Abstract
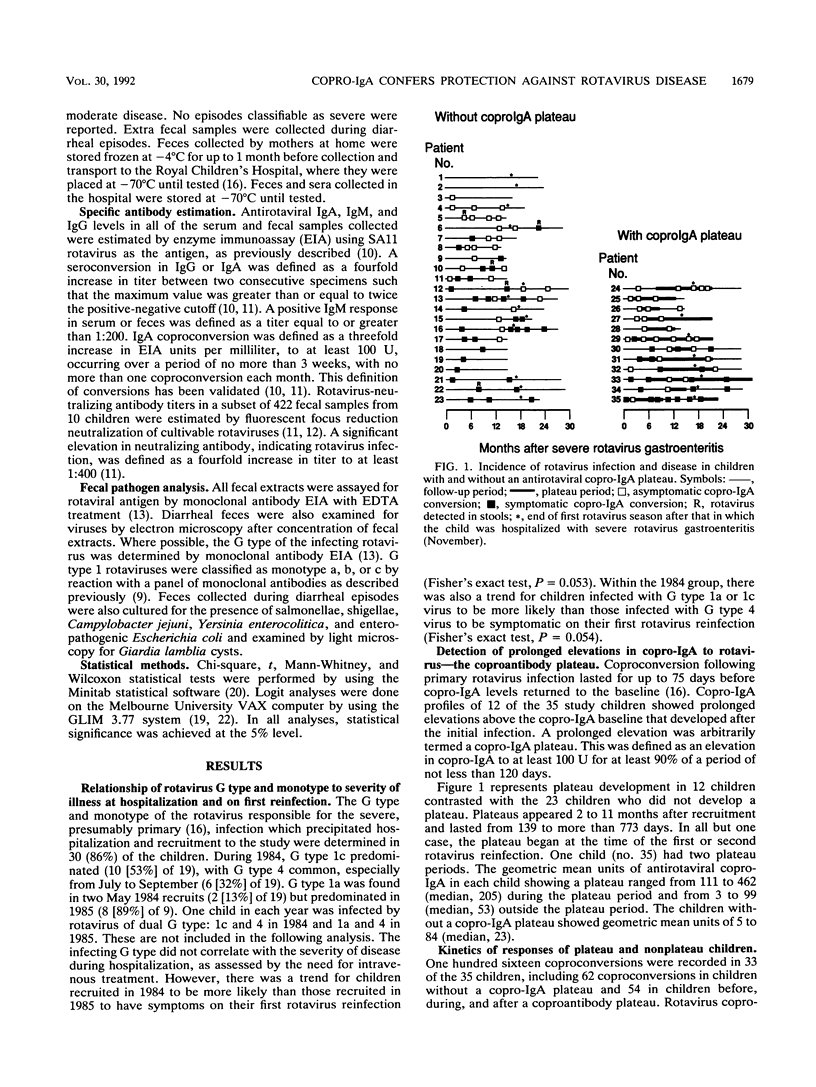
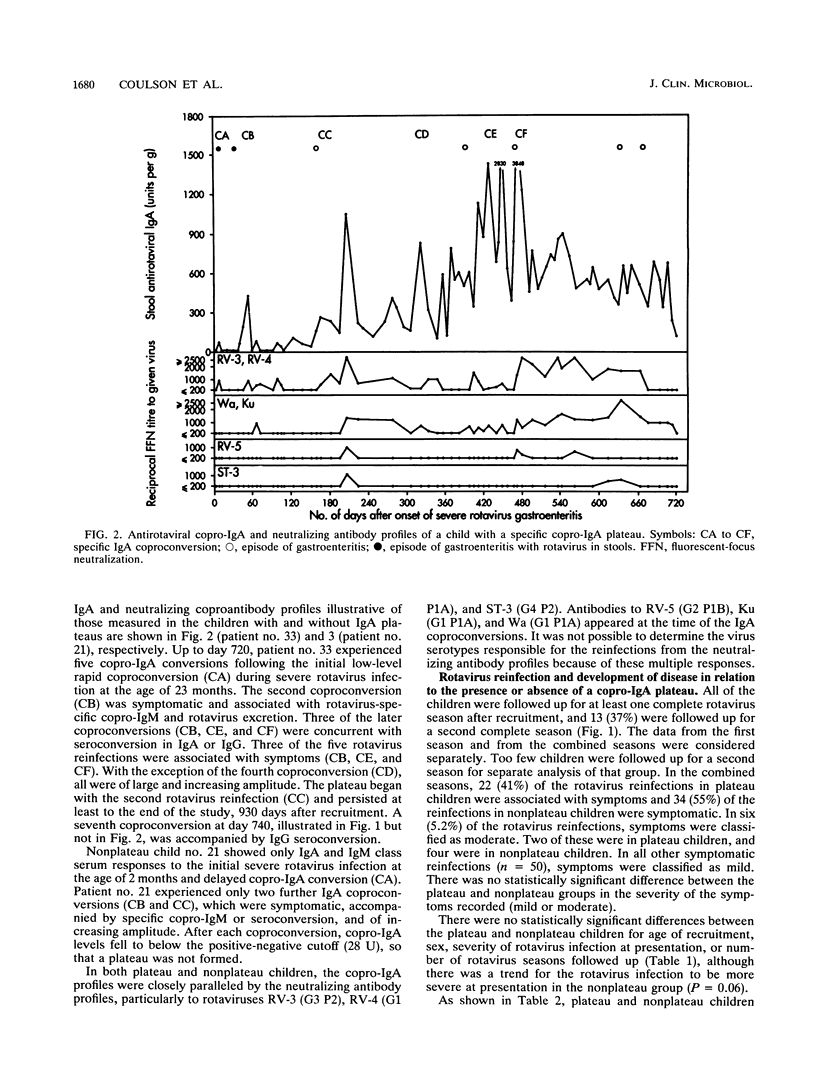
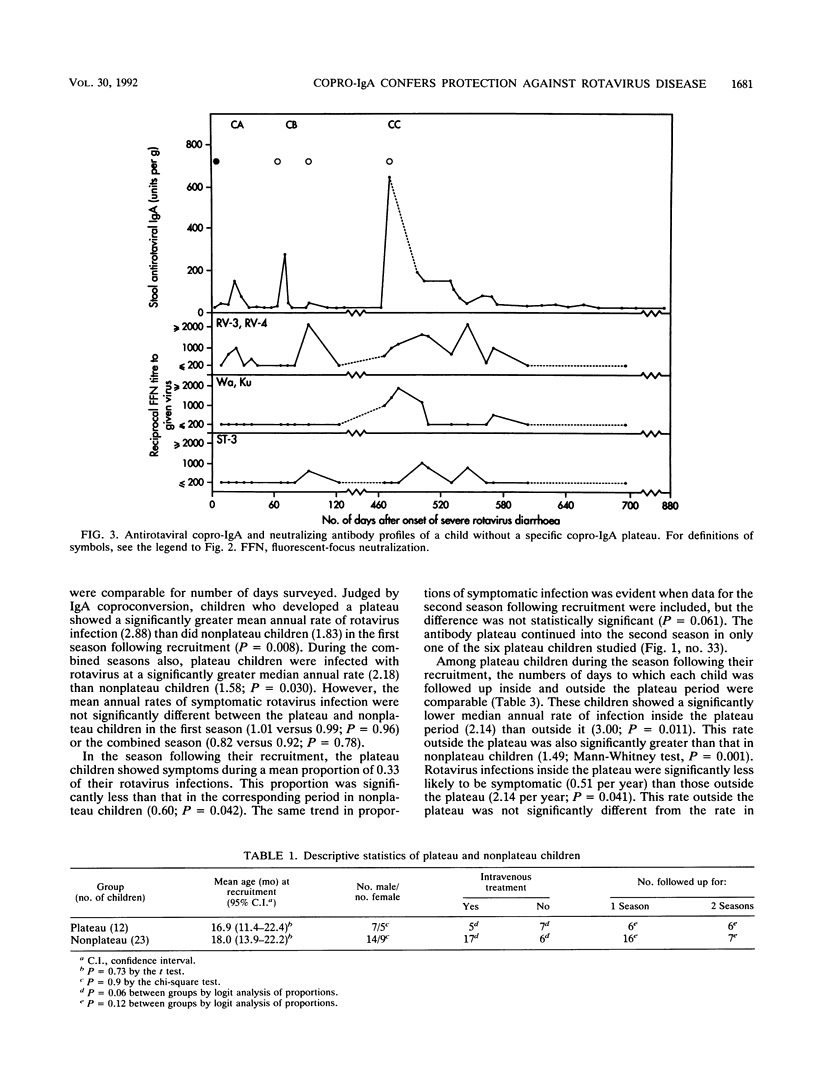
Rotavirus is the major cause of severe, dehydrating infantile gastroenteritis. Infection is limited to the gut, but the relative roles of serum and secretory copro-immunoglobulin A (IgA) in protection are unclear. Specific copro-IgA is predictive of duodenal antirotaviral IgA and correlates with virus-neutralizing coproantibody. Copro-IgA conversion is a more sensitive marker of rotavirus reinfection than seroconversion. We measured rotavirus reinfections by copro-IgA conversion prospectively in 35 children recruited at a time of severe rotavirus illness. The children were followed up longitudinally for 14 to 31 months to determine whether high coproantibody levels correlated with clinical protection against rotavirus disease. Ninety-four percent of the children experienced reinfection, and 38% developed persistent elevations in specific copro-IgA termed plateaus. Plateau children had a higher mean annual rate of rotavirus infection and a lower ratio of symptomatic to total number of rotavirus reinfections than did nonplateau children. The annual rates of rotavirus infection and disease were significantly higher outside the plateau than inside it in children experiencing antirotavirus copro-IgA plateaus. Frequent rotavirus infection of children appears to stimulate production of a specific copro-IgA plateau which correlates with protection against an excess of infection and symptomatic disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein D. I., Sander D. S., Smith V. E., Schiff G. M., Ward R. L. Protection from rotavirus reinfection: 2-year prospective study. J Infect Dis. 1991 Aug;164(2):277–283. doi: 10.1093/infdis/164.2.277. [DOI] [PubMed] [Google Scholar]
- Bishop R. F., Barnes G. L., Cipriani E., Lund J. S. Clinical immunity after neonatal rotavirus infection. A prospective longitudinal study in young children. N Engl J Med. 1983 Jul 14;309(2):72–76. doi: 10.1056/NEJM198307143090203. [DOI] [PubMed] [Google Scholar]
- Bishop R. F., Tzipori S. R., Coulson B. S., Unicomb L. E., Albert M. J., Barnes G. L. Heterologous protection against rotavirus-induced disease in gnotobiotic piglets. J Clin Microbiol. 1986 Dec;24(6):1023–1028. doi: 10.1128/jcm.24.6.1023-1028.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop R. F., Unicomb L. E., Barnes G. L. Epidemiology of rotavirus serotypes in Melbourne, Australia, from 1973 to 1989. J Clin Microbiol. 1991 May;29(5):862–868. doi: 10.1128/jcm.29.5.862-868.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Yokoyama T., Nakata S., Morita Y., Urasawa T., Taniguchi K., Urasawa S., Nakao T. Protective effect of naturally acquired homotypic and heterotypic rotavirus antibodies. Lancet. 1986 Aug 23;2(8504):417–421. doi: 10.1016/s0140-6736(86)92133-1. [DOI] [PubMed] [Google Scholar]
- Coulson B. S., Grimwood K., Bishop R. F., Barnes G. L. Evaluation of end-point titration, single dilution and capture enzyme immunoassays for measurement of antirotaviral IgA and IgM in infantile secretions and serum. J Virol Methods. 1989 Oct;26(1):53–65. doi: 10.1016/0166-0934(89)90074-8. [DOI] [PubMed] [Google Scholar]
- Coulson B. S., Grimwood K., Masendycz P. J., Lund J. S., Mermelstein N., Bishop R. F., Barnes G. L. Comparison of rotavirus immunoglobulin A coproconversion with other indices of rotavirus infection in a longitudinal study in childhood. J Clin Microbiol. 1990 Jun;28(6):1367–1374. doi: 10.1128/jcm.28.6.1367-1374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S., Masendycz P. J. Measurement of rotavirus-neutralizing coproantibody in children by fluorescent focus reduction assay. J Clin Microbiol. 1990 Jul;28(7):1652–1654. doi: 10.1128/jcm.28.7.1652-1654.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S., Unicomb L. E., Pitson G. A., Bishop R. F. Simple and specific enzyme immunoassay using monoclonal antibodies for serotyping human rotaviruses. J Clin Microbiol. 1987 Mar;25(3):509–515. doi: 10.1128/jcm.25.3.509-515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S. Variation in neutralization epitopes of human rotaviruses in relation to genomic RNA polymorphism. Virology. 1987 Aug;159(2):209–216. doi: 10.1016/0042-6822(87)90457-0. [DOI] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., Torsellini M., Torre D., Parea M., Battaglia M. Group- and type-specific serologic response in infants and children with primary rotavirus infections and gastroenteritis caused by a strain of known serotype. J Infect Dis. 1990 Jun;161(6):1105–1111. doi: 10.1093/infdis/161.6.1105. [DOI] [PubMed] [Google Scholar]
- Gouvea V., Glass R. I., Woods P., Taniguchi K., Clark H. F., Forrester B., Fang Z. Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990 Feb;28(2):276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood K., Lund J. C., Coulson B. S., Hudson I. L., Bishop R. F., Barnes G. L. Comparison of serum and mucosal antibody responses following severe acute rotavirus gastroenteritis in young children. J Clin Microbiol. 1988 Apr;26(4):732–738. doi: 10.1128/jcm.26.4.732-738.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio O., Ishihara Y., Isomura S., Inoue H., Inouye S. Long-term follow-up of infants from birth for rotavirus antigen and antibody in the feces. Acta Paediatr Jpn. 1988 Aug;30(4):497–504. doi: 10.1111/j.1442-200x.1988.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F. Protection against rotavirus-induced gastroenteritis in a murine model by passively acquired gastrointestinal but not circulating antibodies. J Virol. 1985 Apr;54(1):58–64. doi: 10.1128/jvi.54.1.58-64.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Cunningham S. L., Dudzik K. I. Memory and distribution of virus-specific cytotoxic T lymphocytes (CTLs) and CTL precursors after rotavirus infection. J Virol. 1991 Mar;65(3):1318–1324. doi: 10.1128/jvi.65.3.1318-1324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D. R., Wells P. W. Rotavirus infection in lambs: studies on passive protection. Arch Virol. 1976;52(3):201–205. doi: 10.1007/BF01348017. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Bernstein D. I., Shukla R., McNeal M. M., Sherwood J. R., Young E. C., Schiff G. M. Protection of adults rechallenged with a human rotavirus. J Infect Dis. 1990 Mar;161(3):440–445. doi: 10.1093/infdis/161.3.440. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., Mebus C. A., Yolken R. H., Kalica A. R., James H. D., Jr, Kapikian A. Z., Chanock R. M. Rotaviral immunity in gnotobiotic calves: heterologous resistance to human virus induced by bovine virus. Science. 1979 Feb 9;203(4380):548–550. doi: 10.1126/science.216077. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Inouye S., Yamauchi M., Morishima T., Matsuno S., Isomura S., Suzuki S. Anamnestic response in fecal IgA antibody production after rotaviral infection of infants. J Infect Dis. 1985 Aug;152(2):398–400. doi: 10.1093/infdis/152.2.398. [DOI] [PubMed] [Google Scholar]
- Yasukawa M., Nakagomi O., Kobayashi Y. Rotavirus induces proliferative response and augments non-specific cytotoxic activity of lymphocytes in humans. Clin Exp Immunol. 1990 Apr;80(1):49–55. doi: 10.1111/j.1365-2249.1990.tb06440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



