Abstract
Cilia function as critical sensors of extracellular information, and ciliary dysfunction underlies diverse human disorders including situs inversus, polycystic kidney disease, retinal degeneration and Bardet-Biedl syndrome. Importantly, mammalian primary cilia have recently been shown to mediate transduction of Hedgehog (Hh) signals, which are involved in a variety of developmental processes. Mutations in several ciliary components disrupt the patterning of the neural tube and limb bud, tissues that rely on precisely coordinated gradients of Hh signal transduction. Numerous components of the Hh pathway, including Patched, Smoothened and the Gli transcription factors, are present within primary cilia, indicating that key steps of Hh signaling may occur within the cilium. Because dysregulated Hh signaling promotes the development of a variety of human tumors, cilia may also have roles in cancer. Together, these findings have shed light on one mechanism by which primary cilia transduce signals critical for both development and disease.
Hh signals pattern diverse developing tissues
Hh proteins are secreted lipoproteins that specify cell patterning in many metazoa and in various developmental contexts (Lum and Beachy 2004; Huangfu and Anderson 2006; Wang et al. 2007b). Investigations into Hh signal transduction have proved to be a rich source of insight into fundamental mechanisms by which cells coordinate their functions during development and homeostasis, and have also revealed that dysregulated Hh signaling underlies a variety of human congenital diseases and cancer (Ruiz i Altaba et al. 2002; McMahon et al. 2003; Hooper and Scott 2005). Indeed, studies on Hh signaling have continued to yield unanticipated surprises, one of which, the involvement of the primary cilium in vertebrate Hh signaling, is the focus of this review.
Hh was initially identified by Nϋsslein-Volhard and Wieschaus in a systematic screen for larval segmentation defects in Drosophila melanogaster (Nüsslein-Volhard and Wieschaus 1980). Whereas flies possess a single Hh gene, mammals have three, the best studied of which encodes Sonic hedgehog (Shh), an important regulator of both embryonic development and post-natal homeostasis (Echelard et al. 1993; Chiang et al. 1996). The other two members of this family, Indian hedgehog and Desert hedgehog, participate in bone development and spermatogenesis, respectively (Bitgood et al. 1996; Vortkamp et al. 1996; St-Jacques et al. 1999).
All Hh proteins are initially synthesized as inactive precursors that possess an amino-terminal signaling domain and a carboxy-terminal intein-like domain which is later removed by autocatalytic cleavage (Perler 1998). In the choanoflagellate Monosiga brevicollis, separate proteins containing domains homologous to the Hh signaling domain and the intein-like domain have recently been identified, suggesting that Hh proteins evolved through shuffling of domains found in our unicellular antecedents (Snell et al. 2006; King et al. 2008). However, some organisms, most notably Caenorhabditis elegans, lack proteins with homology to the Hh amino-terminal signaling domain, suggesting that the Hh pathway has subsequently diverged in some metazoan phyla (Bürglin 1996).
In addition to its role in segment polarity, Drosophila Hh regulates the development of a variety of other tissues, including the wing, eye, muscle and gut (McMahon et al. 2003; Hooper and Scott 2005). Similarly, vertebrate Shh is important for proper morphogenesis of organs such as the skin, eye, lung, muscle and pancreas (McMahon et al. 2003). Perhaps the best understood roles of Shh are in the patterning of the neural tube and limb bud.
In the developing mouse embryo, Shh is first expressed at embryonic day (E) 7.5 in the ventral node (Echelard et al. 1993). By E9.5, Shh is expressed by cells in the ventral forebrain, notochord, floor plate of the neural tube, and posterior limb buds. In the mouse neural tube, Shh is critical for specifying neuronal cell fates in a concentration- and time-dependent manner (Martí et al. 1995; Roelink et al. 1995; Ericson et al. 1997; Dessaud et al. 2007). Shh is initially produced by cells in the notochord and is essential for the specification of cells in the overlying neural tube as floor plate (or, in zebrafish, as lateral floor plate) (Martí et al. 1995; Roelink et al. 1995; Chiang et al. 1996; Schauerte et al. 1998). Once specified, the floor plate also expresses Shh, becoming a second source of axial midline Shh that patterns the remainder of the ventral neural tube.
Shh produced by the notochord, the floor plate and possibly the gut moves away from these sources, establishing a dorsoventral gradient in the neural tube that specifies the fate of five additional neuronal subtypes (Figure 1A) (Ericson et al. 1997). The ventral-most cells in the neural tube—those of the floor plate and the V3 interneurons—are normally exposed to and specified by the highest concentrations of Shh, whereas more dorsal cells are exposed to successively lower concentrations of Shh. Thus, Shh mutants display a loss of the floor plate and almost all ventral neural subtypes, with a concomitant expansion of more dorsal neural subtypes into the ventral neural tube (Figure 1B) (Chiang et al. 1996). Conversely, ectopic placement of Shh sources is sufficient to specify ventral neural cell types in the lateral neural tube (Roelink et al. 1994).
Figure 1. Proper patterning of the neural tube and limb buds is mediated by a gradient of Shh.
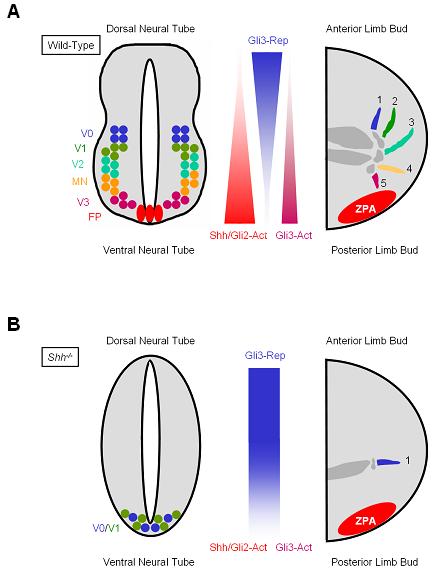
(A) In the neural tubes of wild-type embryos, Shh produced by the notochord, floor plate (FP) and possibly gut forms a gradient along the dorsal-ventral axis. Similarly, Shh released by cells in the zone of polarizing activity (ZPA) in the limb buds forms a gradient along the anterior-posterior axis. Increased Shh concentration and duration of action are associated with increased Gli2 activator (Gli2-Act) and Gli3 activator (Gli3-Act) functions, and reduced Gli3 repressor (Gli3-Rep) formation. High level Hh signaling is critical for specifying the ventral-most cell types in the neural tube, including FP and V3 interneurons (V3). More dorsal cell types (V0, V1, V2 and motorneurons (MN)) are specified by lower levels of Hh signaling. In limb buds, the Shh and Gli3-Rep gradients are interpreted for the specification of digit identity (digits 1-5). (B) In Shh mutant embryos, the gradient of Hh pathway activation is absent. Gli3 is mostly converted into Gli3-Rep along the dorsal-ventral axis of the neural tube and the anterior-posterior axis of the limb buds, although Ihh may still activate the pathway to some degree. In mutant neural tubes, only the V0 and V1 neuronal subtypes are specified, and in mutant limb buds, only the anterior-most digit (digit 1) is formed.
In addition to concentration of Shh, duration of Hh signaling is also an important factor in determining neural cell fates. Neural explants can be induced to become progressively more ventral neural subtypes either by exposing these cells to increasing concentrations of Shh or by lengthening the time of exposure (Dessaud et al. 2007). Similarly, ventral neural cells that eventually become V3 interneurons initially express a marker of the more dorsal motor neuron fate, suggesting that ventral cell fates are sequentially determined in vivo by the duration of Hh pathway activity (Dessaud et al. 2007). Together, these results provide evidence that both concentration and duration of Hh signaling generate the diversity of ventral neural tube precursors.
A similar scenario occurs in the limb buds, where Shh specifies digit identity and number in a concentration- and time-dependent manner. During embryonic development, three organizing centers are crucial for establishing the axes of the nascent limb bud: the apical ectodermal ridge, which is necessary for the outgrowth of the limb and patterning of the proximal-distal axis; the surface ectoderm, which regulates dorsal-ventral patterning; and the zone of polarizing activity (ZPA), which specifies digit number and identity. The ZPA is located in the posterior mesenchyme of the limb bud and secretes Shh, which forms a gradient as it moves anteriorly (Figure 1A) (Riddle et al. 1993). Posterior cells within the ZPA are exposed to high concentrations of Shh for the longest period of time and are specified to form digit five (the little finger in the human hand), whereas anterior cells in the limb bud are exposed to little or no Shh, and are specified into digit one (thumb) (Ahn and Joyner 2004; Harfe et al. 2004; Scherz et al. 2007). The rest of the digits are specified by intermediate levels and/or durations of Shh signaling. In Shh mutant embryos, only a single digit similar to digit one is formed (Figure 1B) (Chiang et al. 1996).
Although vertebrate Hh signaling impinges on many aspects of development, the importance of this pathway later in life appears more limited. During adulthood, the Hh pathway maintains normal tissue homeostasis and plays regulatory roles for stem cells found, for instance, in the central nervous system and hair follicle (Lai et al. 2003; Levy et al. 2005; Han et al. 2008). Improper activation of the Hh pathway may lead to cancer, and in two tumor types in particular—basal cell carcinoma and medulloblastoma—dysregulated Hh signaling appears to play a causative role.
Hh signal transduction: activating an activator and repressing a repressor
Hh initiates signaling by binding to its receptor, the twelve-transmembrane protein Patched (Ptch). Unlike receptors in many signaling pathways, in the absence of Hh, Ptch tonically inhibits the downstream pathway by repressing the function of Smoothened (Smo), a seven-transmembrane protein that acts as the central positive mediator of Hh signaling (McMahon et al. 2003; Hooper and Scott 2005). Vertebrates have two homologs of Ptch. Consistent with its function as a negative regulator of the Hh pathway, Ptch1 mutant embryos display increased and ectopic Hh pathway activity, leading to an expansion of ventral neural cell types and polydactyly (Goodrich et al. 1997; Milenkovic et al. 1999; Motoyama et al. 2003).
In Drosophila, Ptch normally retains Smo in cytoplasmic vesicles. Upon binding of Hh to Ptch, inhibition of Smo is relieved, leading to phosphorylation and plasma membrane accumulation of Smo (Denef et al. 2000; Jia et al. 2004; Zhang et al. 2004). Activated Smo at the cell surface subsequently transduces signals through Cubitus interruptus (Ci), a zinc finger transcription factor that serves as a pivotal switch for downstream pathway activity.
In an intriguing demonstration of biological parsimony, the Drosophila Hh signaling pathway uses Ci both to repress and activate the Hh transcriptional program. In the absence of Hh signals, Ci is processed into a smaller repressor form (CiR) that inhibits the expression of Hh target genes (Aza-Blanc et al. 1997). This processing is facilitated by a kinesin-related protein known as Costal-2 (Cos2), which complexes with Ci and helps exclude it from the nucleus (Robbins et al. 1997; Sisson et al. 1997; Wang and Holmgren 1999; Zhang et al. 2005; Farzan et al. 2008). This Ci-Cos2 complex is also bound by additional proteins in the absence of Hh signals, including unphosphorylated Smo, the serine/threonine kinase Fu, and Sufu (Lum et al. 2003). Importantly, Cos2 recruits several kinases, including Protein kinase A (PKA), Casein kinase I (CKI) and Glycogen synthase kinase 3 (GSK3), which phosphorylate full length Ci (Price and Kalderon 1999; Price and Kalderon 2002). The sequential action of these kinases creates phosphopeptide binding motifs that recruit the Supernumerary limbs (Slimb) subunit of the Skp1/Cullin1/F-box (SCF) E3 ubiquitin ligase complex, which ubiquitinates Ci to direct it for processing into CiR (Jiang and Struhl 1998; Smelkinson and Kalderon 2006). The proteolytic processing required for formation of CiR is mediated by the proteasome, which acts in a regulated manner to degrade only the carboxy-terminal portion of Ci containing the transcriptional activator domain (Chen et al. 1999; Wang and Price 2008). Consistent with their roles in mediating Ci processing, loss of Cos2 or any one of the kinases that phosphorylate Ci can lead to accumulation of full length Ci and increased activity of the Hh pathway (Wang and Holmgren 1999; Price and Kalderon 2002; Zhang et al. 2005).
In the presence of Hh, Drosophila Smo traffics to the membrane and induces phosphorylation and activation of Fu, leading to subsequent phosphorylation of Cos2 and Sufu (Lum et al. 2003; Ruel et al. 2003). Phosphorylation of Cos2 weakens its interactions with Ci and Smo, permitting dissociation of Ci from the complex (Liu et al. 2007; Ruel et al. 2007). Sufu, which suppresses the Hh pathway through its direct binding to Ci, is also inhibited by phosphorylation (Dussillol-Godar et al. 2006). Once freed of its interactions with Cos2 and Sufu, an activator form of Ci can enter the nucleus, where it induces expression of downstream target genes (Chen et al. 1999). In addition to its role in promoting CiR formation, PKA also appears to have a positive role in Hh pathway activation (Zhou et al. 2006). However, how PKA mediates these positive effects and whether additional posttranslational events activate Ci are currently unknown.
Vertebrates possess three homologs of Ci, called Gli transcription factors (Gli1-3). Like Ci, Gli2 and Gli3 possess an amino-terminal repressor domain and a carboxy-terminal activator domain flanking the central DNA-binding zinc fingers (Ruiz i Altaba 1999; Sasaki et al. 1999). Gli1, however, lacks the amino-terminal repressor domain and functions only as a transcriptional activator. Gli2 similarly functions mainly as an activator of Hh signaling, whereas Gli3 functions primarily as a repressor. Ultimately, it is the balance of the collective activator and repressor functions of these Gli transcription factors that determines the status of the Hh transcriptional program.
As with Ci, proteasome-mediated proteolysis regulates the abundance and transcriptional activity of the vertebrate Gli proteins. In the absence of Hh signals, Gli3 is phosphorylated by PKA, CKI and GSK3, generating a phosphopeptide motif bound by β-transducin repeat-containing protein (β-TrCP), the vertebrate homolog of Slimb (Figure 2A) (Wang et al. 2000; Wang and Li 2006). β-TrCP recruits the SCF ubiquitin ligase complex, which targets Gli3 to the proteasome for partial proteolysis into a truncated form that functions as a repressor (Tempé et al. 2006). Gli1 and Gli2 are also phosphorylated by these kinases prior to binding by β-TrCP, but are mainly targeted for complete degradation by the proteasome (Kaesler et al. 2000; Pan et al. 2006). In some cases, Gli2 has been observed to be processed into truncated forms that might serve as transcriptional repressors (Aza-Blanc et al. 1997; Ruiz i Altaba 1999), although the significance of these less abundant species is currently unclear.
Figure 2. A model of mammalian Hh signal transduction.
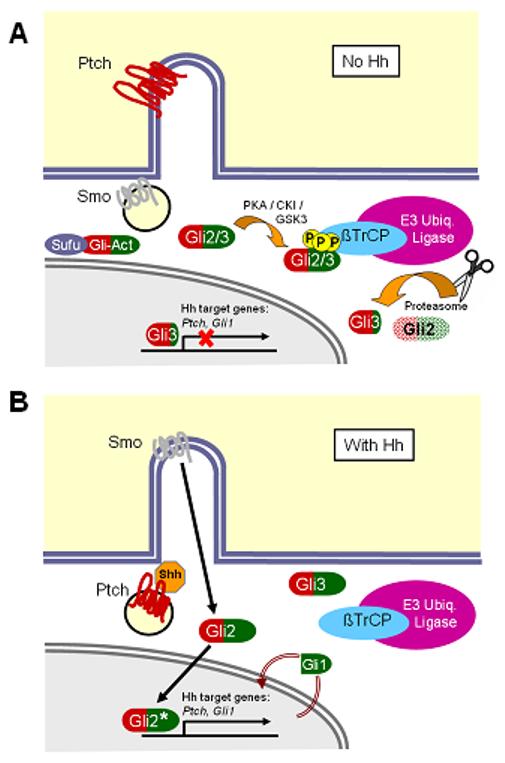
(A) In the absence of Shh, Ptch localizes to the primary cilium and, through an unknown mechanism, prevents Smo from entering the cilium. Gli2 and Gli3 are phosphorylated by kinases, including PKA, CKI and GSK3, to generate phosphopeptide binding motifs for β-TrCP, which recruits an E3 ubiquitin ligase complex. Ubiquitination of Gli2 and Gli3 targets these proteins to the proteasome, which processes Gli3 into a carboxy-terminal-truncated repressor form and degrades Gli2. Gli3 subsequently enters the nucleus and inhibits transcription of downstream Hh target genes such as Ptch1 and Gli1. (B) In the presence of Shh, Ptch is internalized, allowing Smo to traffic into the primary cilium. It is unclear whether Smo traffics directly to the cilium, or accumulates first in the plasma membrane. At the cilium, Smo inhibits the formation of Gli3 repressor and activates Gli2, which enters the nucleus to promote transcription of Ptch1 and Gli1, whose protein products negatively and positively feed back on this pathway, respectively.
SCF is not the only ubiquitin ligase complex that regulates Ci and Gli activity. In Drosophila, a second complex which includes HIB/Roadkill and Cullin 3 (Cul3) also binds full-length Ci proteins in the eye and wing discs to target them for proteasome-mediated degradation (Kent et al. 2006). A mammalian homolog of HIB, SPOP, can functionally substitute for HIB in flies, suggesting that the same complex may also regulate Gli stability (Zhang et al. 2006). Additionally, Gli1, but not Gli2 or Gli3, can interact with Numb, which recruits another E3 ubiquitin ligase, Itch, leading to degradation (Marcotullio et al. 2006).
Upon activation of Hh signaling, both degradation of Gli2 and proteolytic processing of Gli3 into its repressive form are inhibited (Wang et al. 2000; Pan et al. 2006), thereby permitting Gli2 to function as a strong activator of the Hh transcriptional program and allowing full length Gli3 to serve as a activator in some circumstances (Figure 2B) (Bai et al. 2004; Wang et al. 2007a). Overexpression of full length Gli2 causes weak activation of the Hh pathway, whereas overexpression of a truncated form of Gli2 lacking its amino-terminal repressor domain leads to constitutive activation of Hh signaling both in vitro and in vivo (Sasaki et al. 1999; Sheng et al. 2002; Meyer and Roelink 2003). Overexpression of a truncated Gli3 lacking its carboxy-terminal activator domain causes constitutive repression of downstream signaling (Ruiz i Altaba 1999; Meyer and Roelink 2003). Although these artificial constructs may not faithfully recapitulate the normal post-translational regulation of these transcription factors, these results suggest that the presence of activator and repressor domains critically determines the functions of Gli2 and Gli3, and that modulating the activity of these domains may be one mechanism by which the same proteins may exert both positive and negative effects on the Hh transcriptional program.
Functions of Gli proteins in development
Of the three Gli transcription factors, Gli2 plays the most important role in mediating the effects of Hh signaling in the neural tube. Gli2-deficient mouse embryos display loss of ventral neuronal subtypes normally specified by high levels of Hh signaling (the floor plate and some V3 interneurons), and exhibit an expansion of more dorsal cell types normally specified by lower levels of Hh signaling (Figure 3A) (Ding et al. 1998; Matise et al. 1998). In contrast, Gli1-deficient mice manifest no obvious developmental defects (Park et al. 2000), and Gli3-deficient animals exhibit only a subtle expansion of V0, V1 and V2 neural subtypes (Figure 3B) (Persson et al. 2002). These differences suggest that, in the neural tube, cells rely primarily on Gli2 to activate the Hh pathway in response to Shh, whereas Gli1 and Gli3 play supportive roles. Consistent with this, both Gli1; Gli2, and Gli2; Gli3 double mutant embryos display more severe deficits in ventral neuronal subtype specification than do embryos lacking Gli2 alone (Park et al. 2000; Motoyama et al. 2003; Bai et al. 2004; Lei et al. 2004).
Figure 3. The neural tube and limb buds differ in their reliance on Gli2 and Gli3 for proper patterning.
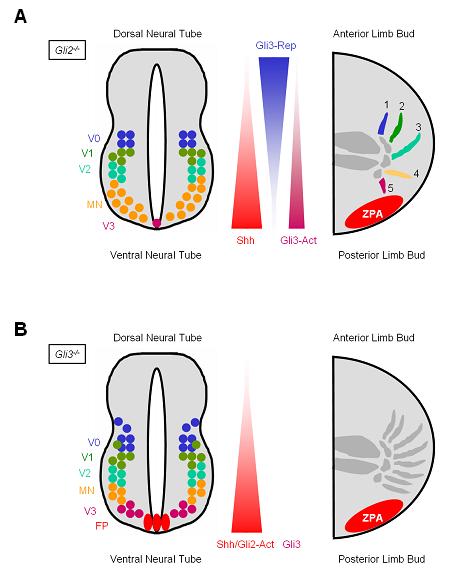
(A) In Gli2 mutant embryos, gradients of Shh and Gli3-Rep/Act are established, but downstream signaling is not properly activated. In the neural tube, which relies predominantly on Gli2 activator function, specification of neuronal subtypes that require the highest levels of Hh pathway activity (FP and V3) is deficient. Because proper patterning of the limb bud depends upon a gradient of Gli3-Act/-Rep, and not on Gli2-Act, digit specification is normal in Gli2 mutants. (B) In Gli3 mutant embryos, gradients of Shh and Gli2-Act are established, but formation of Gli3-Rep is absent. Gli3-deficient neural tubes display only a subtle expansion of V0 and V1 neuronal subtypes. Because proper limb patterning depends on a gradient of Gli3-Rep, Gli3 mutant limb buds upregulate genes normally repressed by Gli3-Rep, including Gremlin and Hoxd13, and exhibit polydactyly.
A different situation occurs in the limb buds, where absence of Gli2 has no effect on digit patterning, but loss of Gli3 leads to polydactyly (Hui and Joyner 1993), a phenotype also caused by increased or ectopic Shh in the limb bud (Figure 3A, B) (Riddle et al. 1993). Embryos lacking both Shh and Gli3 exhibit polydactyly similar to mutants lacking only Gli3, suggesting that Shh patterns the limb bud by suppressing Gli3 repressor formation (Litingtung et al. 2002; Welscher et al. 2002). Indeed, a gradient of the repressor form of Gli3 inverse to that of Shh forms in the limb bud (Wang et al. 2000; Litingtung et al. 2002). At posterior sites proximal to the source of Shh secretion (the future site of digit five), Gli3 repressor levels are lowest, whereas in the anterior limb bud farthest from the source of Shh (the future site of digit one), Gli3 repressor levels are highest. Thus, tissues can respond to Hh signals in two fundamentally different ways. In the first way, exemplified by the neural tube, the principle action of Shh is to induce the activator function of Gli2. In the second way, exemplified by the limb bud, Shh acts primarily to inhibit the formation of Gli3 repressor.
In many tissues, Hh signaling activates the expression of Ptch1 and Gli1, which, in addition to being integral components of the Hh signal transduction pathway, are direct transcriptional targets of Hh signaling (Goodrich et al. 1996; Marigo and Tabin 1996; Bai et al. 2002). As mentioned previously, the developmental role of Gli1 is limited, although double mutant, gain-of-function and gene replacement experiments indicate that Gli1 can act as a positive mediator of the Hh transcriptional program (Park et al. 2000; Bai and Joyner 2001; Bai et al. 2002). Induction of Ptch1 expression has more complex effects on Hh patterning. Increased Ptch limits the movement of Hh proteins in a developmental field, presumably by directly binding and internalizing Hh proteins (Hepker et al. 1997; Jeong and McMahon 2005). Given its role in Smo inhibition, upregulation of Ptch may also be an important feedback by which cells become desensitized to Hh signals (Casali and Struhl 2004; Dessaud et al. 2007).
It is currently unclear the degree to which the functions of the Drosophila Ci interacting proteins Fu, Cos2 and Sufu are conserved in vertebrates. In zebrafish, inhibition of the Cos2 homolog Kif7 induces ectopic Hh signaling in the myotome and neural tube, consistent with data from flies that Cos2 acts as a suppressor of the Hh pathway (Tay et al. 2005). The role of Cos2 homologs in mammals, however, remains unclear, as inhibition of Kif7 and its paralog Kif27 in cell culture assays did not affect Hh signaling (Varjosalo et al. 2006). Mice deficient for Fu also do not display overt Hh-related defects (Chen et al. 2005; Merchant et al. 2005), although inhibition of Fu in zebrafish suppresses the specification of myotome subtypes that rely on high level Hh signaling (Wolff et al. 2003). In contrast, Sufu functions as a negative regulator of Hh signaling in both zebrafish and mice (Cooper et al. 2005; Koudijs et al. 2005; Svärd et al. 2006). This is somewhat unexpected, given that Sufu plays only a minor role in Drosophila Hh signaling (Préat 1992; Ohlmeyer and Kalderon 1998). The molecular mechanism by which Sufu inhibits vertebrate Hh signaling is unclear, although it may both restrict Gli nuclear import and inhibit the activity of Gli transcriptional activators in the nucleus (Cheng and Bishop 2002; Paces-Fessy et al. 2004; Ding et al 1999; Pearse et al. 1999; Stone et al. 1999).
Thus, the homologs of the Ci interacting proteins appear to manifest varied functions in vertebrate Hh signaling. Fu, a critical regulator of Hh signaling in Drosophila, is important for Hh signaling in zebrafish but not in mouse. On the other hand, Sufu, a minor regulator of Hh signaling in fly, is a critical repressor of Hh signaling in both zebrafish and mouse. Together, these observations indicate that whereas much of the Hh signal transduction pathway is remarkably conserved, the mechanisms by which Hh signals are transduced between Smo and Gli have diverged considerably during metazoan evolution (Huangfu and Anderson 2006).
Defective intraflagellar transport disrupts mammalian Hh signaling
A recent screen for N-ethyl-N-nitrosourea (ENU)-induced mouse mutants that display neural tube defects yielded the surprising observation that mammalian Hh signaling relies on intraflagellar transport (IFT) (Huangfu et al. 2003). First visualized over a decade ago in Chlamydomonas flagella, IFT consists of large, multi-subunit complexes that commute bi-directionally between the flagellar base and tip (Kozminski et al. 1993). IFT proteins are conserved among ciliated organisms, and IFT movement along cilia has also been observed in C. elegans chemosensory neuronal cilia (Orozco et al. 1999; Snow et al. 2004; Ou et al. 2005) and in mammalian primary cilia (Tran et al. 2008).
IFT plays a critical role in assembling flagella and cilia, as protein synthesis does not occur within these organelles (Rosenbaum and Witman 2002). Thus, the building blocks required for generating these structures must be transported from the cell cortex to the distal end of the cilium. In Chlamydomonas, IFT is mediated by at least 17 proteins organized into two complexes, A and B (Cole et al. 1998). IFT from the base of the cilium to its tip (anterograde IFT) is powered by Kinesin II (Kozminski et al. 1995; Cole et al. 1998), whereas IFT from the ciliary tip to the base (retrograde IFT) is mediated by a Dynein motor (Pazour et al. 1998; Porter et al. 1999). Disruption of either Chlamydomonas Kinesin II or one of several IFT complex B proteins prevents flagellar assembly (Pazour et al. 2000; Deane et al. 2001). In contrast, mutation of genes encoding components of the Chlamydomonas retrograde Dynein motor or several IFT complex A proteins do not completely abrogate flagellum formation. Instead, these mutants have short flagella that accumulate flagellar proteins at the distal end due to defective retrograde IFT (Pazour et al. 1998; Piperno et al. 1998; Porter et al. 1999).
Consistent with their involvement in Chlamydomonas flagellum biogenesis, mutations that inhibit the function of mouse IFT also disrupt ciliogenesis (Nonaka et al. 1998; Marszalek et al. 1999; Pazour et al. 2000). The first cilia known to form during mouse development are those of the ventral node. These motile cilia generate a leftward flow in the node that is essential for proper left-right axis determination (Nonaka et al. 2002). Disruption of cilia blocks establishment of this flow, leading to left-right axis defects (Nonaka et al. 1998; Marszalek et al. 1999). Similarly, defects in ciliogenesis in the zebrafish embryo disrupt flow in Kupffer's vesicle, the ventral node equivalent, randomizing left-right axis formation (Essner et al. 2005). Paralysis of motile cilia also presumably causes the situs inversus that often accompanies human primary ciliary dyskinesia (Carlén and Stenram 2005; Storm van's Gravesande and Omran 2005).
In addition, mutations in ciliary proteins are linked to human polycystic kidney disease (PKD), a common genetic disorder characterized by the formation of fluid-filled renal cysts (Pan et al. 2005; Simons and Walz 2006). Although disruption of ciliogenesis in mice can cause mid-gestation lethality, preventing analysis of kidney phenotypes, animals bearing a hypomorphic allele of Ift88, which encodes a Complex B protein, survive longer and develop stunted primary cilia in the kidneys and PKD (Pazour et al. 2000; Yoder et al. 2002). Similarly, kidney-specific deletion of Kif3a, which encodes an essential component of the Kinesin II motor required for ciliogenesis, also causes PKD (Lin et al. 2003). Together, these results establish links between defects in IFT function and the pathogenesis of important human diseases.
In addition to left-right axis defects, mutant mouse embryos lacking Kif3a display an open neural tube, as do embryos lacking the IFT complex B components Ift188 or Ift172 (Marszalek et al. 1999; Huangfu et al. 2003; Huangfu and Anderson 2005). Kif3a, Ift88 and Ift172 mutants also display loss of ventral cell types in the neural tube, including loss of the floor plate, V3 interneurons and motor neurons (Figure 4A). These deficiencies are accompanied by an expansion of lateral neural markers into more ventral domains. Unlike Shh mutant embryos (Chiang et al. 1996), neural subtypes such as the V2 precursors are specified in Ift88 and Ift172 mutants, and the dorsal neural progenitor marker Pax7 is not expanded (Huangfu et al. 2003). Thus, the neural tube phenotypes of Kif3a, Ift88 and Ift172 mutants are reminiscent of those caused by abrogated Hh signaling, but are more severe than those caused by loss of Gli2 and less severe than those observed in the absence of Shh.
Figure 4. Many IFT mutations cause polydactyly and loss of ventral neural tube fates.
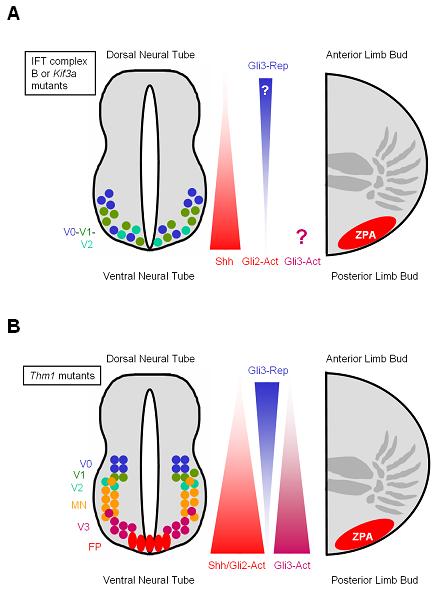
(A) In embryos lacking IFT components such as IFT88, IFT172 and Kif3a, a gradient of Shh is established, but signaling through Gli2-Act and formation of Gli3-Rep are disrupted. Consequently, these mutants do not properly activate the Hh transcriptional targets, and display loss of ventral cell types (FP, V3, MN) and expansion of more dorsal subtypes (V0, V1, V2) in the neural tube. In IFT mutant limb buds, disruption of Gli3-Rep formation leads to polydactyly. (B) In contrast to other IFT mutants, THM1 mutants display increased Hh signaling mediated by Gli2-Act and Gli3-Act, leading to an increase in ventral subtypes (FP, V3, MN) in the neural tube. THM1 is also essential for Gli3 processing, defects of which in THM1 mutants result in polydactyly.
Analyses of additional mice defective for various complex B components have yielded concordant results. For example, disruption of Ift57 or Ift52 causes ventral neural tube patterning defects resembling those observed in Ift88 and Ift172 mutants (Liu et al. 2005; Houde et al. 2006). In the cases in which it has been examined, loss of complex B proteins is associated with an absence of cilia in the ventral node. These results suggest that all IFT complex B proteins may be essential for mammalian Hh signaling and that their loss perturbs this pathway through a common mechanism.
Disruption of retrograde IFT also affects mammalian Hh signaling. Loss of the atypical dynein heavy chain Dnchc2 (also known as Dynch1), a component of the retrograde IFT motor, causes neural tube closure defects and loss of the floor plate and V3 precursors (Huangfu and Anderson 2005; May et al. 2005). Loss of a different dynein subunit, D2lic, also likely interferes with retrograde IFT and is associated with phenotypes indicative of impaired Hh signaling (Rana et al. 2004). In neither case are cilia completely lost; as with Chlamydomonas mutations affecting retrograde IFT transport, the Dnchc2 and D2lic mutant embryos both display stunted, bulged cilia. Defects in retrograde IFT in C. elegans have also been observed to cause a similar bulging (Schafer et al. 2003).
The involvement of IFT proteins in mammalian Hh signaling is surprising, given that mutations in homologous genes do not perturb Drosophila Hh signaling (Han et al. 2003; Sarpal et al. 2003). Indeed, many Drosophila tissues patterned by Hh are not known to possess cilia. It is interesting that epistatic analyses have suggested that IFT functions at a point downstream of Smo and upstream of Gli3 (Huangfu et al. 2003; Huangfu and Anderson 2005). As noted previously, analyses of the vertebrate homologs of Sufu, Fu and Cos2 have suggested that this part of the pathway has diverged most extensively between arthropods and mammals. The contrasting reliance of Drosophila and mouse Hh signaling on cilia appears to be part of this evolutionary divergence. Analysis of whether Hh signals are also transduced through cilia in organisms such as zebrafish, tunicates, and Cnidaria will help reveal whether Drosophila have lost ciliary involvement in Hh signaling or whether mammals have gained this capability.
In addition to neural tube defects, disruption of mouse IFT proteins Dnchc2, Ift88 or Ift52 causes preaxial polydactyly, which is typically associated with increased Hh signaling in the limb bud (Figure 4A) (Haycraft et al. 2005; Liu et al. 2005; May et al. 2005). As discussed previously, Shh mutants form only a single digit (Chiang et al. 1996; Litingtung et al. 2002; Welscher et al. 2002). In contrast, Shh; Ift88 double mutants display polydactyly similar to that of Ift88 single mutants and Shh; Gli3 double mutants (Liu et al. 2005). Thus, as in the neural tube, IFT functions downstream of Shh in limb patterning. However, in contrast to neural tube patterning, loss of IFT results in a limb bud phenotype characteristic of Hh gain of function. How do IFT proteins regulate Hh signaling in this seemingly paradoxical manner? Is it their role in ciliogenesis that mediates Hh signaling, or some alternate activity? Fortunately, recent insights into ciliary function have shed light on these important questions.
The primary cilium as a cellular signaling center
First described by Zimmerman over a hundred years ago, cilia are found in many tissues across diverse phyla (Zimmerman 1898). The recent renewed interest in primary cilia comes from the recognition that these organelles function as critical signaling centers in the cell (Eggenschwiler and Anderson 2007). Indeed, the varied metaphors used to describe cilia in the literature over the past few years—as “cellular antennae,” as “watchtowers,” as “global positioning systems”—underscore the prominent role primary cilia are now regarded to play in the detection and integration of a variety of signaling cues (Benzing and Walz 2006; Marshall and Nonaka 2006; Singla and Reiter 2006; Mans et al. 2008).
Unlike the more familiar motile cilia that abundantly line such surfaces as the airway and oviduct, primary cilia are solitary microtubule-based extensions that protrude from the plasma membrane of most cells and, with the exception of cilia found in the embryonic node, are thought to be non-motile (Satir and Christensen 2007). They are also dynamic structures that are extended during interphase and later resorbed during cell cycle progression (Quarmby and Parker 2005). This regulated assembly and disassembly is thought to be necessary for proper mitosis, as the base of the cilium, known as the basal body, contains a centriole that must be released from the cell membrane to move to the spindle pole.
The formation of a primary cilium initiates during interphase when the centrioles move to the plasma membrane. Vesicles budding from the Golgi deliver proteins that encapsulate the distal end of the mother centriole, leading to an accumulation of pericentriolar material that nucleates microtubule polymerization (Salisbury 2004; Satir and Christensen 2007). These microtubules, which exist as triplets in the basal body, extend as doublets in a 9+0 configuration along the length of the cilium, forming a core structure known as the ciliary axoneme. This microtubule configuration differs from that of most motile cilia, which possess an additional central microtubule pair in a 9+2 arrangement.
At the interface between the axoneme and the basal body are the terminal plate and transition fibers, structures which may function as gateways that regulate the trafficking of proteins into and out of the cilium (Arima et al. 1984). Within the overlying plasma membrane, a condensed lipid zone encircles the base of the cilium, which may also serve to restrict the entry of membrane proteins (Gilula and Satir 1972; Vieira et al. 2006). The basal body, in coordination with these gatekeeper structures, is thought to regulate the loading of ciliary proteins onto IFT particles and their entry into the cilium.
A variety of human diseases are associated with dysfunctional primary cilia. In addition to left-right axis defects and PKD, as discussed above, dysfunctional cilia have been linked to polymorphic diseases such as Bardet-Biedl syndrome (BBS) (Ansley et al. 2003). BBS is characterized by a range of phenotypes including polycystic kidney disease, polydactyly, diabetes, obesity, situs inversus, retinal degeneration, anosmia, sensory defects and cognitive deficits. To date, mutations in at least twelve different Bbs genes have been linked to this disorder, and many of these genes encode proteins that localize to cilia and basal bodies (Nachury et al. 2007; Tobin and Beales 2007). Defective ciliary function may also underlie other multisystem disorders, including Meckel-Gruber syndrome and Joubert syndrome (Adams et al. 2007). Interestingly, like mouse embryos with defects in IFT or Gli3, many BBS- or Meckel-Gruber syndrome-affected individuals exhibit polydactyly. Whether this is a manifestation of altered Hh signaling is currently unclear.
Mammalian Smo activates the Hh pathway at the primary cilium
As discussed previously, double mutant analyses have indicated that IFT proteins function downstream of Smo and upstream of Gli proteins in the Hh pathway. Mechanistic insights into how IFT mediates Hh signal transduction have been provided by recent studies which show that both Ptch and Smo can localize to primary cilia, and that localization of these proteins is mutually exclusive (Corbit et al. 2005; Rohatgi et al. 2007). Thus, in the absence of Hh ligand, Ptch localizes to cilia and inhibits the Hh pathway by keeping Smo out of the cilium. To activate signaling, Hh binds Ptch at the cilium (Rohatgi et al. 2007) and becomes localized to a juxta-ciliary region (Chamberlain et al. 2008). Consequently, Ptch is internalized, allowing Smo to move into the cilium, where it promotes downstream pathway activation by shifting the processing of the Gli transcription factors in favor of activator forms (Figure 2B). How Ptch regulates Smo localization, and how Smo, once in the cilium, transduces signals to the Gli transcription factors remain unclear.
Loss of Ptch1 is sufficient to confer constitutive ciliary localization for Smo (Rohatgi et al. 2007; Ocbina and Anderson 2008). Recent work has focused on determining whether Ptch inhibits Smo localization and activity through small molecule intermediates (Taipale et al. 2002; Rohatgi et al. 2007). Suggestive evidence for this hypothesis comes from the fact that Ptch acts catalytically to inhibit Smo, and is structurally related to the resistance-nodulation-cell division (RND) family of bacterial pumps and the Niemann-Pick C1 cholesterol transporter (Taipale et al. 2002). Thus, Ptch may modulate the concentration of a small molecule, possibly related to cholesterol, which affects Smo trafficking into and/or out of the cilium.
Further support for this hypothesis comes from the fact that sterols affect cellular response to Shh. Treatment of cultured fibroblasts with specific oxysterol derivatives of cholesterol promotes localization of Smo into the cilium (Rohatgi et al. 2007) and can induce differentiation of pluripotent mesenchymal cells into osteoblasts, an effect also observed when these cells are exposed to Shh (Dwyer et al. 2007). Conversely, depleting cholesterol by cyclodextrin or HMG-CoA reductase inhibitor can inhibit pathway response to Shh (Cooper et al. 2003). Furthermore, human genetic disorders such as Smith-Lemli-Opitz syndrome and lathosterolosis are caused by deficiencies in cholesterol biosynthesis, and are characterized by features such as holoprosencephaly and polydactyly, phenotypes that can result from alterations in Hh signaling (Chiang et al. 1996; Kelley et al. 1996; Krakowiak et al. 2003). Pharmacological inhibitors of the sterol synthesis pathway have also been shown to limit the Hh pathway activity and growth of murine Ptch1+/−; p53−/− medulloblastoma cells (Corcoran and Scott 2006). Together, these data suggest that Ptch suppresses Hh signaling either by transporting inhibitory sterols to Smo, or by preventing activating sterols from reaching Smo (Bijlsma et al. 2006; Corcoran and Scott 2006).
It is tempting to speculate that sterols may also affect Smo activity indirectly by altering the membrane dynamics that regulate the trafficking of proteins into and out of the cilium. Another possibility is that oxysterols might act directly on Ptch to inhibit its function. It also currently unclear how Smo reaches the cilium upon activation of Hh signaling. In the presence of Shh, Smo can be phosphorylated by G protein coupled receptor kinase 2 (GRK2), which induces Smo binding to β-arrestin and Kif3a in NIH-3T3 cells (Chen et al. 2004; Meloni et al. 2006; Kovacs et al. 2008). Both β-arrestin and Kif3a are required for Smo to traffic into the cilium, suggesting that Kinesin II may directly transport Smo along the axoneme (Kovacs et al. 2008).
Proper ciliary function is required for processing of Gli proteins
The finding that Smo localization to the cilium is required for activating mammalian Hh signal transduction has provided mechanistic insights into the embryonic phenotypes observed upon disruption of IFT components. In the absence of IFT complex B components, cilia are lost or structurally compromised, likely preventing Smo from signaling through Gli2 and activating the Hh transcriptional program. Although this explanation can account for why disruption of IFT complex B components leads to Hh loss-of-function phenotypes observed in the neural tube, it does not explain why polydactyly is also observed in the limb buds of IFT mutants.
How can IFT mediate Hh pathway activation in certain developmental contexts and Hh pathway inhibition in others? One possible explanation for these dual functions comes from the finding that, in addition to their role in potentiating Gli2 activator formation, IFT components also promote Gli3 repressor activity. This is evidenced by the observation that embryos mutant for genes encoding several IFT components, including Ift88, Ift172, Kif3a and Dnchc2, display decreased processing of Gli3 into its repressor form (Haycraft et al. 2005; Huangfu and Anderson 2005; Liu et al. 2005; May et al. 2005).
As mentioned previously, the principle role of Shh during limb bud patterning is to inhibit formation of Gli3 repressor (Wang et al. 2000). In the absence of Gli3 repressor formation, Shh-dependent genes such as Gremlin and Hoxd13 are ectopically expressed in the limb (Litingtung et al. 2002; Welscher et al. 2002). Consistent with the observed defects in Gli3 repressor formation, Gremlin and Hoxd13 are also ectopically expressed in the limb buds of embryos lacking Ift88 or Dnchc2 (Liu et al. 2005; May et al. 2005). In addition, overexpression of full length Gli3 in IFT-deficient limb bud cells is incapable of suppressing Gli1-mediated Hh pathway activation, although overexpression of a truncated Gli3 lacking its carboxy-terminal activator domain can still function as a repressor in the absence of IFT (Haycraft et al. 2005). Together, these results indicate that IFT plays a critical role in Gli3 repressor formation and activity both in vitro and in vivo.
As also previously mentioned, proper patterning of the neural tube depends primarily on Gli2 activator function, with Gli3 activator playing a supportive role, as evidenced by the observation that Gli2; Gli3 double mutant embryos display more severe ventral neural patterning defects than do Gli2 single mutants (Motoyama et al. 2003; Bai et al. 2004; Lei et al. 2004). IFT complex B mutants display loss of ventral cell types that largely resemble the phenotypes observed in Gli2; Gli3-double mutant embryos (Huangfu et al. 2003; Huangfu and Anderson 2005). However, loss of Gli3 partially mitigates the neural patterning defects observed in either Ift88- or Ift172-deficient embryos by restoring formation of motor neurons (Huangfu et al. 2003). Given the phenotypes observed in the limb bud and neural tube, the most parsimonious explanation for reconciling these results is that, while the cilium is essential for full Gli3 repressor activity, some residual repressor activity remains even in the absence of the cilium.
Finally, all three Gli proteins, as well as Sufu, can localize to the cilium (Haycraft et al. 2005), suggesting that key Gli processing steps do not simply require cilia, but occur within the cilium itself. Interestingly, Gli1, which can localize to cilia, does not require IFT for activity, unlike Gli2 and Gli3 (Haycraft et al. 2005). Proteasomes, which are critical for regulating Gli2 protein levels and for generating Gli3 repressor, have been observed to accumulate near basal bodies (Wigley et al. 1999). Thus, it is possible that Gli2 and Gli3 may be post-translationally modified at the cilium in a Hh-dependent manner, and that proteasomes at the ciliary base may subsequently interpret these cues, prior to Gli2/3 entry into the nucleus.
Additional ciliary components function in Hh signal transduction
If cilia are in fact integral to the transduction of Hh signals during mammalian development, inhibition of critical ciliary proteins such as those involved with proper functioning of the basal body should also be expected to disrupt Hh signal transduction. One such basal body protein is Ofd1, disruption of which causes Oral-facial-digital type I (Ofd1), a human syndrome characterized by craniofacial defects, polydactyly and, in a minority of patients, PKD (Romio et al. 2004). Indeed, mouse embryos lacking Ofd1 display loss of cilia in the node and reduced Hh signaling in the neural tube, as well as anterior expansion of Hoxd genes in the limb buds, suggesting that Gli3 processing is defective (Ferrante et al. 2006).
Similar to Ofd1, loss of another basal body protein, Ftm (also known as Rpgrip1l), compromises ciliary structure and Hh signaling (Vierkotten et al. 2007). Embryos lacking Ftm display fewer cilia in the node and limb buds, and these cilia are bulbous and short (Vierkotten et al. 2007). In addition to left-right axis patterning defects, Ftm mutants display loss of ventral cell types in the neural tube, polydactyly and impaired Gli3 processing. Similar to IFT mutants, the Ftm neural phenotype is partially rescued by loss of Gli3.
As expected, Ftm-deficient fibroblasts are less responsive to Shh compared to wild-type fibroblasts (Vierkotten et al. 2007). Surprisingly, however, both fibroblasts and mesenchymal limb cells cultured from Ftm mutant embryos are ciliated in vitro. Indeed, in spite of the ciliary defects in the node, other tissues such as the trachea and olfactory epithelium appear to have normal cilia in Ftm mutant embryos. These results suggest that some requirements for ciliogenesis may vary among different tissues. For instance, Rpgrip1, a basal body protein similar to Ftm, is required for stabilizing cilia specifically on photoreceptor cells (Hong et al. 2001). It is possible that Rpgrip1 and Ftm may have partially overlapping functions in some tissues.
In addition to IFT and basal body components, many other proteins are likely to be required for proper cilium formation. Arl13b is a small GTPase that localizes to the cilium, and disruption of this protein results in cyst formation in the zebrafish pronephros (Sun et al. 2004). Mouse embryos lacking Arl13b possess shortened nodal cilia with defective assembly of the outer doublet microtubules (Caspary et al. 2007). Similar to mutants with defective IFT or Ofd1, Arl13b mutants display an open neural tube and polydactyly. However, Arl13b mutants exhibit a Hh phenotype that appears unique from the simple gain- or loss-of-function defects described earlier: In the neural tubes of Arl13b mouse mutants, the gradient of the Hh pathway response appears shallower compared to wild-type. The domain of motor neurons, which is normally specified by intermediate levels of Hh, is significantly expanded both dorsally and ventrally in the neural tube. At the same time, Arl13b mutants display defective formation of the floor plate and V3 interneurons, cell fates that require higher levels of Gli activator than do motor neurons. Consistent with a role for Arl13b in Hh pathway activation, loss of both Ptch1 and Arl13b fails to activate the Hh pathway to the same extent as loss of only Ptch1. Together, these results indicate that both full activation and inhibition of the Hh pathway are partially disabled in the absence of Arl13b. Interestingly, however, Gli3 repressor formation is not noticeably diminished in Arl13b mutants.
Whereas the phenotypes of the IFT mutants discussed thus far have generally been associated with impaired Hh signaling, loss of Tetratricopeptide repeat-containing Hedgehog modulator-1 (THM1) increases Hh pathway activity (Tran et al. 2008). In marked contrast to mutations in Ift52, Ift57, Ift88, Ift172, Dnchc2 and Kif3a, the neural tubes of THM1 mutants display a Gli2-dependent expansion of ventral cell types, including floor plate, V3 interneurons and motorneurons (Figure 4B). But like other IFT mutants, THM1-deficient embryos exhibit polydactyly and an increased Gli3 activator-to-repressor ratio. In addition, double mutant analysis indicates that THM1 acts downstream of both Shh and Smo. Thus, THM1 mutants possess phenotypes both similar to and distinct from other IFT mutants.
The Chlamydomonas homolog of THM1 is the complex A protein IFT139 [D. Cole, personal communication]. Disruption of Chlamydomonas complex A IFT components partially impairs retrograde IFT without affecting anterograde IFT, resulting in shortened cilia with bulges at their tips (Piperno et al. 1998). Consistent with this, THM1-deficient mouse embryos possess stunted, bulbous nodal cilia similar to those observed in Dnchc2 mutants (Huangfu and Anderson 2005; May et al. 2005), and ablation of THM1 in cultured cells diminishes retrograde IFT (Tran et al. 2008). It is interesting that more profound defects in retrograde IFT, as presumably found in Dnchc2 and mD2lic mutants, do not result in increased Gli2 activity (Rana et al. 2004; May et al. 2005).
Even in the absence of THM1, Smo displays normal localization to the tips of nodal cilia (Tran et al. 2008). Similarly, overexpressed Gli proteins also localize to cilia in THM1-deficient fibroblasts. These data suggest that whereas complete disruption of retrograde IFT, as seen in Dnchc2 or D2lic mutants, inhibits Hh pathway activity, reduced retrograde IFT velocity can augment Hh signaling. One possibility is that partial abrogation of retrograde IFT prevents Gli repressor activity but preserves some Gli activator activity. Alternatively, IFT complex A members may be required for Gli repressor activity through a function distinct from their role in retrograde IFT. It will be interesting to determine whether loss of other IFT complex A proteins affects the Hh pathway similarly to loss of THM1, and whether increased pathway activation in these IFT complex A mutants also depends on Gli2.
Given the litany of ciliary genes involved in Hh signaling, it might appear as if any defect in ciliary structure is sufficient to perturb Hh pathway activity. However, two cases suggest that this supposition is incorrect. Rfx3 is a transcription factor involved in the expression of many ciliary genes, including D2lic (Bonnafe et al. 2004). Mice mutant for Rfx3 possess shortened nodal cilia but can survive to adulthood, suggesting that Hh signal transduction is not dramatically altered (Bonnafe et al. 2004). Similarly, loss of Stumpy, a highly conserved ciliary protein, causes ependymal and kidney epithelial cilia to be short and reduced in number (Town et al. 2008). Despite these defects in ciliary morphology, Stumpy mutants lack recognized Hh-associated phenotypes.
Additional proteins that have previously been found to modulate the Hh pathway may also have roles in ciliary function. Among these is Rab23, a Rab family GTPase that negatively regulates Hh signaling downstream of Smo and upstream of Gli2 (Eggenschwiler et al. 2001). The immunophilin family member FK506 binding protein 8 (FKBP8) is another intriguing negative regulator of Hh signaling that functions downstream of Smo and upstream of Gli2 in neural tissues (Bulgakov et al. 2004; Cho et al. 2008). Sil1, a cytosolic protein of unknown function, is a positive mediator of Hh signaling that acts downstream of Ptch (Izraeli et al. 2001). Evc, which localizes to the base of the cilium, potentiates Indian Hh-regulated bone development (Ruiz-Perez et al. 2007). Finally, genetic analyses of Tectonic have suggested that this protein may potentiate Hh signaling in the presence of Hh ligands, but may also suppress the pathway in the absence of upstream signals (Reiter and Skarnes 2006). In summary, these studies have provided tantalizing clues that suggest the involvement of additional novel components in ciliogenesis and Hh signaling.
Are cilia involved in Hh pathway-mediated tumorigenesis?
During many developmental processes, activation of the Hh pathway is mediated through paracrine interactions between the epithelium and mesenchyme. In the adult, however, Hh signaling plays a more limited role, although upregulation of Hh pathway activity, either through autocrine signaling or mutations that act cell-autonomously, can give rise to cancer. Indeed, a diversity of human tumors express targets of the Hh pathway, and there is evidence that inappropriate activation of Hh signaling may contribute to the development of lung cancer, pancreatic cancer, prostate cancer and glioma (Ruiz i Altaba et al. 2002; Pasca di Magliano and Hebrok 2003; Rubin and Sauvage 2006). However, for two human cancers in particular—basal cell carcinoma (BCC) and medulloblastoma—there is extensive evidence that increased Hh signaling causes these malignancies (Athar et al. 2006; Tang et al. 2007).
Humans diagnosed with Basal cell nevus syndrome (also known as Gorlin's syndrome) are prone to developing widespread BCCs. These patients have been found to be heterozygous for Ptch1, and Ptch1+/− mice exhibit many features of this disease, including a propensity for developing medulloblastomas and sarcomas, and BCCs after irradiation (Hahn et al. 1998; Aszterbaum et al. 1999). Mutations in the Ptch1 locus are also detected in 10-20% of sporadic medulloblastomas in humans, and in 50-60% of sporadic BCCs (Tang et al. 2007). BCCs without Ptch1 mutations often harbor mutations in Smo (Xie et al. 1998; Lam et al. 1999; Crowson 2006). These mutations likely render Smo insensitive to the inhibitory action of Ptch, leading to constitutive activation of downstream signaling.
A homolog of at least one oncogenic form of Smo isolated from a human patient with BCC localizes constitutively to the cilium in the absence of Hh ligand (Corbit et al. 2005). This is in contrast to wild-type Smo, which moves to the cilium only upon Hh stimulation. In addition, cyclopamine, which has been used as a chemotherapeutic agent for BCC and medulloblastoma in animal models, removes Smo from the cilium (Corbit et al. 2005). Given the preponderance of data indicating that cilia are required for proper Hh pathway activity downstream of Smo, one important question might be, Are cilia necessary for the growth of tumors, particularly those initiated and sustained by Smo activation? And are human tumors ciliated?
Although there are currently no published reports that have examined ciliary distribution across a panel of different tumor types, we have observed that cilia are frequently absent from established human cancer cell lines (S.Y.W. and J.F.R., unpublished results). However, these cells have been selected for growth under tissue culture conditions and may not be representative of tumors arising in human patients. Indeed, we have recently examined biopsies from human BCCs and found that cells from these tumors are frequently ciliated (Wong et al., manuscript submitted). In addition, we have observed that cilia are also present on cells from BCC-like lesions that arise in transgenic mice induced to express a constitutively active form of Smo (SmoM2) specifically in the skin. Others have recently observed that cells from a subset of human medulloblastomas can also be ciliated (Han et al., manuscript submitted).
Because cilia play critical roles in modulating Hh signaling during development, it is likely that these organelles also serve important functions during tumorigenesis, particularly for Hh-dependent cancers such as BCC and medulloblastoma. As discussed previously, while cilia are necessary for propagating signals initiated by Smo and transduced downstream through Gli activators, these organelles are also required for Gli3 repressor formation, which may inhibit the expression of a host of genes, including those that promote cell proliferation. Thus, the functions of cilia during tumorigenesis are likely to be complex (Figure 5). In addition, cilia may have roles in modulating a multitude of other signaling pathways important for cancer, including Wnt and PDGF (Schneider et al. 2005; Gerdes et al. 2007; Corbit et al. 2008).
Figure 5. The primary cilium likely coordinates a variety of signaling pathways important for cancer.
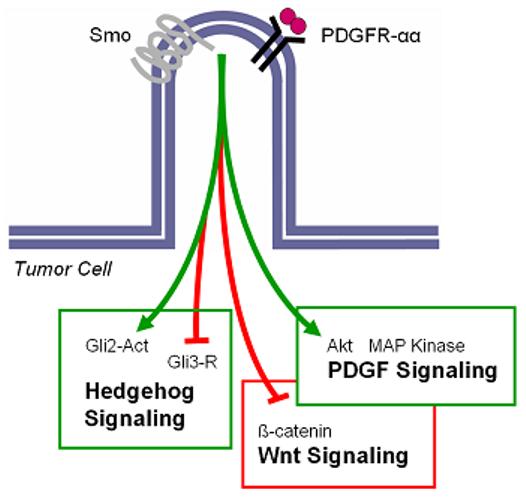
In Hh-dependent cancers such as BCC and medulloblastoma, the cilium may modulate the activity of signaling pathways in addition to Hh. For instance, localization of PDGF receptor-αα to the cilium is important for transducing downstream signals mediated by Akt and MAP kinases. The cilium may also normally restrain Wnt pathway activity by preventing the accumulation of β-catenin. The balance of the downstream effects of these pathways likely governs cellular decisions for proliferation and apoptosis.
Lingering Questions
The identification of the primary cilium as a critical modulator of both positive and negative signals of the Hh pathway has placed this organelle at the crossroads of Hh signal transduction. While many new questions have been raised, many old ones remain to be addressed. For instance, it remains unclear how Ptch restrains Smo activity or how Smo triggers Gli activation. The central involvement of the cilium in these processes suggests that understanding how Ptch alters the cilium, what proteins move Smo into the cilium, and what Gli processing components function within the cilium may provide significant insights into these difficult problems.
It is also currently unknown how general a role cilia play in Hh signaling in other organisms. Although it is clear that mouse Hh signaling relies on cilia and Drosophila Hh signaling does not, the involvement of cilia in Hh signaling in other organisms has not been well explored. For example, does Hh signaling act through cilia in ascidians or Cnidaria? Are cilia important for Hh signaling in fish? Although loss of IFT components causes kidney cysts in zebrafish (Sun et al. 2004), Hh-related phenotypes have not yet been observed, possibly because cilia persist in these mutants through early development, presumably due to maternal deposition of wild-type IFT mRNA or protein (Tsujikawa and Malicki 2004). Thus, it will be interesting in future studies to assess the phenotypes of fish lacking both maternal and zygotic IFT contributions.
Even in mammals, it is currently unclear whether all Hh signaling is transduced through the cilium, or whether some cell types or developmental events respond to Hh signals through alternative mechanisms. One possible example of cilium-independent Hh signaling might be in the guidance of commissural axons. Shh functions as a midline signal that guides commissural axons towards their intermediate target, the floor plate (Charron et al. 2003). As Shh presumably acts at the growth cone to induce rapid cytoskeletal changes, it is difficult to imagine how the cilium could mediate this guidance. Intriguingly, the correct projection of commissural axons depends on Boc, a cell surface protein that binds Shh (Okada et al. 2006). Could Ptch mediate cilium-dependent Hh activity, while Boc, or a similar protein, Cdo, mediate cilium-independent Hh activity?
Perhaps a more philosophical question is, Why is the cilium required for Hh signaling at all? As noted above, work from Drosophila has shown that organisms can clearly solve the problem of Hh signal transduction through other means. Cilia have evolutionarily ancient roles in sensing and transducing environmental information, extending back to our unicellular ancestors. Upon achieving multicellularity, there would have been new demands for coordinating cellular behaviors. Perhaps there was a low barrier for adapting the signaling machinery already present in the cilium for these purposes. If so, ciliary transduction of Hh signals may represent a truly ancient form of intercellular communication likely to be well-represented throughout the animal kingdom.
ACKNOWLEDGEMENTS
The authors acknowledge the support of grants from the NIH (RO1AR054396), the Burroughs Wellcome Fund, and the Sandler Family Supporting Foundation to J.F.R. S.Y.W. acknowledges the support of the A.P. Giannini Foundation, the Herbert W. Boyer Fund, and the American Cancer Society.
REFERENCES
- Adams NA, Awadein A, Toma HS. The retinal ciliopathies. Ophthalmic Genet. 2007;28:113–125. doi: 10.1080/13816810701537424. [DOI] [PubMed] [Google Scholar]
- Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. doi: 10.1016/j.cell.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, Mah AK, Johnsen RC, Cavender JC, Lewis RA, Leroux MR, Beales PL, Katsanis N. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–633. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- Arima T, Shibata Y, Yamamoto T. A deep-etching study of the guinea pig tracheal cilium with special reference to the ciliary transitional region. J Ultrastruct Res. 1984;89:34–41. doi: 10.1016/s0022-5320(84)80021-0. [DOI] [PubMed] [Google Scholar]
- Aszterbaum M, Epstein J, Oro A, Douglas V, LeBoit PE, Scott MP, Epstein EH., Jr Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med. 1999;5:1285–1291. doi: 10.1038/15242. [DOI] [PubMed] [Google Scholar]
- Athar M, Tang X, Lee JL, Kopelovich L, Kim AL. Hedgehog signalling in skin development and cancer. Exp Dermatol. 2006;15:667–677. doi: 10.1111/j.1600-0625.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- Aza-Blanc P, Ramírez-Weber FA, Laget MP, Schwartz C, Kornberg TB. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89:1043–1053. doi: 10.1016/s0092-8674(00)80292-5. [DOI] [PubMed] [Google Scholar]
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- Bai CB, Joyner AL. Gli1 can rescue the in vivo function of Gli2. Development. 2001;128:5161–5172. doi: 10.1242/dev.128.24.5161. [DOI] [PubMed] [Google Scholar]
- Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by Hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6:103–115. doi: 10.1016/s1534-5807(03)00394-0. [DOI] [PubMed] [Google Scholar]
- Benzing T, Walz G. Cilium-generated signaling: a cellular GPS? Curr Opin Nephrol Hypertens. 2006;15:245–249. doi: 10.1097/01.mnh.0000222690.53970.ca. [DOI] [PubMed] [Google Scholar]
- Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. Repression of Smoothened by Patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 2006;4:1397–1410. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol. 1996;6:298–304. doi: 10.1016/s0960-9822(02)00480-3. [DOI] [PubMed] [Google Scholar]
- Bonnafe E, Touka M, AitLounis A, Baas D, Barras E, Ucla C, Moreau A, Flamant F, Dubruille R, Couble P, Collignon J, Durand B, Reith W. The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification. Mol Cell Biol. 2004;24:4417–4427. doi: 10.1128/MCB.24.10.4417-4427.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgakov OV, Eggenschwiler JT, Hong DH, Anderson KV, Li T. FKBP8 is a negative regulator of mouse sonic hedgehog signaling in neural tissues. Development. 2004;131:2149–2159. doi: 10.1242/dev.01122. [DOI] [PubMed] [Google Scholar]
- Bürglin TR. Warthog and groundhog, novel families related to hedgehog. Curr Biol. 1996;6:1047–1050. doi: 10.1016/s0960-9822(02)70659-3. [DOI] [PubMed] [Google Scholar]
- Carlén B, Stenram U. Primary ciliary dyskinesia: a review. Ultrastruct Pathol. 2005;29:217–220. doi: 10.1080/01913120590951220. [DOI] [PubMed] [Google Scholar]
- Casali A, Struhl G. Reading the Hedgehog morphogen gradient by measuring the ratio of bound to unbound Patched protein. Nature. 2004;431:76–80. doi: 10.1038/nature02835. [DOI] [PubMed] [Google Scholar]
- Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Chamberlain CE, Jeong J, Guo C, Allen BL, McMahon AP. Notochord-derived Shh concentrates in close association with the apically positioned basal body in neural target cells and forms a dynamic gradient during neural patterning. Development. 2008;135:1097–1106. doi: 10.1242/dev.013086. [DOI] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Chen CH, von Kessler DP, Park W, Wang B, Ma Y, Beachy PA. Nuclear trafficking of Cubitus interruptus in the transcriptional regulation of Hedgehog target gene expression. Cell. 1999;98:305–316. doi: 10.1016/s0092-8674(00)81960-1. [DOI] [PubMed] [Google Scholar]
- Chen MH, Gao N, Kawakami T, Chuang PT. Mice deficient in the fused homolog do not exhibit phenotypes indicative of perturbed hedgehog signaling during embryonic development. Mol Cell Biol. 2005;25:7042–7053. doi: 10.1128/MCB.25.16.7042-7053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Ren XR, Nelson CD, Barak LS, Chen JK, Beachy PA, de Sauvage F, Lefkowitz RJ. Activity-dependent internalization of smoothened mediated by beta-arrestin 2 and GRK2. Science. 2004;306:2257–2260. doi: 10.1126/science.1104135. [DOI] [PubMed] [Google Scholar]
- Cheng SY, Bishop JM. Suppressor of Fused represses Gli-mediated transcription by recruiting the SAP18-mSin3 corepressor complex. Proc Natl Acad Sci USA. 2002;99:5442–5447. doi: 10.1073/pnas.082096999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Cho A, Ko HW, Eggenschwiler JT. FKBP8 cell-autonomously controls neural tube patterning through a Gli2- and Kif3a-dependent mechanism. Dev Biol. 2008;321:27–39. doi: 10.1016/j.ydbio.2008.05.558. [DOI] [PubMed] [Google Scholar]
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AF, Yu KP, Brueckner M, Brailey LL, Johnson L, McGrath JM, Bale AE. Cardiac and CNS defects in a mouse with targeted disruption of suppressor of fused. Development. 2005;132:4407–4417. doi: 10.1242/dev.02021. [DOI] [PubMed] [Google Scholar]
- Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, Porter FD, Beachy PA. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 2003;33:508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- Corcoran RB, Scott MP. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci USA. 2006;103:8408–8413. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowson AN. Basal cell carcinoma: biology, morphology and clinical implications. Mod Pathol. 2006;19:S127–S147. doi: 10.1038/modpathol.3800512. [DOI] [PubMed] [Google Scholar]
- Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol. 2001;11:1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- Denef N, Neubüser D, Perez L, Cohen SM. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell. 2000;102:521–531. doi: 10.1016/s0092-8674(00)00056-8. [DOI] [PubMed] [Google Scholar]
- Dessaud E, Yang LL, Hill K, Cox B, Ulloa F, Ribeiro A, Mynett A, Novitch BG, Briscoe J. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450:717–720. doi: 10.1038/nature06347. [DOI] [PubMed] [Google Scholar]
- Ding Q, Motoyama J, Gasca S, Mo R, Sasaki H, Rossant J, Hui CC. Diminished Sonic hedgehog signaling and lack of floor plate differentiation in Gli2 mutant mice. Development. 1998;125:2533–2543. doi: 10.1242/dev.125.14.2533. [DOI] [PubMed] [Google Scholar]
- Ding Q, Fukami S, Meng X, Nishizaki Y, Zhang X, Sasaki H, Dlugosz A, Nakafuku M, Hui C. Mouse suppressor of fused is a negative regulator of sonic hedgehog signaling and alters the subcellular distribution of Gli1. Curr Biol. 1999;9:1119–1122. doi: 10.1016/s0960-9822(99)80482-5. [DOI] [PubMed] [Google Scholar]
- Dussillol-Godar F, Brissard-Zahraoui J, Limbourg-Bouchon B, Boucher D, Fouix S, Lamour-Isnard C, Plessis A, Busson D. Modulation of the Suppressor of fused protein regulates the Hedgehog signaling pathway in Drosophila embryo and imaginal discs. Dev Biol. 2006;291:53–66. doi: 10.1016/j.ydbio.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Dwyer JR, Sever N, Carlson M, Nelson SF, Beachy PA, Parhami F. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J Biol Chem. 2007;282:8959–8968. doi: 10.1074/jbc.M611741200. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggenschwiler JT, Espinoza E, Anderson KV. Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature. 2001;412:194–198. doi: 10.1038/35084089. [DOI] [PubMed] [Google Scholar]
- Ericson J, Briscoe J, Rashbass P, van Heyningen V, Jessell TM. Graded sonic hedgehog signaling and the specification of cell fate in the ventral neural tube. Cold Spring Harb Symp Quant Biol. 1997;62:451–466. [PubMed] [Google Scholar]
- Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost HJ. Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development. 2005;132:1247–1260. doi: 10.1242/dev.01663. [DOI] [PubMed] [Google Scholar]
- Farzan SF, Ascano M, Jr, Ogden SK, Sanial M, Brigui A, Plessis A, Robbins DJ. Costal2 Functions as a Kinesin-like Protein in the Hedgehog Signal Transduction Pathway. Curr Biol. 2008;18:1215–1220. doi: 10.1016/j.cub.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante MI, Zullo A, Barra A, Bimonte S, Messaddeq N, Studer M, Dollé P, Franco B. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat Genet. 2006;38:112–117. doi: 10.1038/ng1684. [DOI] [PubMed] [Google Scholar]
- Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, Beachy PA, Beales PL, DeMartino GN, Fisher S, Badano JL, Katsanis N. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- Gilula NB, Satir P. The ciliary necklace. A ciliary membrane specialization. J Cell Biol. 1972;53:494–509. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the hedgehog/patched signaling pathway from flies to mice: induction of a mouse patched gene by Hedgehog. Genes Dev. 1996;10:301–312. doi: 10.1101/gad.10.3.301. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Milenković L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- Hahn H, Wojnowski L, Zimmer AM, Hall J, Miller G, Zimmer A. Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. Nat Med. 1998;4:619–622. doi: 10.1038/nm0598-619. [DOI] [PubMed] [Google Scholar]
- Han YG, Dlugosz AA, Alvarez-Buylla A. Double-edged roles of primary cilia in oncogenic hedgehog signaling in medulloblastoma. Manuscript submitted. [Google Scholar]
- Han YG, Kwok BH, Kernan MJ. Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr Biol. 2003;13:1679–1686. doi: 10.1016/j.cub.2003.08.034. [DOI] [PubMed] [Google Scholar]
- Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neuroscience. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:480–488. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepker J, Wang QT, Motzny CK, Holmgren R, Orenic TV. Drosophila cubitus interruptus forms a negative feedback loop with patched and regulates expression of Hedgehog target genes. Development. 1997;124:549–558. doi: 10.1242/dev.124.2.549. [DOI] [PubMed] [Google Scholar]
- Hong DH, Yue G, Adamian M, Li T. Retinitis pigmentosa GTPase regulator (RPGRr)-interacting protein is stably associated with the photoreceptor ciliary axoneme and anchors RPGR to the connecting cilium. J Biol Chem. 2001;276:12091–12099. doi: 10.1074/jbc.M009351200. [DOI] [PubMed] [Google Scholar]
- Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- Houde C, Dickinson RJ, Houtzager VM, Cullum R, Montpetit R, Metzler M, Simpson EM, Roy S, Hayden MR, Hoodless PA, Nicholson DW. Hippi is essential for node cilia assembly and Sonic hedgehog signaling. Development. 2006;300:523–533. doi: 10.1016/j.ydbio.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Cilia and hedgehog responsiveness in the mouse. Proc Natl Acad Sci USA. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development. 2006;133:3–14. doi: 10.1242/dev.02169. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Hui CC, Joyner AL. A mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat Genet. 1993;3:241–246. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- Izraeli S, Lowe LA, Bertness VL, Campaner S, Hahn H, Kirsch IR, Kuehn MR. Genetic evidence that Sil is required for the Sonic Hedgehog response pathway. Genesis. 2001;31:72–77. doi: 10.1002/gene.10004. [DOI] [PubMed] [Google Scholar]
- Jeong J, McMahon AP. Growth and pattern of the mammalian neural tube are governed by partially overlapping feedback activities of the hedgehog antagonists patched 1 and Hhip1. Development. 2005;132:143–154. doi: 10.1242/dev.01566. [DOI] [PubMed] [Google Scholar]
- Jia J, Tong C, Wang B, Luo L, Jiang J. Hedgehog signalling activity of Smoothened requires phosphorylation by protein kinase A and casein kinase I. Nature. 2004;432:1045–1050. doi: 10.1038/nature03179. [DOI] [PubMed] [Google Scholar]
- Jiang J, Struhl G. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391:493–496. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- Kaesler S, Lüscher B, Rüther U. Transcriptional activity of GLI1 is negatively regulated by protein kinase A. Biol Chem. 2000;381:545–551. doi: 10.1515/BC.2000.070. [DOI] [PubMed] [Google Scholar]
- Kelley RL, Roessler E, Hennekam RC, Feldman GL, Kosaki K, Jones MC, Palumbos JC, Muenke M. Holoprosencephaly in RSH/Smith-Lemli-Opitz syndrome: does abnormal cholesterol metabolism affect the function of Sonic Hedgehog? Am J Med Genet. 1996;66:478–484. doi: 10.1002/(SICI)1096-8628(19961230)66:4<478::AID-AJMG22>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Kent D, Bush EW, Hooper JE. Roadkill attenuates Hedgehog responses through degradation of Cubitus interruptus. Development. 2006;133:2001–2010. doi: 10.1242/dev.02370. [DOI] [PubMed] [Google Scholar]
- King N, Westbrook MJ, Young SL, Kuo A, Abedin M, Chapman J, Fairclough S, Hellsten U, Isogai Y, Letunic I, Marr M, Pincus D, Putnam N, Rokas A, Wright KJ, Zuzow R, Dirks W, Good M, Goodstein D, Lemons D, Li W, Lyons JB, Morris A, Nichols S, Richter DJ, Salamov A, Sequencing JG, Bork P, Lim WA, Manning G, Miller WT, McGinnis W, Shapiro H, Tjian R, Grigoriev IV, Rokhsar D. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature. 2008;451:783–788. doi: 10.1038/nature06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koudijs MJ, den Broeder MJ, Keijser A, Wienholds E, Houwing S, van Rooijen EM, Geisler R, van Eeden FJ. The zebrafish mutants dre, uki, and lep encode negative regulators of the hedgehog signaling pathway. PLoS Genet. 2005;1:e19. doi: 10.1371/journal.pgen.0010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ. Beta-arrestin-mediated localization of Smoothened to the primary cilium. Science. 2008;320:1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak PA, Wassif CA, Kratz L, Cozma D, Kovárová M, Harris G, Grinberg A, Yang Y, Hunter AG, Tsokos M, Kelley RI, Porter FD. Lathosterolosis: an inborn error of human and murine cholesterol synthesis due to lathosterol 5-desaturase deficiency. Hum Mol Genet. 2003;12:1631–1641. doi: 10.1093/hmg/ddg172. [DOI] [PubMed] [Google Scholar]
- Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- Lam CW, Xie J, To KF, Ng HK, Lee KC, Yuen NW, Lim PL, Chan LY, Tong SF, McCormick F. A frequent activated smoothened mutation in sporadic basal cell carcinomas. Oncogene. 1999;18:833–836. doi: 10.1038/sj.onc.1202360. [DOI] [PubMed] [Google Scholar]
- Lei Q, Zelman AK, Kuang E, Li S, Matise MP. Transduction of graded Hedgehog signaling by a combination of Gli2 and Gli3 activator functions in the developing spinal cord. Development. 2004;131:3593–3604. doi: 10.1242/dev.01230. [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell. 2005;9:855–861. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA. 2003;100:5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litingtung Y, Dahn RD, Li Y, Fallon JF, Chiang C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature. 2002;418:979–983. doi: 10.1038/nature01033. [DOI] [PubMed] [Google Scholar]
- Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cao X, Jiang J, Jia J. Fused-Costal2 protein complex regulates Hedgehog-induced Smo phosphorylation and cell-surface accumulation. Genes Dev. 2007;21:1949–1963. doi: 10.1101/gad.1557407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755–1759. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- Lum L, Zhang C, Oh S, Mann RK, von Kessler DP, Taipale J, Weis-Garcia F, Gong R, Wang B, Beachy PA. Hedgehog signal transduction via Smoothened association with a cytoplasmic complex scaffolded by the atypical kinesin, Costal-2. Mol Cell. 2003;12:1261–1274. doi: 10.1016/s1097-2765(03)00426-x. [DOI] [PubMed] [Google Scholar]
- Mans DA, Voest EE, Giles RH. All along the watchtower: Is the cilium a tumor suppressor organelle? Biochim Biophys Acta. 2008 doi: 10.1016/j.bbcan.2008.02.002. In Press. [DOI] [PubMed] [Google Scholar]
- Marcotullio LD, Ferretti E, Greco A, Smaele ED, Po A, Sico MA, Alimandi M, Giannini G, Maroder M, Screpanti I, Gulino A. Numb is a suppressor of Hedgehog signalling and targets Gli1 for Itch-dependent ubiquitination. Nat Cell Biol. 2006;8:1415–1423. doi: 10.1038/ncb1510. [DOI] [PubMed] [Google Scholar]
- Marigo V, Tabin CJ. Regulation of patched by sonic hedgehog in the developing neural tube. Proc Natl Acad Sci USA. 1996;93:9346–9351. doi: 10.1073/pnas.93.18.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WF, Nonaka S. Cilia: tuning in to the cell's antenna. Curr Biol. 2006;16:R604–R614. doi: 10.1016/j.cub.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci USA. 1999;96:5043–5048. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí E, Bumcrot DA, Takada R, McMahon AP. Requirement of 19K form of Sonic hedgehog for induction of distinct ventral cell types in CNS explants. Nature. 1995;375:322–325. doi: 10.1038/375322a0. [DOI] [PubMed] [Google Scholar]
- Matise MP, Epstein DJ, Park HL, Platt KA, Joyner AL. Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development. 1998;125:2759–2770. doi: 10.1242/dev.125.15.2759. [DOI] [PubMed] [Google Scholar]
- May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, Peterson AS. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol. 2005;287:378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- Meloni AR, Fralish GB, Kelly P, Salahpour A, Chen JK, Wechsler-Reya RJ, Lefkowitz RJ, Caron MG. Smoothened signal transduction is promoted by G protein-coupled receptor kinase 2. Mol Cell Biol. 2006;26:7550–7560. doi: 10.1128/MCB.00546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant M, Evangelista M, Luoh SM, Frantz GD, Chalasani S, Carano RA, van Hoy M, Ramirez J, Ogasawara AK, McFarland LM, Filvaroff EH, French DM, de Sauvage FJ. Loss of the serine/threonine kinase fused results in postnatal growth defects and lethality due to progressive hydrocephalus. Mol Cell Biol. 2005;25:7054–7068. doi: 10.1128/MCB.25.16.7054-7068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer NP, Roelink H. The amino-terminal region of Gli3 antagonizes the Shh response and acts in dorsoventral fate specification in the developing spinal cord. Dev Biol. 2003;257:343–355. doi: 10.1016/s0012-1606(03)00065-4. [DOI] [PubMed] [Google Scholar]
- Milenkovic L, Goodrich LV, Higgins KM, Scott MP. Mouse patched1 controls body size determination and limb patterning. Development. 1999;126:4431–4440. doi: 10.1242/dev.126.20.4431. [DOI] [PubMed] [Google Scholar]
- Motoyama J, Milenkovic L, Iwama M, Shikata Y, Scott MP, Hui CC. Differential requirement for Gli2 and Gli3 in ventral neural cell fate specification. Dev Biol. 2003;259:150–161. doi: 10.1016/s0012-1606(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peränen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Shiratori H, Saijoh Y, Hamada H. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature. 2002;418:96–99. doi: 10.1038/nature00849. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Ocbina PJ, Anderson KV. Intraflagellar transport, cilia, and mammalian Hedgehog signaling: Analysis in mouse embryonic fibroblasts. Dev Dyn. 2008;237:2030–2038. doi: 10.1002/dvdy.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlmeyer JT, Kalderon D. Hedgehog stimulates maturation of Cubitus interruptus into a labile transcriptional activator. Nature. 1998;396:749–753. doi: 10.1038/25533. [DOI] [PubMed] [Google Scholar]
- Okada A, Charron F, Morin S, Shin DS, Wong K, Fabre PJ, Tessier-Lavigne M, McConnell SK. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature. 2006;444:369–373. doi: 10.1038/nature05246. [DOI] [PubMed] [Google Scholar]
- Orozco JT, Wedaman KP, Signor D, Brown H, Rose L, Scholey JM. Movement of motor and cargo along cilia. Nature. 1999;398:674. doi: 10.1038/19448. [DOI] [PubMed] [Google Scholar]
- Ou G, Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature. 2005;436:583–587. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- Paces-Fessy M, Boucher D, Petit E, Paute-Briand S, Blanchet-Tournier MF. The negative regulator of Gli, Suppressor of fused (Sufu), interacts with SAP18, Galectin3 and other nuclear proteins. Biochem J. 2004;378:353–362. doi: 10.1042/BJ20030786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Wang Q, Snell WJ. Cilium-generated signaling and cilia-related disorders. Lab Invest. 2005;85:452–463. doi: 10.1038/labinvest.3700253. [DOI] [PubMed] [Google Scholar]
- Pan Y, Bai CB, Joyner AL, Wang B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol. 2006;26:3365–3377. doi: 10.1128/MCB.26.9.3365-3377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M, Joyner AL. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development. 2000;127:1593–1605. doi: 10.1242/dev.127.8.1593. [DOI] [PubMed] [Google Scholar]
- Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003;3:903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Wilkerson CG, Witman GB. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT) J Cell Biol. 1998;141:979–992. doi: 10.1083/jcb.141.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse RV, 2nd, Collier LS, Scott MP, Tabin CJ. Vertebrate homologs of Drosophila suppressor of fused interact with the gli family of transcriptional regulators. Dev Biol. 1999;212:323–336. doi: 10.1006/dbio.1999.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perler FB. Protein splicing of inteins and hedgehog autoproteolysis: structure, function, and evolution. Cell. 1998;92:1–4. doi: 10.1016/s0092-8674(00)80892-2. [DOI] [PubMed] [Google Scholar]
- Persson M, Stamataki D, te Welscher P, Andersson E, Böse J, Rüther U, Ericson J, Briscoe J. Dorsal-ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity. Genes Dev. 2002;16:2865–2878. doi: 10.1101/gad.243402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Siuda E, Henderson S, Segil M, Vaananen H, Sassaroli M. Distinct mutants of retrograde intraflagellar transport (IFT) share similar morphological and molecular defects. J Cell Biol. 1998;143:1591–1601. doi: 10.1083/jcb.143.6.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Bower R, Knott JA, Byrd P, Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol Cell Biol. 1999;10:693–712. doi: 10.1091/mbc.10.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Préat T. Characterization of Suppressor of fused, a complete suppressor of the fused segment polarity gene of Drosophila melanogaster. Genetics. 1992;132:725–736. doi: 10.1093/genetics/132.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MA, Kalderon D. Proteolysis of cubitus interruptus in Drosophila requires phosphorylation by protein kinase A. Development. 1999;126:4331–4339. doi: 10.1242/dev.126.19.4331. [DOI] [PubMed] [Google Scholar]
- Price MA, Kalderon D. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by Glycogen Synthase Kinase 3 and Casein Kinase 1. Cell. 2002;108:823–835. doi: 10.1016/s0092-8674(02)00664-5. [DOI] [PubMed] [Google Scholar]
- Quarmby LM, Parker JD. Cilia and the cell cycle? J Cell Biol. 2005;169:707–710. doi: 10.1083/jcb.200503053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana AA, Barbera JP, Rodriguez TA, Lynch D, Hirst E, Smith JC, Beddington RS. Targeted deletion of the novel cytoplasmic dynein mD2LIC disrupts the embryonic organiser, formation of the body axes and specification of ventral cell fates. Development. 2004;131:4999–5007. doi: 10.1242/dev.01389. [DOI] [PubMed] [Google Scholar]
- Reiter JF, Skarnes WC. Tectonic, a novel regulator of the Hedgehog pathway required for both activation and inhibition. Genes Dev. 2006;20:22–27. doi: 10.1101/gad.1363606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Robbins DJ, Nybakken KE, Kobayashi R, Sisson JC, Bishop JM, Thérond PP. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell. 1997;90:225–234. doi: 10.1016/s0092-8674(00)80331-1. [DOI] [PubMed] [Google Scholar]
- Roelink H, Augsburger A, Heemskerk J, Korzh V, Norlin S, Ruiz i Altaba A, Tanabe Y, Placzek M, Edlund T, Jessell TM, Dodd J. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell. 1994;76:761–775. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA, Jessell TM. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995;81:445–455. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates Hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Romio L, Fry AM, Winyard PJ, Malcolm S, Woolf AS, Feather SA. OFD1 is a centrosomal/basal body protein expressed during mesenchymal-epithelial transition in human nephrogenesis. J Am Soc Nephrol. 2004;15:2556–2568. doi: 10.1097/01.ASN.0000140220.46477.5C. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- Ruel L, Gallet A, Raisin S, Truchi A, Staccini-Lavenant L, Cervantes A, Thérond PP. Phosphorylation of the atypical kinesin Costal2 by the kinase Fused induces the partial disassembly of the Smoothened-Fused-Costal2-Cubitus interruptus complex in Hedgehog signalling. Development. 2007;134:3677–3689. doi: 10.1242/dev.011577. [DOI] [PubMed] [Google Scholar]
- Ruel L, Rodriguez R, Gallet A, Lavenant-Staccini L, Thérond PP. Stability and association of Smoothened, Costal2 and Fused with Cubitus interruptus are regulated by Hedgehog. Nat Cell Biol. 2003;5:907–913. doi: 10.1038/ncb1052. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A. Gli proteins encode context-dependent positive and negative functions: implications for development and disease. Development. 1999;126:3205–3216. doi: 10.1242/dev.126.14.3205. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Sanchez P, Dahmane N. Gli and Hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361–372. doi: 10.1038/nrc796. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Blair HJ, Rodriguez-Andres ME, Blanco MJ, Wilson A, Liu YN, Miles C, Peters H, Goodship JA. Evc is a positive mediator of Ihh-regulated bone growth that localises at the base of chondrocyte cilia. Development. 2007;134:2903–2912. doi: 10.1242/dev.007542. [DOI] [PubMed] [Google Scholar]
- Salisbury JL. Primary cilia: putting sensors together. Curr Biol. 2004;14:R765–R767. doi: 10.1016/j.cub.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Sarpal R, Todi SV, Sivan-Loukianova E, Shirolikar S, Subramanian N, Raff EC, Erickson JW, Ray K, Eberl DF. Drosophila KAP interacts with the kinesin II motor subunit KLP64D to assemble chordotonal sensory cilia, but not sperm tails. Curr Biol. 2003;13:1687–1696. doi: 10.1016/j.cub.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Nishizaki Y, Hui CC, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999;126:3915–3924. doi: 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- Schafer JC, Haycraft CJ, Thomas JH, Yoder BK, Swoboda P. XBX-1 encodes a dynein light intermediate chain required for retrograde intraflagellar transport and cilia assembly in Caenorhabditis elegans. Mol Biol Cell. 2003;14:2057–2070. doi: 10.1091/mbc.E02-10-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauerte HE, van Eeden FJ, Fricke C, Odenthal J, Strähle U, Haffter P. Sonic hedgehog is not required for the induction of medial floor plate cells in the zebrafish. Development. 1998;125:2983–2993. doi: 10.1242/dev.125.15.2983. [DOI] [PubMed] [Google Scholar]
- Scherz PJ, McGlinn E, Nissim S, Tabin CJ. Extended exposure to Sonic hedgehog is required for patterning the posterior digits of the vertebrate limb. Dev Biol. 2007;308:343–354. doi: 10.1016/j.ydbio.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Sheng H, Goich S, Wang A, Grachtchouk M, Lowe L, Mo R, Lin K, de Sauvage FJ, Sasaki H, Hui CC, Dlugosz AA. Dissecting the oncogenic potential of Gli2: deletion of an NH2-terminal fragment alters skin tumor phenotype. Cancer Res. 2002;62:5308–5316. [PubMed] [Google Scholar]
- Simons M, Walz G. Polycystic kidney disease: cell division without a c (l)ue? Kidney Int. 2006;70:854–864. doi: 10.1038/sj.ki.5001534. [DOI] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Sisson JC, Ho KS, Suyama K, Scott MP. Costal2, a novel kinesin-related protein in the Hedgehog signaling pathway. Cell. 1997;90:235–245. doi: 10.1016/s0092-8674(00)80332-3. [DOI] [PubMed] [Google Scholar]
- Smelkinson MG, Kalderon D. Processing of the Drosophila hedgehog signaling effector Ci-155 to the repressor Ci-75 is mediated by direct binding to the SCF component Slimb. Curr Biol. 2006;16:110–116. doi: 10.1016/j.cub.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Snell EA, Brooke NM, Taylor WR, Casane D, Philippe H, Holland PW. An unusual choanoflagellate protein released by Hedgehog autocatalytic processing. Proc Biol Sci. 2006;273:401–407. doi: 10.1098/rspb.2005.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow JJ, Ou G, Gunnarson AL, Walker MR, Zhou HM, Brust-Mascher I, Scholey JM. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol. 2004;6:1109–1113. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DM, Murone M, Luoh S, Ye W, Armanini MP, Gurney A, Phillips H, Brush J, Goddard A, de Sauvage FJ, Rosenthal A. Characterization of the human suppressor of fused, a negative regulator of the zinc-finger transcription factor Gli. J Cell Sci. 1999;112:4437–4448. doi: 10.1242/jcs.112.23.4437. [DOI] [PubMed] [Google Scholar]
- Storm van's Gravesande K, Omran H. Primary ciliary dyskinesia: clinical presentation, diagnosis and genetics. Ann Med. 2005;37:439–449. doi: 10.1080/07853890510011985. [DOI] [PubMed] [Google Scholar]
- Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development. 2004;131:4085–4093. doi: 10.1242/dev.01240. [DOI] [PubMed] [Google Scholar]
- Svärd J, Heby-Henricson K, Persson-Lek M, Rozell B, Lauth M, Bergström A, Ericson J, Toftgård R, Teglund S. Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev Cell. 2006;10:187–197. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418:892–897. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- Tang JT, So PL, Epstein EH., Jr Novel Hedgehog pathway targets against basal cell carcinoma. Toxicol Appl Pharmacol. 2007;224:257–264. doi: 10.1016/j.taap.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay SY, Ingham PW, Roy S. A homologue of the Drosophila kinesin-like protein Costal2 regulates Hedgehog signal transduction in the vertebrate embryo. Development. 2005;132:625–634. doi: 10.1242/dev.01606. [DOI] [PubMed] [Google Scholar]
- te Welscher P, Zuniga A, Kuijper S, Drenth T, Goedemans HJ, Meijlink F, Zeller R. Progression of vertebrate limb development through Shh-mediated counteraction of Gli3. Science. 2002;298:827–830. doi: 10.1126/science.1075620. [DOI] [PubMed] [Google Scholar]
- Tempé D, Casas M, Karaz S, Blanchet-Tournier MF, Concordet JP. Multisite protein kinase A and glycogen synthase kinase 3beta phosphorylation leads to Gli3 ubiquitination by SCFbetaTrCP. Mol Cell Biol. 2006;26:4316–4326. doi: 10.1128/MCB.02183-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin JL, Beales PL. Bardet-Biedl syndrome: beyond the cilium. Pediatr Nephrol. 2007;22:926–936. doi: 10.1007/s00467-007-0435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town T, Breunig JJ, Sarkisian MR, Spilianakis C, Ayoub AE, Liu X, Ferrandino AF, Gallagher AR, Li MO, Rakic P, Flavell RA. The stumpy gene is required for mammalian ciliogenesis. Proc Natl Acad Sci USA. 2008;105:2853–2858. doi: 10.1073/pnas.0712385105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, Chesebro AL, Qiu H, Scherz PJ, Shah JV, Yoder BK, Beier DR. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet. 2008;40:403–410. doi: 10.1038/ng.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004;42:703–716. doi: 10.1016/s0896-6273(04)00268-5. [DOI] [PubMed] [Google Scholar]
- Varjosalo M, Li SP, Taipale J. Divergence of hedgehog signal transduction mechanism between Drosophila and mammals. Dev Cell. 2006;10:177–186. doi: 10.1016/j.devcel.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci USA. 2006;103:18556–18561. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierkotten J, Dildrop R, Peters T, Wang B, Ruther U. Ftm is a novel basal body protein of cilia involved in Shh signalling. Development. 2007;134:2569–2577. doi: 10.1242/dev.003715. [DOI] [PubMed] [Google Scholar]
- Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Nature. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- Wang B, Li Y. Evidence for the direct involvement of {beta}TrCP in Gli3 protein processing. Proc Natl Acad Sci USA. 2006;103:33–38. doi: 10.1073/pnas.0509927103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ruther U, Wang B. The Shh-independent activator function of the full-length Gli3 protein and its role in vertebrate limb digit patterning. Dev Biol. 2007;305:460–469. doi: 10.1016/j.ydbio.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QT, Holmgren RA. The subcellular localization and activity of Drosophila cubitus interruptus are regulated at multiple levels. Development. 1999;126:5097–5106. doi: 10.1242/dev.126.22.5097. [DOI] [PubMed] [Google Scholar]
- Wang Y, McMahon AP, Allen BL. Shifting paradigms in Hedgehog signaling. Curr Opin Cell Biol. 2007;19:159–165. doi: 10.1016/j.ceb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Wang Y, Price MA. A unique protection signal in Cubitus interruptus prevents its complete proteasomal degradation. Mol Cell Biol. 2008 doi: 10.1128/MCB.00524-08. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigley WC, Fabunmi RP, Lee MG, Marino CR, Muallem S, DeMartino GN, Thomas PJ. Dynamic association of proteasomal machinery with the centrosome. J Cell Biol. 1999;145:481–490. doi: 10.1083/jcb.145.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff C, Roy S, Ingham PW. Multiple muscle cell identities induced by distinct levels and timing of hedgehog activity in the zebrafish embryo. Curr Biol. 2003;13:1169–1181. doi: 10.1016/s0960-9822(03)00461-5. [DOI] [PubMed] [Google Scholar]
- Wong SY, So PL, Dluogsz AA, Epstein EH, Jr., Reiter JF. Primary cilia can both promote and suppress tumorigenesis. Manuscript submitted. [Google Scholar]
- Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, Rosenthal A, Epstein EH, Jr, de Sauvage FJ. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE, Jr, Schafer JA, Balkovetz DF. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol. 2002;282:F541–552. doi: 10.1152/ajprenal.00273.2001. [DOI] [PubMed] [Google Scholar]
- Zhang C, Williams EH, Guo Y, Lum L, Beachy PA. Extensive phosphorylation of Smoothened in Hedgehog pathway activation. Proc Natl Acad Sci USA. 2004;101:17900–17907. doi: 10.1073/pnas.0408093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zhang L, Wang B, Ou CY, Chien CT, Jiang J. A hedgehog-induced BTB protein modulates hedgehog signaling by degrading Ci/Gli transcription factor. Dev Cell. 2006;10:719–729. doi: 10.1016/j.devcel.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhao Y, Tong C, Wang G, Wang B, Jia J, Jiang J. Hedgehog-regulated Costal2-kinase complexes control phosphorylation and proteolytic processing of Cubitus interruptus. Dev Cell. 2005;8:267–278. doi: 10.1016/j.devcel.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Apionishev S, Kalderon D. The contributions of protein kinase A and smoothened phosphorylation to hedgehog signal transduction in Drosophila melanogaster. Genetics. 2006;173:2049–2062. doi: 10.1534/genetics.106.061036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman H. Beitrage zur kenntniss einiger drusen und epithelien. Arch Mikr Anat. 1898;52:552–706. [Google Scholar]


