startle eyeblink reflex. Psychophysiology. 1996;33:691–697. doi: 10.1111/j.1469-8986.1996.tb02365.x. [DOI] [PubMed] [Google Scholar]
- Voronin LG, Sokolov EN. Cortical mechanisms of the orienting reflex and its relation to the conditioned reflex. In: Jasper HH, Smirnov GD, editors. The Moscow Colloquium on Electroencephalography of Higher Nervous Activity. EEG Suppl 13. Amsterdam: Elsevier; 1960. pp. 335–344. [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: A new measure of emotion? Journal of Abnormal Psychology. 1988;97:487–491. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology. 1995;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- Watson D, Wiese D, Vaidya J, Tellegen A. The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology. 1999;76:820–838. [Google Scholar]
- Welch BL. The significance of the difference between two means when the population variances are unequal. Biometrika. 1938;29:350–362. [Google Scholar]
- Welch B. On the comparison of several mean values: An alternative approach. Biometrika. 1951;38:330–336. [Google Scholar]
- Witvliet CV, Vrana SR. Psychophysiological responses as indices of affective dimensions. Psychophysiology. 1995;32:436–443. doi: 10.1111/j.1469-8986.1995.tb02094.x. [DOI] [PubMed] [Google Scholar]
- Witvliet CV, Vrana SR. Emotional imagery, the visual startle, and covariation bias: An affective matching account. Biological Psychology. 2000;52:187–204. doi: 10.1016/s0301-0511(00)00027-2. [DOI] [PubMed] [Google Scholar]
- Yee CM, Miller GA. Emotional information processing: Modulation of fear in normal and dysthymic subjects. Journal of Abnormal Psychology. 1988;97:54–63. doi: 10.1037//0021-843x.97.1.54. [DOI] [PubMed] [Google Scholar]