Abstract
Phosphorylation of S880 within the GluR2 C-terminus has been reported to promote endocytosis of AMPA receptors (AMPARs) by preventing GluR2 interaction with the putative synaptic anchoring proteins GRIP and ABP. It is not yet established however, whether S880 phosphorylation induces removal of AMPARs from synaptic sites, and the trafficking of phosphorylated GluR2 subunits with surface and endocytosed GluR2 has not been directly compared within the same intact neurons. Here we show that phosphorylation of GluR2 subunits by PKC activated with phorbol esters is compartmentally restricted to receptors located at the cell surface. Endogenous AMPARs containing S880-phosphorylated GluR2 remained highly synaptic and colocalized with post-synaptic markers to the same extent as AMPARs which did not contain S880-phosphorylated GluR2. Moreover, following S880 phosphorylation, exogenous GluR2 homomers were found specifically at the cell surface and did not cotraffic with the internalized endosomal GluR2 population. We also show that GluR2 is endogenously phosphorylated by a constitutively active kinase pharmacologically related to PKC, and this phosphorylation is opposed by the protein phosphatase PP1. Our results demonstrate a population of hippocampal AMPARs which do not require interaction with GRIP/ABP for synaptic anchorage.
Keywords: GluR2 phosphorylation, AMPA receptor trafficking, mutagenesis, synaptic plasticity, Sindbis virus, primary neuronal cultures
INTRODUCTION
Dynamic modification of the synaptic abundance of Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) receptors has been shown to underlie activity-dependent synaptic plasticity at glutamatergic synapses (Beattie et al., 2000; Carroll et al., 1999; Isaac et al., 1995; Liao et al., 1995; Liao et al., 1999; Lu et al., 2001; Matsuda et al., 2000; Shi et al., 1999; Xia et al., 2000). AMPA receptors (AMPARs) are mainly heterotetrameric channels assembled from subunits GluR1-GluR4 (Hollmann and Heinemann, 1994). The intracellular C-terminal domain of GluR2 contains a PDZ-binding sequence which interacts with proteins implicated in regulating the synaptic anchorage of AMPARs. In particular, the PDZ proteins GRIP and ABP have been proposed to function as scaffolds that anchor AMPA receptors at postsynaptic sites (Dong et al., 1997; Matsuda et al., 2000; Osten et al., 2000; Srivastava et al., 1998). PKC activated with phorbol esters can phosphorylate S880 within the GluR2 C-terminal PDZ binding site and S880 phosphorylation prevents interaction of ABP and GRIP with GluR2 while leaving PICK1 binding intact (Chung et al., 2000; Perez et al., 2001). Consequently, S880 phosphorylation of synaptic GluR2 subunits has been proposed to induce redistribution of AMPARs away from synapses by interfering with the interaction of GluR2 with its synaptic anchoring proteins, resulting in receptor endocytosis as occurs during long term depression (LTD) (Chung et al., 2000; Matsuda et al., 2000; Perez et al., 2001; Xia et al., 2000). AMPAR endocytosis following GluR2 phosphorylation may result simply from severing interaction with anchoring proteins (Matsuda et al., 2000; Xia et al., 2000), or may be mediated by preferential binding of PICK1 to S880 phosphorylated GluR2 (Lu and Ziff, 2005) (Chung et al., 2000; Xia et al., 2000). In accordance with the latter mechanism, peptides which selectively inhibit PICK1/GluR2 interaction can attenuate LTD (Kim et al., 2001; Xia et al., 2000), however not all studies agree with this model (Daw et al., 2000).
A morphological hallmark of AMPAR endocytosis is the translocation of AMPARs away from dendritic spines to intracellular locations within main dendritic shafts and perikarya (Beattie et al., 2000; Lin et al., 2000; Lissin et al., 1999; Matsuda et al., 2000). Such AMPAR translocation has been observed following several different stimuli which promote AMPAR endocytosis including exposure to NMDA (Beattie et al., 2000; Ehlers, 2000), AMPA (Beattie et al., 2000; Lissin et al., 1999) and insulin (Lin et al., 2000). Despite biochemical and physiological reports which implicate GluR2 phosphorylation in the removal of AMPARs from synapses, it remains unclear whether S880 phosphorylation promotes AMPAR endocytosis or transit away from synaptic sites (Braithwaite et al., 2002; Chung et al., 2000; Daw et al., 2000; Kim et al., 2001; Matsuda et al., 2000). Importantly, the cotrafficking of S880-phosphorylated GluR2 with either endocytosed or surface populations of AMPARs has not been directly compared within the same intact neurons. To obtain a better understanding of the role of GluR2 phosphorylation in AMPAR endocytosis we compared the trafficking of S880-phosphorylated GluR2 with endocytosed and surface GluR2 subunits in intact cultured hippocampal neurons.
We show S880 that phosphorylation of GluR2 is compartmentally restricted to receptors located at the cell surface. Following GluR2 phosphorylation by PKC activated with phorbol esters, a significant majority of AMPA receptors remained anchored in the postsynaptic membrane and did not co-traffic with the endocytosed receptor population. Non-phosphorylated receptors however, did co-traffic with the endocytosed receptor population suggesting that the GluR2 receptor subunits that are phosphorylated on S880 may be preferentially retained in the postsynaptic membrane over non-phosphorylated GluR2. Investigating endogenous mechanisms of GluR2 phosphorylation, we show that GluR2 is constitutively phosphorylated by a kinase pharmacologically related to PKC and this phosphorylation is opposed by the protein phosphatase PP1. Our results suggest a population of hippocampal AMPARs that are not dependent upon GRIP/ABP for anchorage in the postsynaptic membrane.
MATERIALS AND METHODS
Expression Plasmids
CD8R2c was constructed according to the following procedure. The ecto and transmembrane domains of human CD8α (a gift from Dan Littman, The Skirball Institute, New York University School of Medicine) were amplified by polymerase chain reaction using the upstream primer 5’-AAGCAGGATCCTGGGGAGCGCGTCAT-3’ and the downstream primer 5’-TCGGAATTCGTGGTTGCAGTAAAGGG-3’. The GluR2 C-terminus was amplified using the upstream primer 5’-ATTGAATTCTGTTACAAGTCAAGGG-3’ and the downstream primer 5’-CTCAAGGATCCCCCCTAAATTTTAAC-3’. Both PCR products were restricted with BamHI and EcoRI and ligated in a single ligation into the BamHI site of pcDNA 3.1 myc/his B(−) (Invitrogen). All myc-GluR2 constructs have been described previously (Greger et al., 2002; Osten et al., 2000).
Immunocytochemistry
Primary hippocampal neurons were cultured as previously described (Osten et al., 2000). Except where otherwise indicated cells undergoing immunocytochemistry were subjected to the following procedure. For cell surface labeling with antibodies while still living in culture, cells were incubated with antibodies (Santa Cruz 9E10 anti-myc monoclonal, 4µg/ml) for 10–15 minutes, washed in PBS, and fixed as above. For acid stripping experiments, cells undergoing surface labeling were washed quickly once in PBS and then placed in ice cold acid stripping buffer (500mM NaCl + 0.2N glacial acetic acid) for 3 min on ice (Beattie et al., 2000). Cells were then washed in PBS and fixed as above. For internalization assays of myc-GluR2 mutants in neurons, living cells were labeled for 10 minutes with 9E10 anti-myc antibody 4µg/ml, acid stripped as above, and fixed in paraformaldehyde/PBS. Cells then blocked and labeled with polyclonal anti-myc antibodies (Santa Cruz) 0.25 µg/ml overnight at 4°C. After washes, cells were permeablized and labeled with secondary antibodies before mounting onto glass slides.
Quantification of pGluR2 and GluR1 with PSD-95
35µm sections of dendrites were imaged by CCD microscopy and individual punctae of pGluR2 or GluR1 were scored blind as either containing or not containing corresponding punctae of PSD-95. To determine the synaptic localization of AMPARs not exposed to TPA, the localization of GluR1 instead of GluR2 was used as an indicator of AMPAR localization because a high percentage of GluR2 is retained in the ER compared with GluR1 (Greger et al., 2002). These GluR2 subunits retained in the ER have therefore never been exposed to the cell surface, thus making them invalid for quantifying AMPAR transit from synaptic compartments. We note that virtually all GluR1 in synaptic locations is complexed with GluR2 as evinced by the non-rectifying current/voltage relationship of endogenous AMPARs at hippocampal synapses and the observed lack of calcium permeability (Dingledine et al., 1999). 9–10 cells were examined per group. Comparison of the colocalization of pGluR2 or GluR1 with PSD-95 were analyzed by ANOVA.
For testing the effect of PKC activation on AMPA-induced redistribution of GluR1 away from PSD-95 punctae neurons were fixed, permeablized, and stained for PSD-95 and total GluR1 following pharmacological treatments. Epifluorescent PSD-95 and GluR1 signals were acquired under identical acquisition parameters for all cells using a 63x objective. All groups were analyzed blind. PSD-95 punctae were identified by computer using a variable intensity selection threshold which best identified PSD-95 punctae. A standard size exclusion threshold excluding punctae over 2000 pixels was then applied to all images and a mask corresponding to the selected punctae was generated. The average measured area of PSD-95 masks was not significantly different between groups (data not shown). GluR1 intensity under PSD-95 masks was then quantified. 10 cells per group per experiment were analyzed and experiments were performed in duplicate. Results were pooled and analyzed by ANOVA.
Quantitative comparison of the localization of surface and internalized myc-GluR2 with phosphorylated myc-GluR2
Neurons infected with myc-GluR2 were labeled live in culture for 10 minutes with monoclonal anti-myc antibodies while simultaneously being administered TPA 100ng/ml. Cells were then either acid stripped or fixed to examine internalized or surface receptor populations, respectively. Following fixation, acid stripped cells were permeablized and labeled overnight at 4°C with anti-phospho-GluR2 (S880) antibodies (Perez et al., 2001). FITC and Rhodamine-conjugated secondary antibodies were applied to the cells the next day. Cells that did not undergo acid stripping were labeled with anti-mouse secondary antibodies prior to permeablization to label the surface population of receptors. After washing, cells were permeablized and labeled overnight with anti-phospho-GluR2 (S880) antibodies overnight at 4°C. FITC-conjugated anti-rabbit antibodies were applied to the cells the next day.
All cells were scanned with a confocal microscope. Gain settings were chosen on a per-cell basis to maximally delineate and include the specific signal generated by the secondary antibodies without saturating the input. Objects to be quantified by the computer were identified based on a single threshold value for the anti-phospho-GluR2 channel (green) which was applied to all cells. The total intensity of internalized or surface myc-GluR2 within these objects was measured by the computer and normalized to the area of the object. The measured values in each group were then normalized to the value of the average for the surface receptor versus phospho-GluR2 group, thus making the average value for this group equal to 100 to aid interpretation. 11–12 cells were examined per group. All experiments were performed in duplicate. Data were compared using the unpaired Student’s t-test.
Quantification of internalization of myc-GluR2 mutants
Comparison of the rates of internalization of myc-GluR2 mutants was performed as follows. Neurons for each group were identified under epifluorescent illumination of the green channel only (e.g. surface expression, without knowledge of the amount of internalization) and selected based on two criteria; 1) cells had to exceed a minimal threshold of surface expression and 2) all neurons had to be spiny in nature and demonstrate intact and generally healthy cellular morphology. Cells for all groups within an experiment were scanned under identical gain settings and the total intensity of each channel within the cell outline was measured. The intensity value of the red channel (internalization) was normalized to the intensity value of the green channel (surface expression). 10–15 cells per group per experiment were analyzed. Experiments were carried out in quadruplicate (except for myc-GluR2(I883E) which was done in triplicate) and the data were compared as a percentage of the mean value of myc-GluR2 internalization within each experiment. Results from the four experiments were pooled and compared by two way analysis of variance (ANOVA) followed by the Bonferroni-Dunn post-hoc test to correct for multiple comparisons.
Biotinylation of Cell Surface Proteins
Cultured hippocampal neurons were treated with TPA (100ng/ml) for 10 minutes in ACSF followed by two incubation steps carried out in ACSF containing Sulfo-NHS-SS-biotin (1mg/ml) without glycine at 37°C for 5 minutes each. Alternatively, cells were treated with TPA and 100nM okadaic acid for 10 minutes, washed in ACSF and then incubated for an additional 20 minutes with okadaic acid at 37°C prior to undergoing biotinylation. Cells were then washed and collected in cold 50mM Tris pH=7.4 + 150mM NaCl (Tris buffer). Cells were resuspended and triturated in 166µl of the same buffer + 1% SDS. Cells then were diluted with 833 µl of Tris buffer supplemented with 1% triton and solublized for an additional 30 minutes at 4°C. Lysates were centrifuged and the supernatants incubated with 40 µl of streptavidin-agarose to isolate biotinylated proteins. Isolated proteins were electrophoresed in SDS-PAGE gels and immunoblotted as indicated. Densitometry values of biotinylated proteins were normalized to the respective values for total expression. Experiments were conducted in duplicate and gave similar results. GluR1 was used to measure total AMPA receptor surface expression instead of GluR2 because of the concern that phosphorylation of S880 within the GluR2 C-terminus would abrogate binding of GluR2/3 C-terminal antibodies to this epitope. GluR2 N-terminal antibodies were similarly unsuitable due to the possibility that the epitope for these antibodies, which is unknown, may contain lysine residues which would be modified by the NHS ester chemistry used for biotinylation.
Quantification of S880 phosphorylation in the Presence of Kinase Inhibitors
Cultured hippocampal neurons were treated for 10 minutes with the indicated kinase inhibitor in ACSF and then this solution was exchanged with ACSF containing the kinase inhibitor and okadaic acid (100nM). After 45 minutes neurons were washed in PBS, lysed in 2X sample buffer with gentle trituration through a 26 gauge needle to sheer genomic DNA, and electrophoresed in SDS-PAGE gels. All experiments involving kinase inhibitors were examined in triplicate or quadruplicate using one-way ANOVA.
RESULTS
Stable synaptic retention of AMPARs following phosphorylation of S880 of GluR2
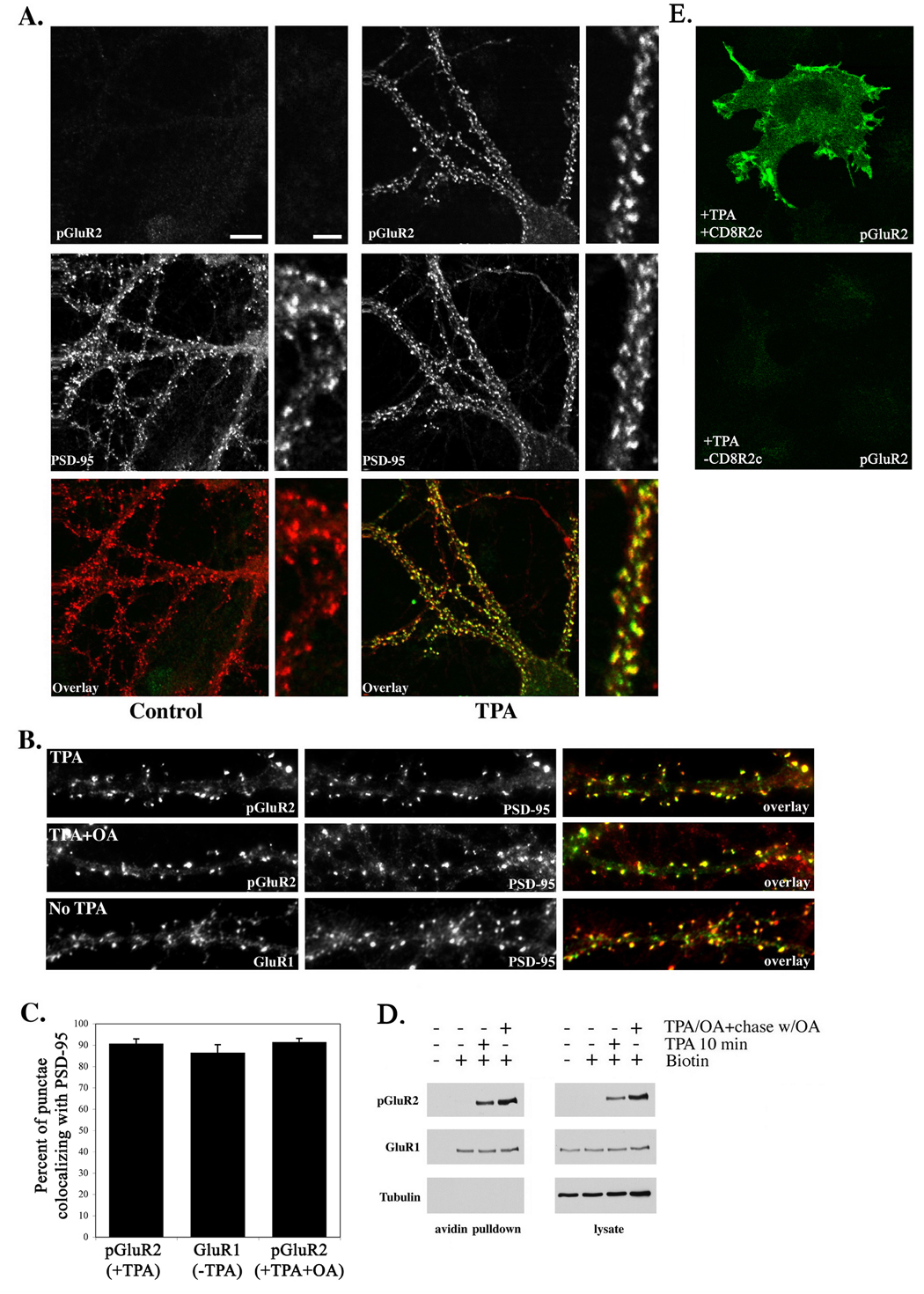
We first investigated whether GluR2-containing AMPARs transit away from synaptic sites following S880 phosphorylation, consistent with other reports of the trafficking of endocytosed AMPARs (Beattie et al., 2000; Lin et al., 2000; Lissin et al., 1999; Matsuda et al., 2000). Using phosphospecific antibodies against phospho-S880 of GluR2 (Fu et al., 2003; Perez et al., 2001) we compared the localization of endogenous phosphorylated GluR2 subunits with that of the postsynaptic protein PSD-95 following a 10 minute exposure to the phorbol ester TPA (100ng/ml) at 37°C under normal culture conditions. A control group received the same volume of vehicle (DMSO) but was not exposed to TPA. Endogenous phosphorylated GluR2 exhibited a punctate distribution (Figure 1a and b) which colocalized extensively with PSD-95 (90.7% ± 2.25 pGluR2 punctae overlapped with PSD-95, mean ± SEM), indicating that, following S880 phosphorylation, GluR2 subunits remain highly synaptic. Untreated cells displayed levels of GluR2 phosphorylation which were not detectable above background (Figure 1a), indicating that basal levels of phosphorylation of GluR2 are low. The extent of colocalization of S880-phosphorylated GluR2 with PSD-95 in TPA-treated cells did not differ from that observed between AMPARs (GluR1) and PSD-95 in cells not exposed to TPA (86.5% ± 3.7, p=0.21 vs. TPA group, Figure 1b,c) suggesting that, for these receptors, the synaptic localization of AMPARs remains unchanged following S880-phosphorylation, and that hippocampal AMPARs containing S880 phosphorylated GluR2 may localize to synapses to the same extent as AMPARs which do not contain S880-phosphorylated GluR2. Because, in theory, it is possible that the reason we did not see more phosphorylated GluR2 subunits in non-synaptic locations might be due to efficient and rapid dephosphorylation of GluR2 subunits after exiting the synapse, we inhibited the phosphatase PP1, which we found dephosphorylates S880 (described in detail below), before and during TPA treatment. Inhibition of PP1 did not alter the localization of phosphorylated GluR2 or the extent to which it colocalized with PSD-95 (91.5% ± 1.7, p=0.28 vs. control, p=0.84 vs. TPA group) from that observed following TPA alone (Figure 1b,c) indicating that rapid dephosphorylation of internalized S880-phosphorylated subunits is not the reason why non-synaptic phosphorylated GluR2 was not observed. Phospho-S880 antibody staining was specific for phosphorylated GluR2, as non-specific staining was not observed in non-transfected COS cells after treatment with TPA (Figure 1e, bottom), however COS cells transfected with a chimeric protein, CD8R2c, containing the GluR2 C-terminus, were stained with this antibody following TPA treatment (Figure 1e, top). Additionally, as noted above, the antibody did not react with non-phosphorylated GluR2 subunits in neurons in the absence of TPA (Figure 1a), demonstrating specificity for the phospho-form of GluR2.
Figure 1. Stable synaptic retention of AMPARs following phosphorylation of S880 of GluR2.
(A) Antibodies which specifically detect the phosphorylated form of GluR2 (phosphoserine 880) were used to determine the localization of endogenous phosphorylated GluR2 (green) following treatment of cultured hippocampal neurons with TPA (100ng/ml) for 10 minutes (right panels). Cells were counterstained with antibodies to PSD-95 (red) to label excitatory synapses. Untreated controls are shown at left. Phospho-serine 880 was not detected in the absence of TPA treatment (left panels). Treatment with TPA revealed a strong increase in phosphorylated GluR2 (A, right). Both groups in A were scanned under identical gain settings with a confocal microscope. Phosphorylated GluR2 demonstrated strong colocalization with PSD-95 which did not differ from that of GluR1 in untreated controls (see text and C for values). (B) Inhibition of PP1 with okadaic acid for 10 minutes prior to and during TPA administration did not significantly alter pGluR2/PSD-95 colocalization or the synaptic localization of AMPARs (see text and C for values). Colocalization of GluR1 (green) with PSD-95 (red) in the absence of TPA was similar to that of pGluR2 (green) and PSD-95 (red) following TPA administration (see text and C for values). (C) Histogram depicting the percentage of GluR1 or pGluR2 punctae which colocalized with PSD-95 punctae (mean±SEM). (D) Pull down of biotinylated endogenous cell surface proteins after the indicated treatments confirmed the presence of phosphorylated GluR2 on the cell surface as long as 20 minutes (the longest chase period examined) after the 10 minute TPA treatment. ‘Chase’ denotes incubation of neurons in okadaic acid for 20 minutes following removal of TPA. Densitometric values indicating the amount of surface GluR1 from two independent experiments were as follows (represented as percentage of controls not treated with TPA): TPA=97, 110; TPA/OA+chase w/OA=129, 103. Scales bar in A, large panel=10µm, bar in small panel=3µm. (E) Representative controls demonstrating the specificity of pS880 antibodies for the phosphorylated form of GluR2. Top, COS cells transfected with CD8R2c, a chimeric protein containing the GluR2 C-terminus (see methods), treated with TPA, and labeled with pS880 antibodies. Bottom, untransfected COS cells treated with TPA and labeled with pS880 antibodies.
We confirmed the localization of phosphorylated GluR2 subunits at the plasmalemma using a cell surface biotinylation assay. Cultured neurons were treated with TPA for 10 minutes, washed briefly, and then immediately incubated with a cell-impermeable biotinylating reagent for 10 minutes at 37°C to label receptors remaining on the cell surface following the 10 minute exposure to TPA. Where indicated, TPA was also used together with okadaic acid to inhibit the phosphatase PP1 due to this enzyme’s ability to dephosphorylate GluR2 (described below). Following PKC activation for 10 minutes, phosphorylated GluR2 was easily visualized in the biotinylated (cell surface) receptor pool (Figure 1d). When dephosphorylation of GluR2 was blocked by inhibiting PP1, phosphorylated receptors were found in abundance in the cell surface fraction as long as 20 minutes after removal of TPA (the longest time point examined) (Figure 1d). Additionally, the level of GluR1 on the cell surface (the greater part of which of which is complexed with GluR2) (Wenthold et al., 1996) following GluR2 phosphorylation did not differ from that in untreated controls (see figure legend for densitometric values). The intracellular protein tubulin was not biotinylated and did not precipitate with cell surface proteins demonstrating the biotinylation to be specific for cell surface proteins. Precipitation of cell surface proteins was dependent upon biotinylation as AMPARs were not precipitated if the biotinylation step was omitted. Taken together these results reveal a population of AMPARs which remain localized at the cell surface and which do not traffic away from postsynaptic locations following GluR2 S880 phosphorylation.
Cotrafficking of S880-phosphorylated GluR2 with surface but not internalized AMPARs
Given that redistribution of AMPARs away from synaptic sites is a morphological hallmark of AMPAR endocytosis, the synaptic localization of phosphorylated GluR2 described above is inconsistent with a role for GluR2 phosphorylation in AMPAR endocytosis, at least for the receptor population studied here. To understand in more precise detail the relationship between GluR2 phosphorylation and AMPAR endocytosis, we compared directly, within the same intact neurons, the trafficking of phosphorylated GluR2 with endocytosed and surface populations of GluR2 following PKC activation. For these assays we employed exogenously expressed GluR2 subunits containing a myc epitope tag in the extracellular N-terminus (myc-GluR2) which we have used in previous studies of GluR2 trafficking (Osten et al., 2000). The use of exogenous subunits here conferred two main advantages; 1) myc-GluR2 expressed on the cell surface is homomeric (Osten et al., 2000), thus allowing us to isolate the trafficking contributions of GluR2 phosphorylation by itself, without the influence of other, potentially dominant subunits (e.g. GluR1), and 2) we found detection of exogenous internalized GluR2 with anti-myc 9E10 antibodies to be more robust and reliable than detection of endogenous internalized GluR2 using commercially available N-terminal anti-GluR2 antibodies (data not shown).
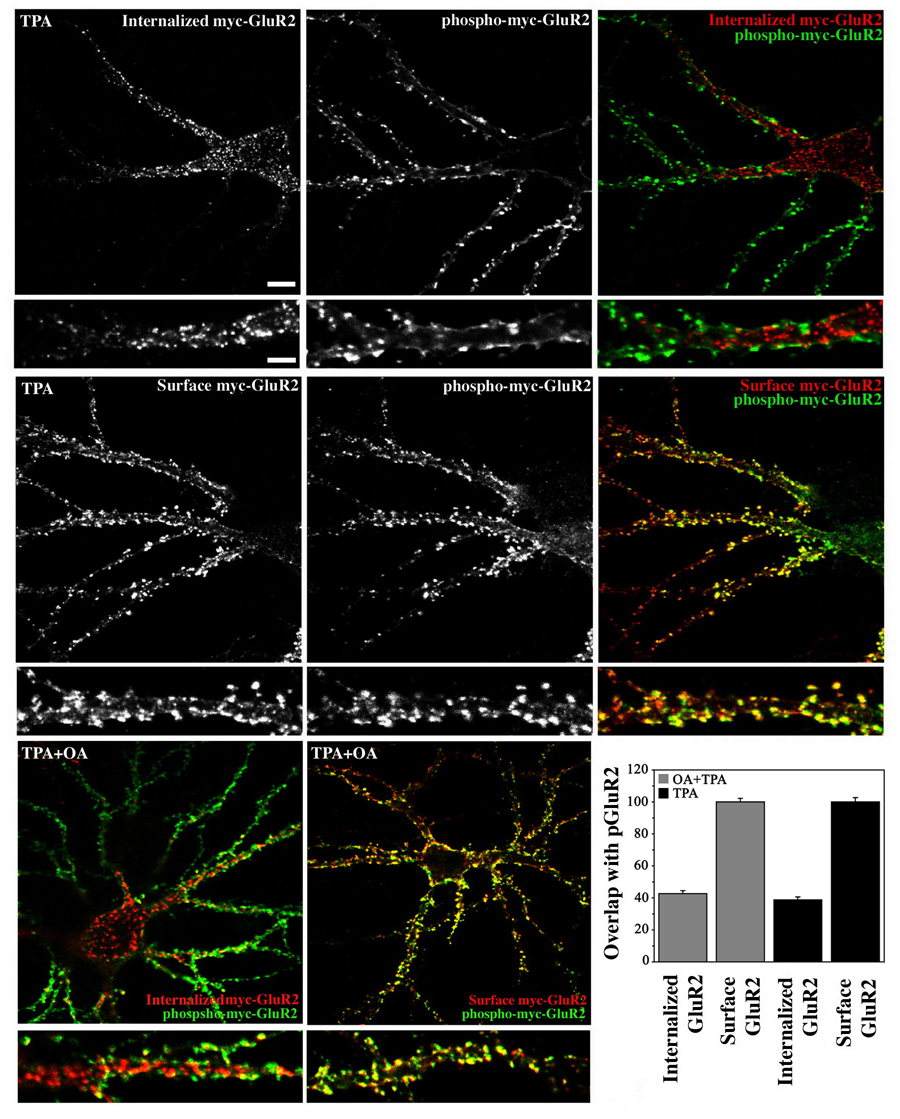
Neurons expressing myc-GluR2 were treated with TPA for 10 minutes while simultaneously being labeled with anti-myc antibodies during which period receptor/anti-myc antibody complexes endocytosed. Neurons then either underwent acid stripping and staining to identify internalized receptors, or were fixed immediately and stained under non-permeant conditions to label the surface receptor population. Phosphorylated myc-GluR2 was subsequently detected in both groups with phosphospecific antibodies. Examination of internalized and phosphorylated myc-GluR2 revealed the two receptor populations to be largely separate. The internalized population of receptors was found within nonsynaptic intracellular locations within the soma and main dendritic shafts (Figure 2, top). In contrast, phosphorylated myc-GluR2 was found prominently at the cell periphery where it colocalized extensively, in both location and shape of individual punctae, with staining of the surface population of myc-GluR2 (Figure 2, middle). The difference in colocalization of phosphorylated myc-GluR2 with surface versus internalized receptor populations was highly significant (p<.0001, Student’s t-test) (Figure 2, graph). We also examined the trafficking of phosphorylated myc-GluR2 subunits in the presence of okadaic acid to confirm that the reason we did not see phosphorylated myc-GluR2 among the endocytosed receptor population was not due to rapid dephosphorylation of myc-GluR2 subunits following endocytosis. When treated with TPA in the presence of okadaic acid, phosphorylated myc-GluR2 subunits exhibited a similar distribution as those that were treated with TPA only (Figure 2, bottom) and the difference in colocalization of phosphorylated GluR2 with surface versus internalized myc-GluR2 populations was not significantly different in the presence or absence of okadaic acid (p=0.20, Figure 2, graph). We note that while some of the phospho-GluR2 label likely reflects endogenous phosphorylated GluR2, the fluorescent intensity observed with anti-phospho-GluR2 antibodies was always greatly enhanced in infected neurons compared with uninfected cells indicating that much of the signal was derived from exogenous receptors (data not shown). Although we were obligated to permeablize cells to detect the phosphorylated GluR2 C-terminus and thus would not have been able to conclude in the absence of counterlabeling that phosphorylated subunits were on the cell surface, our ability to counterlabel phospho-GluR2 reliably with surface and internalized receptor populations effectively circumvented this problem. The high degree of overlap in location, and also the shape, of surface and phosphorylated GluR2 punctae strongly suggests that the phosphorylated and surface GluR2 subunits were part of the same receptor population, and indeed were likely the same molecules. This interpretation is further substantiated by the fact that it is unlikely that the same phosphorylated receptors which were strongly labeled in the cell periphery by antipGluR2 antibodies would remain undetected in acid stripping experiments if these phosphorylated receptors had been endocytosed. By this logic, it is also unlikely that endocytosed receptors which were easily visualized with anti-myc antibodies in acid stripping experiments would remain unlabeled by phospho-GluR2 antibodies if these internalized receptors were phosphorylated. We therefore conclude that the homomeric phosphorylated GluR2 observed here remained on the cell surface following phosphorylation, and did not endocytose. Indeed, these results suggest that the phospho-GluR2 observed here was preferentially retained at the cell surface over non-phosphorylated GluR2 because our antibody feeding protocol labeled both phosphorylated and non-phosphorylated GluR2 subunits, yet only non-phosphorylated GluR2 subunits were found among the endocytosed receptor population.
Figure 2. Cotrafficking of S880-phosphorylated GluR2 with surface but not internalized AMPARs.
(A) Cultured hippocampal neurons expressing myc-GluR2 were treated with TPA for 10 minutes and the localization of surface or internalized receptors was compared quantitatively with that of phosho-myc-GluR2 using confocal microscopy and Simple PCI software (see Methods). Internalized and phosphorylated receptors populations were largely separate and did not overlap (upper panels). Internalized receptors were found in dendritic shafts and the soma but were largely absent near the cell periphery. In contrast, phosphorylated receptors were highly localized to the cell periphery and colocalized extensively with the surface receptor population (middle panels). The difference in colocalization of surface and internalized receptors with phospho-myc-GluR2 was highly significant (graph, p<0.0001, ANOVA; mean ± SEM). Inclusion of okadaic acid 10 minutes prior to and during TPA administration did not alter the difference in colocalization of pGluR2 with surface versus internalized myc-GluR2 populations (lower panels and graph; p=0.20). Scale bar in large panel=10µm, bar in small panel=5µm.
Mutation of the GluR2 juxtamembrane region, but not the PDZ-binding domain, increases receptor endocytosis
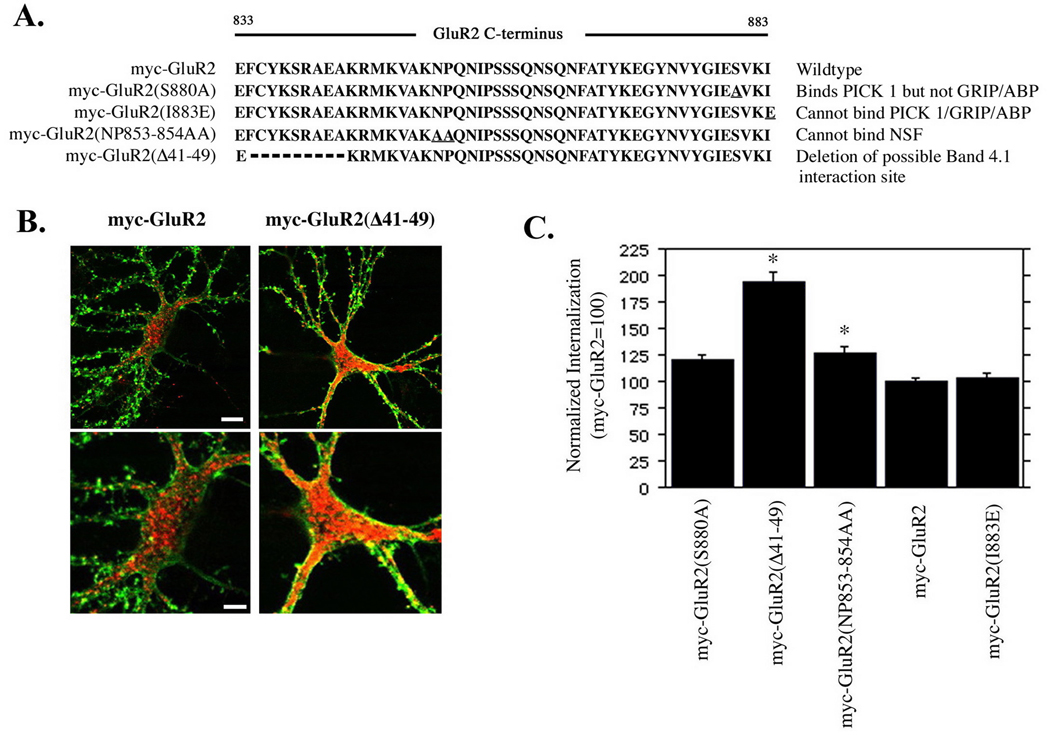
The stable retention of phosphorylated GluR2 subunits in synaptic locations at the neuronal surface was surprising since disruption of GluR2 interaction with GRIP/ABP by S880 phosphorylation has been linked to removal of AMPARs from synapses (Kim et al., 2001; Osten et al., 2000; Xia et al., 2000). To examine directly whether the ability to bind to GRIP/ABP was influential in regulating internalization of GluR2 subunits, we quantified internalization of myc-GluR2 subunits containing mutations in the PDZ-binding site which disrupt binding of GRIP/ABP and PICK1, or GRIP/ABP. As a positive control, myc-GluR2 containing a double point mutation in the membrane proximal GluR2 C-terminus that interferes with NSF binding (Nishimune et al., 1998; Osten et al., 1998), and which increases GluR2 internalization approximately 26–40% (Braithwaite et al., 2002; Lee et al., 2002) was also examined. Additionally, a mutant in which the membrane-proximal 10 amino acids of the GluR2 C-terminus were deleted was also included in these experiments (Figure 3a) (Osten et al., 2000). We used a protocol of live labeling of neurons and acid stripping to analyze AMPA receptor internalization (Lee et al., 2002) which allowed us to visualize and compare receptors internalized during the labeling period with receptors remaining on the cell surface in the same cell. Internalization was quantified and normalized to surface expression on a per-cell basis. All myc-GluR2 mutants were expressed on the cell surface and surface receptors were highly concentrated in punctae on dendritic spine-like structures (shown for myc-GluR2 and myc-GluR2(Δ41–49) in Figure 3b) where they colocalized with PSD-95 (not shown) indicative of synaptic targeting. The myc-GluR2(S880A) and myc-GluR2(I883E) mutations, which abolish GRIP/ABP binding or GRIP/ABP/PICK1 binding, respectively (Osten et al., 2000), did not significantly alter receptor internalization although there was a trend towards increased internalization which did not reach significance for the myc-GluR2(S880A) mutant (myc-GluR2=100±3.1%, myc-GluR2(S880A)=119±5.7%;p=0.022, myc-GluR2(I883E) = 103.2±4.2%; p=0.74, mean±SEM, Bonferroni-Dunn α level=0.0125) (Figure 3c) confirming that the inability to bind GRIP/ABP does not enhance GluR2 internalization. The myc-GluR2(NP853-854AA) mutant displayed a significant increase in internalization compared with wild type myc-GluR2 (126±5.5%;p<0.002) consistent with previous reports on the effect of disruption of NSF-GluR2 interactions on AMPAR internalization (Braithwaite et al., 2002; Lee et al., 2002). Interestingly, a novel finding of these experiments was the approximate doubling in internalization of the myc-GluR2(Δ41–49) mutant (194±9.0%;p<0.0001). This region, which is highly conserved between the GluR1-4 C-termini, interacts with PI3-kinase and is required for the stable synaptic insertion of AMPARs during LTP (Man et al., 2003). These results indicate that for these receptors, disruption of the ability of GluR2 to interact with PDZ proteins was not influential in determining receptor endocytosis, but intriguingly suggest a novel involvement of the GluR2 juxtamembrane 10 amino acids in mediating AMPAR endocytosis.
Figure 3. Mutation of the GluR2 juxtamembrane region, but not the PDZ-binding domain, increases receptor endocytosis.
(A) Cultured hippocampal neurons were infected with Sindbis viruses encoding mutant or wild type GluR2 subunits with a myc epitope tag in the extracellular N-terminus as indicated. Levels of internalization were acquired with a confocal microscope under identical parameters for all groups and quantified with Simple PCI software. (B) Representative cells from myc-GluR2 and myc-GluR2(Δ41–49) are shown (red=internalized receptors, green=surface receptors). Internalization was normalized to surface expression on a per-cell basis and is expressed in arbitrary units (mean +/− SEM, myc-GluR2=100) (C). myc-GluR2(Δ41–49) demonstrated a 94% increase in internalization compared with myc-GluR2 (p<.0001). myc-GluR2(NP853-854AA) demonstrated a 26% increase in internalization compared with myc-GluR2 (p<.005). Mutations within the PDZ binding site did not significantly increase GluR2 endocytosis. Asterisks denote statistical significance vs. myc-GluR2 (ANOVA with Bonferroni-Dunn correction; see text for means, standard errors, and p values). Scale bars in B 7.5µm (top panels) 4µm (bottom panels).
GluR2 phosphorylation is restricted to AMPARs at the cell surface
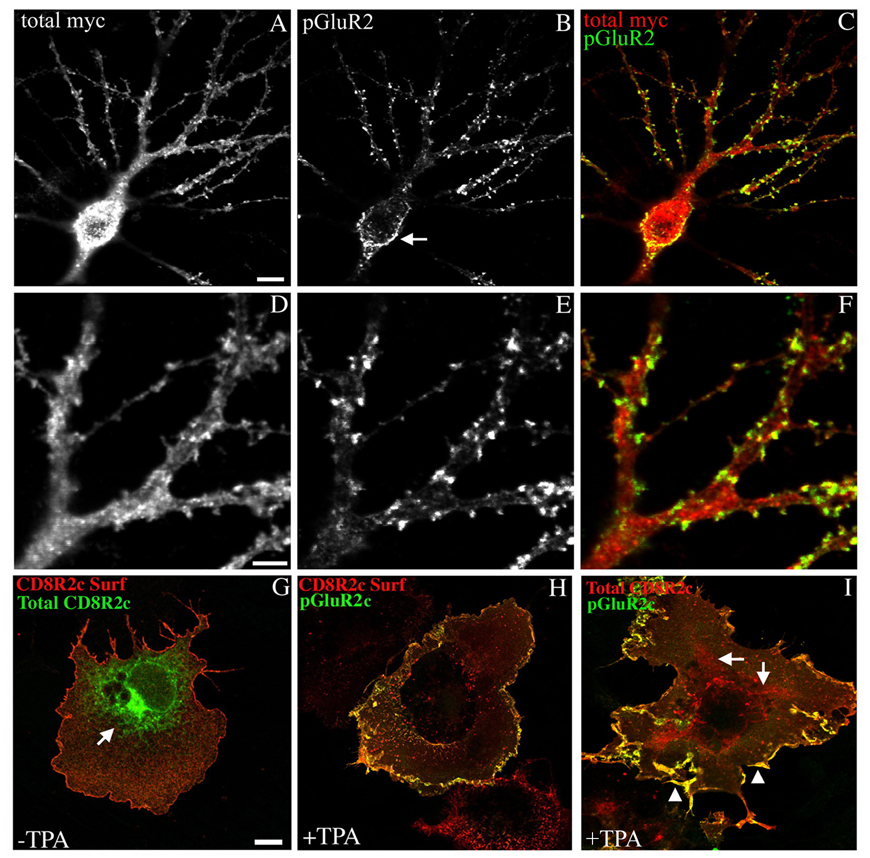
We have previously shown that a significant proportion of both endogenously and exogenously expressed GluR2 subunits are localized at non-synaptic intracellular locations within neurons. This intracellular localization is controlled by the edited R607 residue in the GluR2 pore loop which results in retention of GluR2 subunits in early compartments of the secretory pathway including the endoplasmic reticulum (Greger et al., 2002). The selective localization of pGluR2 at the cell surface in the current study suggests that this intracellular population of receptors is not a substrate for activated PKC. In theory however, the pGluR2 which we observed at the cell surface could have been phosphorylated intracellularly resulting in the rapid transit of newly phosphorylated receptors to the cell surface. Indeed, although phosphorylation of GluR2 at the cell surface has been suggested as a mechanism of removing AMPARs from synapses, several studies have proposed that certain isoforms of GRIP/ABP, non-palmitoylated isoforms for example, may sequester GluR2 subunits intracellularly, and that S880 phosphorylation of these intracellular GluR2 subunits may relieve such retention and allow AMPARs to traffic to the postsynaptic surface (Braithwaite et al., 2002; Daw et al., 2000; DeSouza et al., 2002). Such a mechanism could be expected to deplete the reservoir of intracellular GluR2 following S880 phosphorylation and subsequent movement of phosphorylated receptors to the neuronal surface, thus explaining the absence of phosphorylated GluR2 at intracellular sites observed here. We therefore asked whether GluR2 within endomembrane compartments associated with early stages of the secretory pathway is phosphorylated by activated PKC. We treated neurons expressing myc-GluR2 with TPA for 10 minutes, fixed the cells immediately, and compared the localization of total myc-GluR2 and phosphorylated myc-GluR2 in permeablized cells (Figure 4a–f). Following administration of TPA, neurons expressing myc-GluR2 still demonstrated considerable intracellular staining with anti-myc antibodies (Figure 4a,c,d,f) indicating that PKC activation does not grossly reduce the amount of intracellular GluR2. Despite the presence of intracellular GluR2, anti-phospho-GluR2 antibodies did not label this receptor population. As before, only receptors within spine-like protuberances and in the plasma membrane of proximal dendrites and the soma were labeled (Figure 4 arrow in b,c,e,f). We also confirmed this result in transfected COS cells where endomembrane compartments of the early secretory pathway are more easily identified than in neurons. Because GluR2 is not expressed on the cell surface in transfected COS cells (data not shown) we used a chimeric protein, termed CD8R2c, consisting of the ecto- and transmembrane domains of human CD8α fused to the entire GluR2 C-terminus at the point at which the CD8 transmembrane domain exits the lipid bilayer into the cytosol. CD8R2c trafficked efficiently to the cell surface when transfected into COS cells (Figure 4g,h). There was also an easily visualized intracellular fraction which was reticular in appearance and which reflects chimeras within the endoplasmic reticulum and early stages of the secretory pathway (arrows Figure 4g and i). COS cells expressing CD8R2c were treated with TPA for 10 minutes, fixed immediately, and the localization of phosphorylated chimeras was compared with either the surface (Figure 4h) or total (Figure 4i) chimeric receptor population. Similar to our results in neurons, anti-phospho-GluR2 antibodies only labeled the receptor population at the cell surface (Figure 4h and i, arrow heads in i) and did not label receptors located in internal endomembrane compartments (Figure 4h and arrows in i). These results demonstrate a novel subcellular compartmentalization to AMPA receptor phosphorylation and indicate that GluR2 located at the synaptic plasma membrane is the selective substrate of activated PKC, and that internal receptors in transit at early stages of the secretory pathway such as the ER, are not phosphorylated following PKC activation. Therefore, any effect on AMPAR trafficking conferred by S880 phosphorylation by PKC is likely limited to the population of AMPARs at the cell surface which are susceptible to S880 phosphorylation.
Figure 4. GluR2 phosphorylation is restricted to AMPARs at the cell surface.
(A–F) Neurons expressing myc-GluR2 were treated for 10 minutes with TPA and then fixed and processed immunocytochemically to detect phosphorylated and total myc-GluR2 receptor populations as indicated. Localization of phosphorylated and total myc-GluR2 was evaluated qualitatively. Representative cells demonstrating that S880-phosphorylated myc-GluR2 was found at the cell surface in perikarya (arrow in B) and in spine-like structures (B and E). Internal myc-GluR2 within endomembrane compartments including early stages of the secretory pathway was not phosphorylated on S880 however. (G–I) Chimeric proteins encoding the human CD8α ecto- and transmembrane domains fused to the GluR2 C-terminus (CD8R2c) were used to examine phosphorylation of the GluR2 C-terminus at internal and cell surface locations in COS cells. CD8R2c was expressed on the cell surface in COS cells (G and H), and was also found in an internal reticular compartment reflective of chimeric receptors at early stages of the secretory pathway (arrows in G and I). Treatment of COS cells expressing CD8R2c with TPA for 10 minutes demonstrated that the surface chimera population (H and arrowheads in I) but not the internal chimera population (arrows in I) was phosphorylated by PKC (H and I). Images are representative of three independent experiments which gave similar results. Scale bar in A–C=10µm, bar in D–F=3µm, bar in G-I=20µm.
Stabilization of AMPARs at synapses following PKC activation
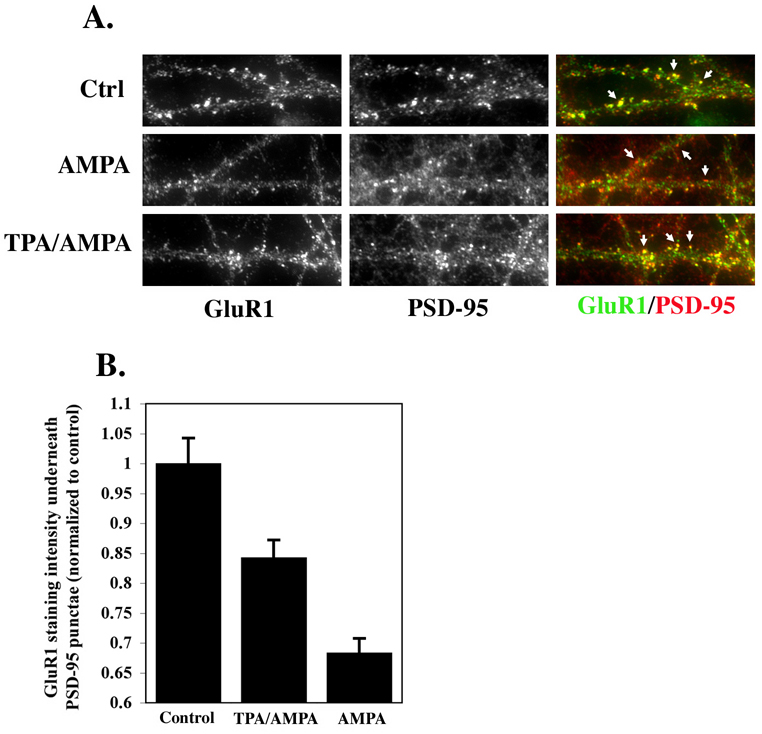
Our results to this point suggest a population of AMPARs in which GluR2 phosphorylation does not induce trafficking of AMPARs away from the synapse. We have also shown that GluR2 phosphorylation is compartmentalized to subunits on the cell surface. What then, might be the function of phosphorylation of GluR2 subunits on the cell surface if not to induce AMPAR trafficking away from the synapse? One possibility is that GluR2 phosphorylation could stabilize AMPARs at synapses. This would be consistent with our results showing that following labeling of both phosphorylated and non-phosphorylated GluR2 on the neuronal surface, only non-phosphorylated GluR2 is found in the internalized receptor pool suggesting that phosphorylated GluR2 may be preferentially retained at the cell surface over non-phosphorylated GluR2. This is also consistent with one report which found that synaptic AMPAR currents increased and LTD was abolished following disruption of GRIP/ABP interaction with GluR2 in hippocampal neurons (Daw et al., 2000). We tested this hypothesis by examining the effect of GluR2 phosphorylation on the movement of AMPARs away from synaptic locations induced by brief application of AMPA (Ehlers, 2000; Lissin et al., 1999). Cultured hippocampal neurons were treated with TPA or ACSF for 10 minutes followed by AMPA (10µM) for 10 minutes and then fixed and labeled with antibodies to GluR1 and PSD-95. A control group was treated with ACSF for 20 minutes and was not exposed to TPA or AMPA. Treatment with AMPA alone caused a 32±2.3% (mean±SEM) reduction in intensity of GluR1 labeling associated with PSD-95 punctae compared with controls (Figure 5), consistent with published results of AMPAR internalization and a reduction in overlap of AMPARs with synaptic markers following pharmacological activation of AMPARs in neuronal cultures (Ehlers, 2000; Lissin et al., 1999). Treating neurons with TPA prior to AMPA administration reduced this decrease by 50% (16±2.8% reduction in GluR1 labeling under PSD-95) (Figure 5). These results suggest that activation of PKC can stabilize AMPARs at synapses, possibly by either offsetting or directly preventing endocytosis of AMPARs induced by AMPA.
Figure 5. Stabilization of AMPARs at synapses following PKC activation.
Cultured hippocampal neurons were treated with TPA or ACSF for 10 minutes followed by AMPA (10µM) for 10 minutes and then fixed and labeled with antibodies to GluR1 and PSD-95. Controls were treated with ACSF for 20 minutes and were not exposed to TPA or AMPA. (A) Representative images of PSD-95 and AMPARs (GluR1) labeling in dendrites acquired using identical acquisition settings for all groups under epifluorescent illumination. Arrows indicate PSD-95 punctae which either colocalized with AMPAR punctae (top and bottom rows) or which did not colocalize with AMPAR punctae (middle row) (B) Results of quantification of the intensity of GluR1 overlap with PSD-95 punctae following treatments described above (n=2 experiments, 10 cell per group per experiment). *=significant vs. controls, **=significant vs. TPA/AMPA group (p<0.05).
Endogenous regulation of GluR2 phosphorylation
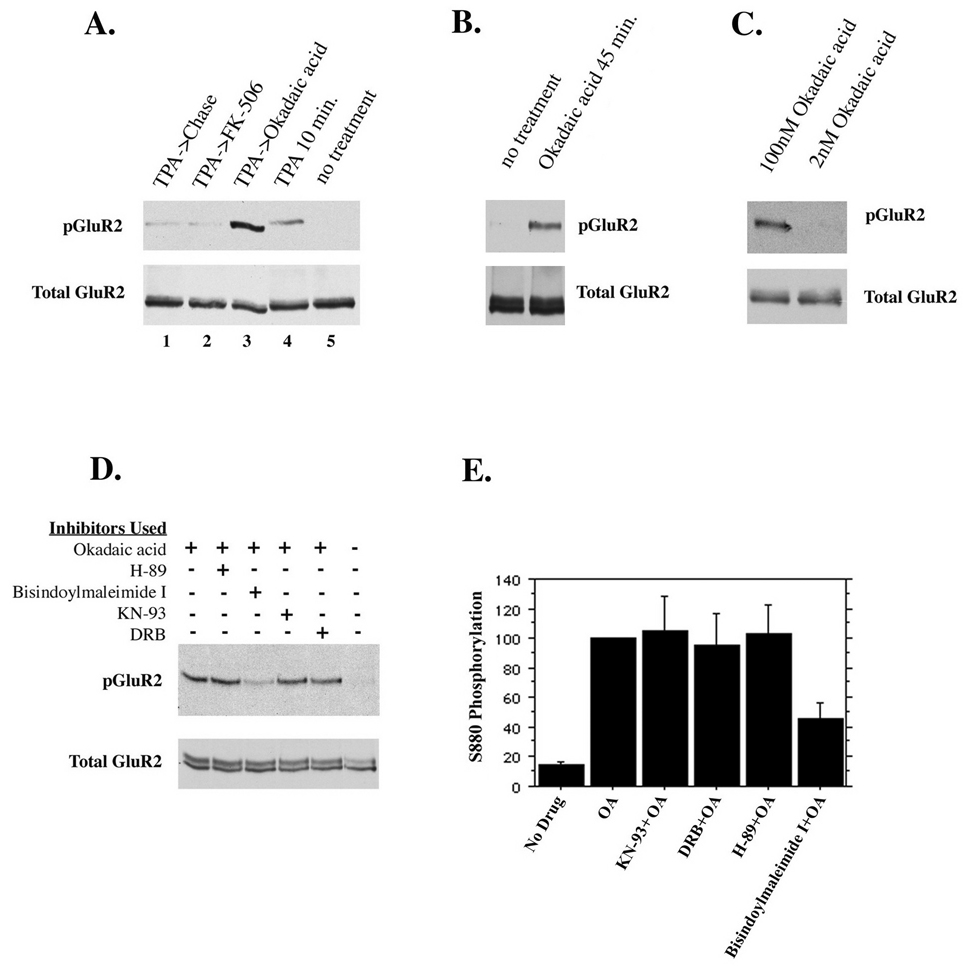
If phosphorylation of GluR2 is important in AMPAR trafficking then endogenous (e.g. non-phorbol ester-induced) phosphorylation of the receptor should be evident in vivo. In the current study however, immunocytochemistry of untreated cultured hippocampal neurons using phosphospecific anti-GluR2 antibodies revealed virtually no phosphorylated receptors (Figure 1). Other studies, however, have detected phosphorylated GluR2 in neurons that had not been administered phorbol esters (Kim et al., 2001; Xia et al., 2000). We therefore considered the possibility that a phosphatase may be active in controlling the levels of phosphorylated GluR2. To test this, we inhibited several different serine/threonine phosphatases in cultured hippocampal neurons in the presence or absence of TPA. Neurons were incubated for 30 minutes with either 1µM FK-506 to inhibit calcineurin, or 100nM okadaic acid, which inhibits PP1 and PP2A at the concentration used. Following this, neurons were treated with TPA and the indicated phosphatase inhibitor for 10 minutes and then chased in the presence of the indicated phosphatase inhibitor without TPA for an additional hour. Treatment of cells with okadaic acid resulted in a strong increase in the level of TPA-induced phosphorylation of GluR2 compared with cells which were treated with TPA but in which no phosphatase inhibitor was used (Figure 6a). This suggests that PP1 or PP2A is active in regulating the amount of phosphorylated GluR2 in vivo. Inhibition of calcineurin with FK-506 had no effect on GluR2 phosphorylation. These experiments do not confirm, however, that there is an endogenous kinase activity that acts on GluR2, as PKC was artificially stimulated with TPA in this instance. It was hypothesized that if GluR2 were phosphorylated by an endogenous kinase activity that was masked by PP1 or PP2A activity, then inhibiting the active phosphatase should reveal this activity. We therefore inhibited PP1 and PP2A in cultured neurons for 45 minutes with 100nM okadaic acid without treating cells with TPA. Treatment of neurons with okadaic acid for 45 minutes resulted in a significant increase in the phosphorylation of GluR2 over untreated controls (Figure 6b). This suggested that GluR2 is phosphorylated by a kinase in vivo, but that this phosphorylation is not detected due to the activity of PP1 and/or PP2A. To distinguish between the activities of these two phosphatases we used two different concentrations of okadaic acid (2nM, 100nM) which selectively inhibit PP2A at the lower concentration but which inhibit both phosphatases at the higher concentration. Okadaic acid used in concentrations that block PP2A selectively did not increase phosphorylation of GluR2 (Figure 6c), indicating that PP1 is responsible for dephosphorylating GluR2.
Figure 6. Endogenous regulation of GluR2 phosphorylation.
(A) Cultured hippocampal neurons were treated for 10 minutes with TPA in the presence of 1µM FK-506 (to inhibit PP2B) or 100nM okadaic acid (to inhibit PP1 and PP2A) or without phosphatase inhibitors. Neurons were then chased for 1 hour in the absence or presence of the phosphatase inhibitors as indicated. (B) Inhibition of PP1 and PP2A with okadaic acid (100nM) for 45 minutes reveals an endogenous kinase activity which phosphorylates GluR2. (C) PP2A alone or PP2A and PP1 were inhibited with 2nM and 100nM okadaic acid for 45 minutes, respectively. Inhibition of PP2A alone did not increase GluR2 phosphorylation, indicating that PP1 dephosphorylates GluR2. (D and E) To identify the endogenous kinase activity, different cell-permeable serine/threonine kinase inhibitors were used in the presence of okadaic acid for 45 minutes. Control groups either were untreated or treated with okadaic acid but no kinase inhibitors. Kinase inhibitors used were 2µM H-89 (inhibits PKA), 1µM bisindolylmaleimide I (inhibits PKC), 7.4µM KN-93 (inhibits CaMKII), 100µM 5,6 dichloro-1-beta-D-ribofuranosylbenzimidazole I or DRB (inhibits casein kinase II). Only inhibition of PKC diminished phosphorylation of GluR2 in the presence of okadaic acid (45% of controls treated with okadaic acid but no kinase inhibitor).
Although activation of PKC with phorbol esters results in robust phosphorylation of GluR2, the identity of the kinase responsible for endogenous S880 phosphorylation in vivo is still unresolved (Chung et al., 2000; Kim et al., 2001; Matsuda et al., 2000; Matsuda et al., 1999; Perez et al., 2001; Sheng and Hyoung Lee, 2003). To identify the kinase or kinases involved in phosphorylation of S880 of GluR2, we inhibited several different serine/threonine kinases in the presence of okadaic acid. Cell-permeable kinase inhibitors were applied to cultured hippocampal neurons 10 minutes before the addition of okadaic acid and the indicated inhibitor for an additional 45 minutes. As controls, neurons received either no treatment, or were treated with okadaic acid for 45 minutes without kinase inhibitors. The inhibitors used were; 2µM H-89 (inhibits PKA), 1µM bisindolylmaleimide I (inhibits PKC), 7.4 µM KN-93 (inhibits CaMKII), 100µM 5,6 dichloro-1-beta-D-ribofuranosylbenzimidazole, or DRB (inhibits casein kinase II). Of the preceding inhibitors, only bisindolylmaleimide I significantly reduced phosphorylation of GluR2 in the presence of okadaic acid (45.6±11.0% of controls which were administered okadaic acid only; KN-93=104.8±23.6, DRB=95.1±21.9, H-89=103.1±19.1%, mean±SEM, p≤0.05), indicating that a PKC isoform is responsible for the phosphorylation of GluR2 observed when PP1 is inhibited (Figure 6d and e). These results suggest that a PKC isoform and PP1 may have opposing roles in regulating GluR2 phosphorylation in vivo.
DISCUSSION
In this report we have examined the trafficking of S880-phosphorylated GluR2 in dissociated hippocampal neurons. Phosphorylation of S880 within the GluR2 C-terminus is reported to regulate interactions between GluR2 and PDZ proteins, and such interactions have been shown to play an important role in directing the synaptic trafficking of AMPARs (Chung et al., 2000; Matsuda et al., 1999; Perez et al., 2001; Sheng and Hyoung Lee, 2003; Xia et al., 2000). Here we show that following PKC activation with phorbol esters phosphorylation of GluR2 is compartmentally restricted to receptors located at the cell surface. S880-phosphorylated GluR2 subunits remained selectively at the cell surface in synaptic locations and were not mobilized into a pool of endocytosed receptors. Because S880 phosphorylation prevents GluR2 binding to GRIP/ABP, this suggests that for this population of GluR2, interaction with GRIP/ABP was not required for synaptic anchorage, and preventing this interaction was not associated with AMPA receptor endocytosis. Our results instead suggest that activation of PKC, which induces GluR2 phosphorylation, may lead to enhanced stabilization of a population of receptors containing GluR2 subunits in the postsynaptic membrane.
Phosphorylation of S880 within the GluR2 C-terminal domain abrogates binding of AMPARs to the putative synaptic anchoring proteins GRIP/ABP while leaving PICK 1 binding intact (Chung et al., 2000; Daw et al., 2000). S880 phosphorylation has thus been proposed to disengage AMPARs from synaptic anchoring proteins, resulting in internalization of AMPARs from synaptic locations which has been shown to occur during LTD (Chung et al., 2000; Matsuda et al., 2000; Matsuda et al., 1999; Xia et al., 2000). Although severing GluR2 interaction with GRIP/ABP may, of itself, free AMPARs from PSD proteins and allow AMPAR movement out of the synapse, several studies have suggested that active recruitment of PICK 1 to S880-phosphorylated GluR2 plays a role in AMPAR endocytosis and LTD (Lu and Ziff, 2005) (Kim et al., 2001; Matsuda et al., 2000; Xia et al., 2000). Peptides which inhibit PICK1 but not GRIP/ABP interactions with GluR2, prevented LTD in hippocampus (Kim et al., 2001) and cerebellum (Xia et al., 2000). In contrast however, others found that specific inhibition of the PICK1/GluR2 interaction had no effect on LTD or basal synaptic transmission in hippocampus, but peptides that blocked binding of both GRIP/ABP and PICK 1 to GluR2 prevented LTD and increased basal neurotransmission (Daw et al., 2000). This suggests that abrogating GluR2 interaction with GRIP/ABP, as occurs following S880 phosphorylation, may under certain circumstances increase synaptic levels of GluR2. The complexity of this control is further illustrated by two studies in permeablized cells, one of which found that PKC activation with phorbol esters caused AMPARs to translocate from synaptic to non-synaptic locations within dendrites (Matsuda et al., 2000), while another found that S880 phosphorylation changed the subcellular localization of GluR2 subunits from diffuse non-synaptic locations to synaptic (Chung et al., 2000).
In the current study we compared the trafficking of phosphorylated GluR2 with that of surface and internalized GluR2 populations within the same neurons, which has not been reported previously. We found that endogenous phosphorylated GluR2 subunits were localized almost exclusively to synapses, colocalizing with PSD-95 to a high degree (over 90%) which was equal to that of AMPARs that did not contain phosphorylated GluR2. Furthermore, S880-phosphorylated GluR2 colocalized extensively with the surface GluR2 population but not the internalized endosomal GluR2 population indicating that phosphorylated GluR2 remained on the surface and did not endocytose. Interestingly, non-phosphorylated GluR2 subunits initially on the cell surface did, however, endocytose indicating that phosphorylated GluR2 subunits may be preferentially retained at the neuronal surface compared with non-phosphorylated subunits. Inhibition of PP1, which we show effectively prevents GluR2 dephosphorylation, did not reveal a population of endocytosed phosphorylated receptors indicating that rapid dephosphorylation of GluR2 following endocytosis is unlikely to be the reason phosphorylated GluR2 subunits were not found among the endocytosed receptor population. The selective retention of phosphorylated GluR2 at the cell surface is also consistent with our observation that activation of PKC before treating neurons with AMPA mitigates the loss of synaptic AMPARs that normally results from exposure to AMPA (Beattie et al., 2000; Ehlers, 2000; Lissin et al., 1999). We cannot rule out the possibility that the population of phosphorylated GluR2 that is stably retained in the neuronal plasmalemma might be more susceptible to S880 phosphorylation than the GluR2 population that is more readily endocytosed. This would suggest a coincidental but not causal correlation between PKC activation/S880 phosphorylation and stabilization of AMPARs at synapses. However, the existence of two such populations of GluR2 at the neuronal surface, which are differentially susceptible to phosphorylation by PKC and, independently, endocytosis, has not been reported.
GluR2 C-terminal domain mutants
The S880A and I883E GluR2 mutants do not bind ABP/GRIP and the latter also does not bind PICK1 (Osten et al., 2000). Notably, we did not observe a decrease in GluR2 internalization with these mutants as might be predicted if S880 phosphorylation stabilizes AMPARs at the neuronal surface. Indeed, myc-GluR2(S880A) displayed a trend towards an increase in internalization which did not reach significance. It is possible that the myc-GluR2(S880A) mutation, although effective at preventing interaction with GRIP/ABP while preserving PICK1 interaction similarly to S880 phosphorylation, does not completely mimic the effects on AMPAR trafficking of PKC activation. Phosphorylation of other substrates by PKC could influence AMPAR trafficking in ways not replicated by mutation of the GluR2 C-terminus alone. Phosphorylation of stargazin by PKC, for example, is required for LTP in hippocampal synapses (Tomita et al., 2005). Stargazin phosphorylated by activated PKC might have stabilized the AMPARs observed in our study following TPA administration but would not have been expected to stabilize the myc-GluR2(S880A) or myc-GluR2 (I883E) mutants in the absence of PKC activation. Phosphorylation of S818 of GluR1 by PKC has also been shown to be required for LTP, and acute phosphorylation of this site increases delivery of AMPARs to synapses (Boehm et al., 2006). Although anchorage by S818 phosphorylated GluR1 could be responsible for the stabilization of endogenous GluR1/2 heteromers observed following PKC activation in our study, it is unlikely to explain the similar results we observed using exogenous receptors which we (Osten et al., 2000) and others (Hayashi et al., 2000) have shown do not contain other endogenous GluR subunits. Such findings together with our results, however, indicate that PKC regulation of AMPAR trafficking is complex and likely involves phosphorylation of multiple substrates in addition to GluR2. The complexity of regulation of AMPAR trafficking by PKC likely underlies, in part, the differing results which have been reported concerning the role of this kinase and AMPAR trafficking. It is difficult to envision, for example, how phosphorylation of GluR2 by PKC would cause AMPAR internalization from synapses and LTD, while PKC-mediated phosphorylation of GluR1 and stargazin in the same oligomeric complex with GluR2, would cause the opposite, videlicet synaptic accumulation of AMPARs and LTP. Adding to the complexity of interpreting such results is the fact that the two brain regions where the role of PKC in AMPAR trafficking and synaptic plasticity has been most well characterized, the cerebellum and hippocampus, likely employ different mechanisms to regulate this plasticity. LTD, for example, is absent in cerebellar purkinje cells lacking functional GluR2 and can be reconstituted in such cells by transfection of wild type GluR2 indicating that GluR2 is critical for LTD in cerebellum (Chung et al., 2003). In the hippocampus however, LTD is unaffected by genetic deletion of GluR2 or by double knockout of GluR2 and GluR3 (Meng et al., 2003), indicating the hippocampal LTD is not reliant upon GluR2. Moreover, these regions differ in their expression of individual AMPAR subunits, in particular GluR1, the expression of which is considerably lower in cerebellum compared with hippocampus (Petralia and Wenthold, 1992; Zhao et al., 1998). In theory hippocampal GluR1/GluR2 heteromers with S880 phosphorylated GluR2 subunits might be stabilized at synapses through GluR1 interaction with band 4.1 proteins (Coleman et al., 2003; Shen et al., 2000), while this mechanism might be unavailable in cerebellum due to the lower expression of GluR1 (Petralia and Wenthold, 1992; Zhao et al., 1998). Because the current study also employed exogenously expressed homomeric GluR2 however, anchorage by GluR1 is unlikely to account fully for the surface stability of phosphorylated GluR2 observed here.
GluR2 kinase and phosphatase identities
We have also examined regulation of endogenous GluR2 phosphorylation. Little is known about the extent to which GluR2 is phosphorylated in vivo or the enzymes involved in regulating such phosphorylation. Indeed, the identity of the kinase which phosphorylates GluR2 in vivo is not firmly established (Chung et al., 2000; Kim et al., 2001; Matsuda et al., 2000; Matsuda et al., 1999; Perez et al., 2001; Sheng and Hyoung Lee, 2003). Chung et al. (2000) noted a diffuse staining of S880-phosphorylated GluR2 in untreated cells, and we find by immunoblot and immunocytochemistry that levels of GluR2 phosphorylation in untreated cells are very low and not easily detected. Following selective inhibition of PP1 with okadaic acid however, we observed an increase in S880-phosphorylated GluR2, which indicates that an endogenous kinase activity phosphorylates GluR2 in the absence of phorbol ester treatment. This finding agrees with the results of Kim et al. (2000) who noted a similar increase in GluR2 phosphorylation following administration of okadaic acid, although it was not established in that study whether this increase was due to inhibition of PP1 or PP2A. The increase in endogenously phosphorylated GluR2 which we observe following PP1 inhibition was attenuated by inhibition of PKC but not by inhibition of other candidate serine/threonine kinases suggesting that S880 is in fact a substrate for PKC in vivo. Kim et al. (2000) also reported that S880 phosphorylation increases following hippocampal LTD but found also that this increase was not prevented by the PKC inhibitor Gö6976. We note that bisindolylmaleimide I inhibits a broader array of PKC isoforms, including constitutively active isoforms, than does Gö6976 (Martiny-Baron et al., 1993) which could explain the difference in sensitivity to PKC inhibitors used in these two studies. Notably, the 55% inhibition of endogenous phosphorylation of S880 observed with 1 µM bisindolylmaleimide I in the current study is consistent with the reported IC50 of this inhibitor for the constitutively active atypical PKC isoform PKMς (IC50 = 5.8±1.1 µM), but is not consistent with that for calcium-dependent PKC isoforms (IC50 = 8.4-18 nM) (Martiny-Baron et al., 1993), suggesting that PKMς activity may underlie the endogenous S880 phosphorylation observed here. Interestingly, intracellular infusion of PKMς into hippocampal pyramidal neurons increases postsynaptic AMPAR currents by stabilizing AMPARs in the postsynaptic membrane (Ling et al., 2006) and the persistent activity of this kinase is critical for the maintenance phase of LTP in vitro and in vivo (Pastalkova et al., 2006; Serrano et al., 2005). These findings agree with our observation of increased synaptic stabilization of AMPARs following PKC activation. Because infusion of PKMς inhibitors into the hippocampus prevents the retention but not the formation of spatial memory in living animals, the synaptic stabilization of AMPARs by a PKC activity may have a role in memory retention in vivo (Pastalkova et al., 2006).
The mechanism of synaptic anchorage of phosphorylated GluR2 subunits which cannot bind to GRIP/ABP remains unclear, however it is likely to involve the protein stargazin or its homologues which interact with AMPARs through domains outside of the AMPAR C-terminus and which therefore should not be influenced by GluR2 S880 phosphorylation (Chen et al., 2000; Schnell et al., 2002). Stargazin and PSD-95 cooperate in a two step mechanism to anchor AMPARs at postsynaptic sites (Chen et al., 2000; Chetkovich et al., 2002; Schnell et al., 2002). Specifically, stargazin/AMPAR interactions are believed to be required for delivery of AMPARs to the cell surface, and subsequent interaction of the stargazin C-terminus with PSD-95 anchors the surface-localized stargazin/AMPAR complexes at synapses. The extremely high colocalization of phosphorylated GluR2 with PSD-95 that we observe is consistent with a role for stargazin or its homologues and PSD-95 in synaptic anchorage of phosphorylated GluR2.
In summary, our results identify a population of GluR2 that remains anchored at the postsynaptic surface and does not co-traffic with the endocytosed GluR2 population following S880 phosphorylation by PKC. This indicates that for this receptor population, GluR2 phosphorylation and prevention of GRIP/ABP interaction with GluR2 is not correlated with enhanced endocytosis. Interestingly, PKC activation may stabilize these receptors in the postsynaptic membrane. Characterization of the mechanism of synaptic anchorage of S880-phosphorylated GluR2 subunits will require further study, including investigation of the roles of other possible PKC targets or interacting proteins, such as stargazin/PSD-95 complexes.
Acknowledgments
We thank I. Greger for critical reading of the manuscript and T. Serra for help in preparation of the manuscript. This work was supported by NIH grant MH067229 to E.B.Z.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic Incorporation of AMPA Receptors during LTP Is Controlled by a PKC Phosphorylation Site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Braithwaite SP, Xia H, Malenka RC. Differential roles for NSF and GRIP/ABP in AMPA receptor cycling. Proc Natl Acad Sci U S A. 2002;99:7096–7101. doi: 10.1073/pnas.102156099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 1999;2:454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazing regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Chetkovich DM, Chen L, Stocker TJ, Nicoll RA, Bredt DS. Phosphorylation of the postsynaptic density-95 (PSD-95)/discs large/zona occludens-1 binding site of stargazin regulates binding to PSD-95 and synaptic targeting of AMPA receptors. J Neurosci. 2002;22:5791–5796. doi: 10.1523/JNEUROSCI.22-14-05791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science. 2003;300:1751–1755. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domaincontaining proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman SK, Cai C, Mottershead DG, Haapalahti JP, Keinanen K. Surface expression of GluR-D AMPA receptor is dependent on an interaction between its C-terminal domain and a 4.1 protein. J Neurosci. 2003;23:798–806. doi: 10.1523/JNEUROSCI.23-03-00798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw MI, Chittajallu R, Bortolotto ZA, Dev KK, Duprat F, Henley JM, Collingridge GL, Isaac JT. PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC- dependent regulation of AMPA receptors at hippocampal synapses. Neuron. 2000;28:873–886. doi: 10.1016/s0896-6273(00)00160-4. [DOI] [PubMed] [Google Scholar]
- DeSouza S, Fu J, States BA, Ziff EB. Differential Palmitoylation Directs the AMPA Receptor-Binding Protein ABP to Spines or to Intracellular Clusters. J Neurosci. 2002;22:3493–3503. doi: 10.1523/JNEUROSCI.22-09-03493.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Dong H, O'Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Fu J, deSouza S, Ziff EB. Intracellular membrane targeting and suppression of Ser880 phosphorylation of glutamate receptor 2 by the linker I-set II domain of AMPA receptor-binding protein. J Neurosci. 2003;23:7592–7601. doi: 10.1523/JNEUROSCI.23-20-07592.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Ziff EB. RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron. 2002;34:759–772. doi: 10.1016/s0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Kim CH, Chung HJ, Lee HK, Huganir RL. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci U S A. 2001;98:11725–11730. doi: 10.1073/pnas.211132798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Liu L, Wang YT, Sheng M. Clathrin Adaptor AP2 and NSF Interact with Overlapping Sites of GluR2 and Play Distinct Roles in AMPA Receptor Trafficking and Hippocampal LTD. Neuron. 2002;36:661–674. doi: 10.1016/s0896-6273(02)01024-3. [DOI] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Liao D, Zhang X, O'Brien R, Ehlers MD, Huganir RL. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat Neurosci. 1999;2:37–43. doi: 10.1038/4540. [DOI] [PubMed] [Google Scholar]
- Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Sacktor TC. Protein kinase Mzeta enhances excitatory synaptic transmission by increasing the number of active postsynaptic AMPA receptors. Hippocampus. 2006;16:443–452. doi: 10.1002/hipo.20171. [DOI] [PubMed] [Google Scholar]
- Lissin DV, Carroll RC, Nicoll RA, Malenka RC, von Zastrow M. Rapid, activation-induced redistribution of ionotropic glutamate receptors in cultured hippocampal neurons. J Neurosci. 1999;19:1263–1272. doi: 10.1523/JNEUROSCI.19-04-01263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Lu W, Ziff EB. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron. 2005;47:407–421. doi: 10.1016/j.neuron.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Man HY, Wang Q, Lu WY, Ju W, Ahmadian G, Liu L, D'Souza S, Wong TP, Taghibiglou C, Lu J, Becker LE, Pei L, Liu F, Wymann MP, MacDonald JF, Wang YT. Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron. 2003;38:611–624. doi: 10.1016/s0896-6273(03)00228-9. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- Matsuda S, Launey T, Mikawa S, Hirai H. Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. Embo J. 2000;19:2765–2774. doi: 10.1093/emboj/19.12.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Mikawa S, Hirai H. Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J Neurochem. 1999;73:1765–1768. doi: 10.1046/j.1471-4159.1999.731765.x. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Jia Z. Synaptic transmission and plasticity in the absence of AMPA glutamate receptor GluR2 and GluR3. Neuron. 2003;39:163–176. doi: 10.1016/s0896-6273(03)00368-4. [DOI] [PubMed] [Google Scholar]
- Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- Osten P, Khatri L, Perez JL, Kohr G, Giese G, Daly C, Schulz TW, Wensky A, Lee LM, Ziff EB. Mutagenesis reveals a role for ABP/GRIP binding to GluR2 in synaptic surface accumulation of the AMPA receptor. Neuron. 2000;27:313–325. doi: 10.1016/s0896-6273(00)00039-8. [DOI] [PubMed] [Google Scholar]
- Osten P, Srivastava S, Inman GJ, Vilim FS, Khatri L, Lee LM, States BA, Einheber S, Milner TA, Hanson PI, Ziff EB. The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and alpha- and beta-SNAPs. Neuron. 1998;21:99–110. doi: 10.1016/s0896-6273(00)80518-8. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Perez JL, Khatri L, Chang C, Srivastava S, Osten P, Ziff EB. PICK1 targets activated protein kinase Calpha to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA- type glutamate receptor subunit 2. J Neurosci. 2001;21:5417–5428. doi: 10.1523/JNEUROSCI.21-15-05417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci U S A. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P, Yao Y, Sacktor TC. Persistent phosphorylation by protein kinase Mzeta maintains late-phase long-term potentiation. J Neurosci. 2005;25:1979–1984. doi: 10.1523/JNEUROSCI.5132-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Liang F, Walensky LD, Huganir RL. Regulation of AMPA receptor GluR1 subunit surface expression by a 4. 1N-linked actin cytoskeletal association. J Neurosci. 2000;20:7932–7940. doi: 10.1523/JNEUROSCI.20-21-07932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Hyoung Lee S. AMPA receptor trafficking and synaptic plasticity: major unanswered questions. Neurosci Res. 2003;46:127–134. doi: 10.1016/s0168-0102(03)00040-3. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Osten P, Vilim FS, Khatri L, Inman G, States B, Daly C, DeSouza S, Abagyan R, Valtschanoff JG, Weinberg RJ, Ziff EB. Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron. 1998;21:581–591. doi: 10.1016/s0896-6273(00)80568-1. [DOI] [PubMed] [Google Scholar]
- Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45:269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- Zhao HM, Wenthold RJ, Petralia RS. Glutamate receptor targeting to synaptic populations on Purkinje cells is developmentally regulated. J Neurosci. 1998;18:5517–5528. doi: 10.1523/JNEUROSCI.18-14-05517.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]