Abstract
Models of the differentiation of memory CD8+ T cells that replicate during secondary infections differ over whether such cells had acquired effector function during primary infections. We created a transgenic mouse line that permits mapping of the fate of granzyme B (gzmB)-expressing CD8+ T cells and their progeny by indelibly marking them with EYFP. Virus-specific CD8+ T cells express gzmB within the first two days of a primary response to infection with influenza without impairing continued primary clonal expansion. On secondary infection, virus-specific CD8+ T cells that became EYFP+ during a primary infection clonally expand as well as all virus-specific CD8+ T cells. Therefore, CD8+ T cells that have acquired an effector phenotype during primary infection may function as memory cells with replicative function.
During a primary immune response, naïve, pathogen-specific CD8+ T cells replicate and generate effector cells that control the primary infection, and “memory” cells that persist after resolution of the primary infection and respond to secondary infections. Two types of memory cells have been identified: a subset that resides in the peripheral tissues and has immediate effector function, such as production of IFN-γ and cytolytic activity, but cannot replicate, and a subset that maintains a capacity for clonal expansion and generation of effector cells that are required for control secondary or persistent infections (1). Since a single, antigen-specific CD8+ T cell can give rise to primary effector cells and both types of memory cells (2), only two models are possible for the development of memory cells with replicative potential: they arise directly from naïve CD8+ T cells and avoid effector differentiation (3), perhaps by a process of asymmetrical division (4), or they come from proliferating cells that have acquired effector function but have not irreversibly lost replicative capability (5, 6). Determining which model is correct is necessary to guide experimental approaches to defining optimal vaccine strategies. We generated a mouse model that enables conditional, irreversible marking of CD8+ T cells that have acquired an effector function, the expression of the cytolytic granule protein, granzyme B (gzmB). In the asymmetrical division model, gzmB expression is considered to identify the daughter T cell that is committed to loss of secondary replicative function (4).
We created a transgenic mouse line using a bacterial artificial chromosome (BAC) containing the gzmB gene, which had been modified by inserting at the start codon the tamoxifen-inducible, site-specific recombinase, CreERT2 (Fig. S1) (7, 8). We crossed this gzmBCreERT2 BAC transgenic line with the ROSA26EYFP reporter line in which EYFP is expressed following Cre-mediated excision of a loxP-flanked stop codon (9). Thus, in gzmBERT2/ROSAEYFP mice, cells that transcribe the gzmB gene will express CreERT2, but such cells may become EYFP+ only in the presence of tamoxifen. This enables the fate-mapping of such cells without the need for adoptive transfer, which may alter the dynamics of clonal expansion (10). Furthermore, a BAC transgene containing the gzmB gene for regulating expression of the Cre recombinase circumvents the problem of earlier studies (11) using a truncated human GzmB promoter that did not accurately reflect expression of the endogenous gzmB gene (12).
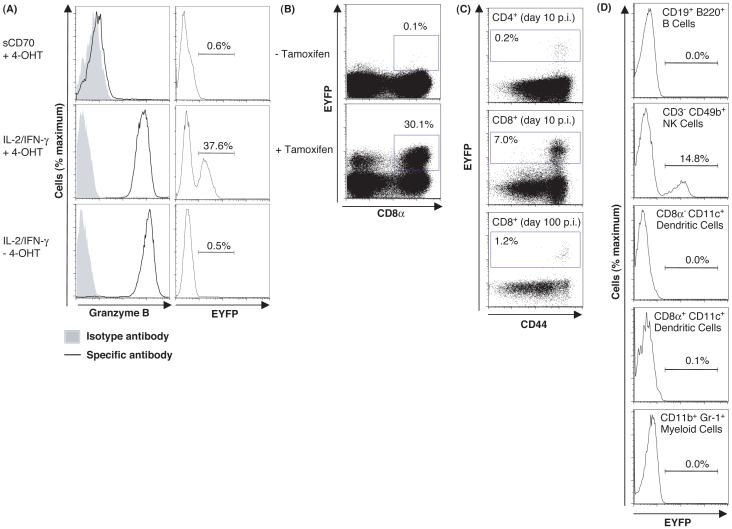
A requirement for both gzmB transcription and tamoxifen to induce EYFP was assessed by culturing CD8+ T cells from gzmBCreERT2/ROSA26EYFP mice in gzmB inducing or non-inducing conditions, with or without 4-hydroxytamoxifen (4-OHT). EYFP was observed only with culture conditions that induced gzmB synthesis by CD8+ T cells in the presence of 4-OHT (Fig. 1A). CD8+ T cells from the lungs of mice on day 10 of influenza infection were EYFP+ also only if tamoxifen had been present (Fig. 1B). EYFP was expressed only by antigen-experienced, CD44high CD4+ and CD8+ T cells at day 10 and day 100 post-infection (p.i.) (Fig. 1C), and by NK cells, but not by B cells, dendritic cells or myelomonocytic cells (Fig. 1D). Therefore, induction of EYFP is stringently restricted to cells expressing gzmB in the presence of tamoxifen. The proportion of EYFP+ cells correlated with the magnitude of gzmB expression (Fig. S2), suggesting that inefficient Cre-mediated recombination accounted for the occurrence of gzmB-expressing CD8+ T cells that were EYFP-, despite the presence of tamoxifen.
Fig. 1.
Requirements for expression of EYFP by cells from gzmBCreERT2/ROSA26EYFP mice. (A) Naive CD8+ T cells from gzmBCreERT2/ROSA26EYFP mice were stimulated for 6 days under gzmB-inducing (anti-CD3ε, IL-2, IFN-γ, and IL-7) or non-inducing conditions (anti-CD3ε, sCD70, IL-7, and antibodies to IL-2, CD25, and IFN-γ), in the presence or absence of 4-OHT and assessed for expression of gzmB and EYFP. (B) gzmBCreERT2/ROSA26EYFP mice were infected intranasally with the HKx/31 strain of influenza and received tamoxifen or carrier alone on days 1-8 p.i. The lungs were assessed on day 10 p.i. for EYFP+ CD8+ T cells. (C) Splenic CD4+ and CD8+ T cells were evaluated for expression of EYFP and CD44 on days 10 and 100 p.i., and (D) splenic B cells, NK cells, dendritic cells, and myeloid cells were assessed for EYFP on day 10 p.i.
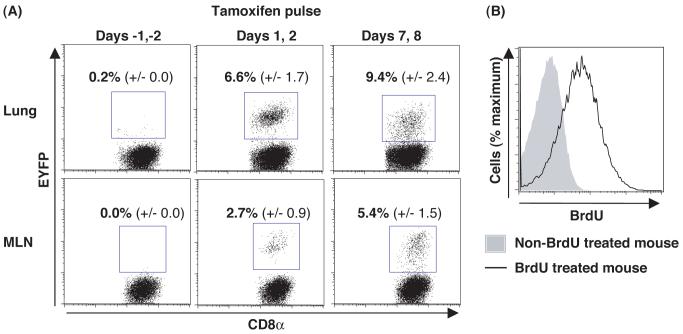
To determine whether gzmB expression by CD8+ T cells early during a primary response impairs clonal expansion, mice were infected intranasally with influenza, pulsed with tamoxifen on days 1 and 2 or days 7 and 8 p.i., and assessed on day 10 p.i. for the presence in the lungs and mediastinal lymph nodes (MLNs) of EYFP+ CD8+ T cells that were specific for the H-2Db/nucleoprotein (NP) peptide complex. Db/NP-specific CD8+ T cells in the MLNs were EYFP+ even when tamoxifen was given only during the initial phase of clonal expansion, indicating that gzmB is expressed by virus-specific CD8+ T cells in the first few cell cycles, and that such cells continue to proliferate and generate EYFP+ cells that migrate to the lungs (Fig. 2A). Their continued replication was confirmed by incorporation of BrdU during days 5-9 (Fig. 2B). Administering tamoxifen on days 7 and 8 induced only slightly higher percentages of EYFP+ cells among the Db/NP-specific populations, corroborating that early gzmB expression does not impair subsequent clonal expansion. Finally, the pulse characteristics of tamoxifen-mediated CreERT2 function were confirmed by the absence of EYFP+ Db/NP-specific CD8+ T cells in mice that had received tamoxifen on the two days preceding viral infection (Fig. 2A).
Fig 2.
Clonal expansion by CD8+ T cells that had expressed gzmB during the first days of influenza infection. (A) gzmBCreERT2/ROSA26EYFP mice were infected intranasally with the HKx/31 strain of influenza and were given tamoxifen 2 days prior to infection, on days 1 and 2 p.i., or on days 7 and 8 p.i.. On day 10 p.i. we determined the proportion (mean ± SEM) of Db/NP-pentamer-binding CD8+ T cells that was EYFP+. (B) Influenza-infected mice that had received tamoxifen on days 1 and 2 were given BrdU on days 5-9 p.i., and EYFP+ CD8+ T cells from the lungs were assessed on day 10 p.i for incorporation of BrdU.
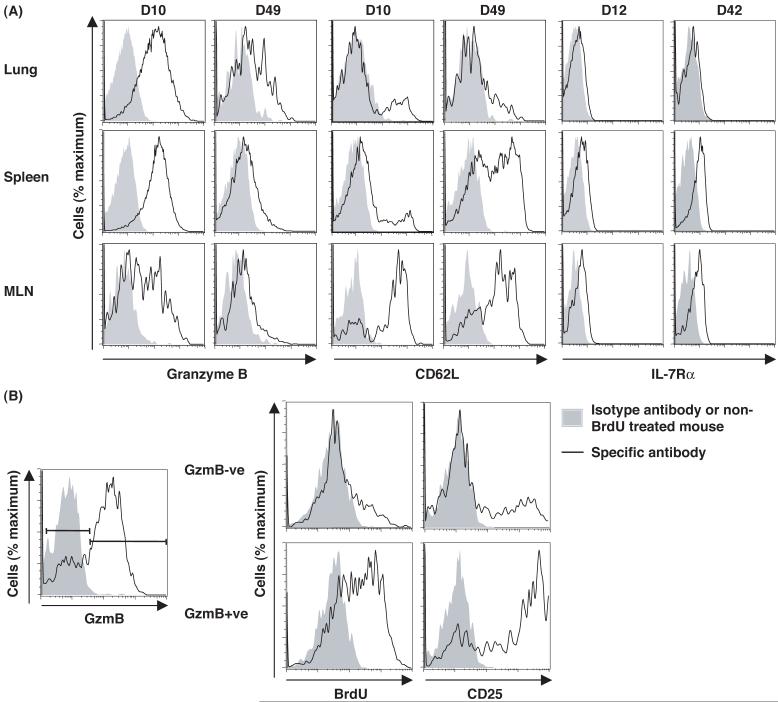
The capacity to mark irreversibly a cohort of cells that had expressed gzmB permitted an analysis of changes in their phenotypic characteristics over time. On day 10 of the primary response to influenza with tamoxifen administered on days 1-8, almost all EYFP+ CD8+ T cells in the lungs and spleen expressed gzmB, whereas a subset of EYFP+ cells in the MLNs had become gzmB- (Fig. 3A). By day 49, most EYFP+ CD8+ T cells had lost expression of gzmB except for a small sub-population in the lungs. A decrease in gzmB expression among virus-specific CD8+ T cells during the phase following clearance of a primary infection has been considered to exemplify a requirement for a “rest” period during which effector cells convert to memory cells (5). The present finding that CD8+ T cells have the capacity to switch gzmB expression on and off during the acute phase of the primary response suggests that decreased biosynthesis may be caused by diminished inducing signals, at least within the MLN. Consistent with this possibility, when tamoxifen was administered on days 1-4 p.i. and BrdU during the last 12 hours, before analysis on day 8 p.i., the EYFP+ CD8+ T cells lacking expression of gzmB were mainly CD25- and BrdU-, whereas the gzmB+ cells expressed high levels of CD25 and were BrdU+ (Fig. 3B). Thus, loss of gzmB expression may reflect interrupted signaling through the IL-2 receptor.
Fig 3.
Expression of gzmB, CD62L, IL-7Rα, and CD25 by EYFP+ CD8+ T cells responding to influenza infection. (A) gzmBCreERT2/ROSA26EYFP mice were infected intranasally with the HKx/31 strain of influenza and treated with tamoxifen on days 1-8 p.i. The EYFP+ CD8+ T cells from the lungs, MLNs and spleen were analyzed for intracellular gzmB, CD62L, and IL-7Rα at the peak of the primary response and during the memory phase (6 or 7 weeks p.i.). (B) Influenza-infected mice were given tamoxifen on days 1-4 p.i. and BrdU 12 hours prior to analysis on day 8 for BrdU incorporation and CD25 expression by EYFP+ CD8+ T cells from the MLNs.
The loss of expression of CD62L and IL-7Rα by CD8+ T cells during the primary response correlates with diminished survival and replicative capability in the memory phase of the response (5, 6). On day 10, EYFP+ CD8+ T cells in the MLNs, the site of clonal expansion, but not in the spleen or lungs, remained CD62Lhigh (Fig. 3A). Therefore, diminished expression of CD62L is not necessarily linked to expression of gzmB, as has been suggested (4). By day 49, CD62Lhigh EYFP+ CD8+ T cells were also present in the spleen, but not in the lung, indicating either that during the memory phase CD62Lhigh cells from the MLNs migrate to the lymphoid areas of the spleen, that there is selective loss of CD62Llow cells in the spleen, or that both occur. Similarly, by day 42, EYFP+ CD8+ T cells expressing IL-7Rα were present in the MLNs and spleen, but all EYFP+ cells in the lungs lacked this receptor (Fig. 3A). Therefore, the phenotype of CD62Lhigh and IL-7Rα+ of memory cells that have expressed gzmB correlates with their anatomic site, and not with whether they have exhibited this differentiated function.
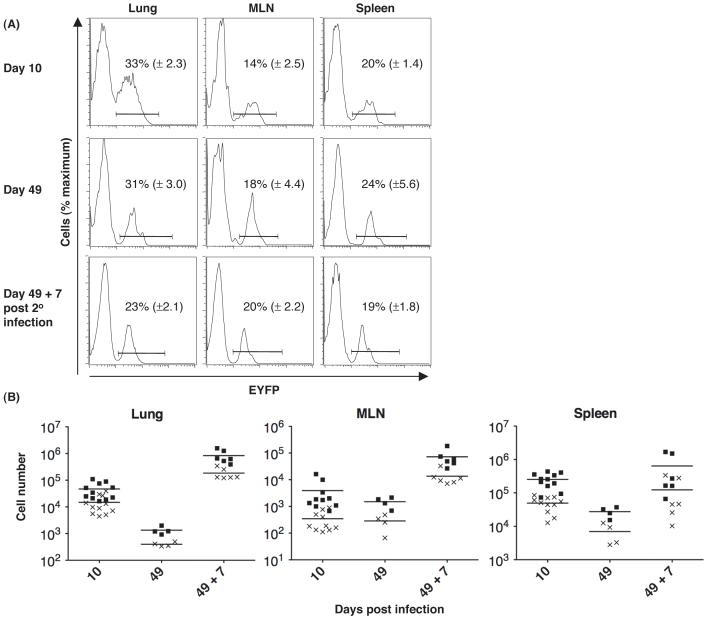
We examined whether EYFP+ Db/NP-specific CD8+ T cells persisted into the memory phase and responded secondarily to influenza infection. The proportion of Db/NP-specific CD8+ T cells in the lungs, MLNs and spleen that were EYFP+ did not change between days 10 and 49 p.i. (p > 0.4), indicating that expression of gzmB during the primary response does not destine a cell for contraction (Fig. 4A). Furthermore, the proportion of EYFP+ Db/NP-specific cells in the MLNs remained the same after secondary viral infection, suggesting that these cells replicated as well as all Db/NP-specific memory CD8+ T cells. The modest decrease in the percent of EYFP+ cells in the lungs after secondary expansion may reflect the presence at day 49 of cells that had been labeled at this peripheral site during the primary infection and were unable to replicate during the secondary infection.
Fig 4.
Absence of impaired secondary clonal expansion by CD8+ T cells that had expressed gzmB during primary influenza infection. gzmBCreERT2/ROSA26EYFP mice were intranasally infected with the HKx/31 strain of influenza and given tamoxifen on days 1-8 p.i. On days 10 and 49 p.i., CD8+ T cells from the lungs, MLNs, and spleens were assessed for the binding of Db/NP pentamers and expression of EYFP+. The mice were challenged on day 49 p.i. with the PR8 strain of influenza in the absence of tamoxifen, and the same measurements were performed 7 days later. (A) The proportions (mean ± SEM) of Db/NP-specific CD8+ T cells that were EYFP+ are shown. (B) The numbers of total (squares) and EYFP+ (crosses) Db/NP-specific CD8+ T cells in the lungs, MLNs, and spleens of each mouse are shown.
Equivalent replicative capability of the EYFP+ and total Db/NP-specific CD8+ T cells was confirmed by finding approximately 500-fold expansion of both populations in the lungs on day 7 post-secondary infection (Fig. 4B). There were lower, although again comparable, increases in these two populations in the MLNs (Fig. 4B) where most new effector cells in the lungs are likely to be generated, and also in the spleen.
The capacity to replicate secondarily was also determined for EYFP+ Db/NP-specific CD8+ T cells that had been marked by tamoxifen pulses on days 1-3 and days 7-9, respectively, during primary influenza infection. On day 7 post-secondary infection, the EYFP+ Db/NP-specific CD8+ T cells in the two groups had similar fold increments in the MLNs, spleens and lungs, and these were comparable to those of the total antigen-specific cells (Fig. S3). This finding further excludes models of differentiation in which early expression of gzmB marks a CD8+ T cell that has lost a capacity for secondary replicative function.
In summary, the conditional and indelible marking of CD8+ T cells that had previously expressed gzmB permitted their identification among all subsets of CD8+ T cells in the primary and memory phases of an anti-viral response, including the subset that mediates secondary clonal expansion. Combined with the observation that a CD8+ T cell may express gzmB early in the primary response without preventing continued expansion (Fig. 2A), one may conclude that such cells can self-renew and serve as progenitors of the more differentiated, senescent cells that have diminished expression of CD62L and IL-7Rα (Fig. 3A). Although EYFP was not induced in all gzmB-expressing cells because of inefficient Cre-mediated recombination, the EYFP+ memory CD8+ T cell population was representative of the entire memory population with respect to survival and secondary expansion. Therefore, memory CD8+ T cells that proliferate during secondary infections can be derived from cells that had acquired an effector phenotype during the primary response, as has been proposed (5, 6). This conclusion is consistent with the IL-2R dependence of gzmB expression (Fig. 3B) and development of memory cells with replicative function (13), and with the secondary replication of IFN-γ-expressing memory CD4+ T cells (14). However, it does not support the asymmetrical division model in which the gzmB-expressing daughter cell of the first division of the activated naïve CD8+ T cell is restricted to a non-replicative memory cell fate (4). Thus, as yet undefined signals, in addition to those leading to acquisition of gzmB-dependent effector functions and that cannot be defined in this experimental system, must account for terminal differentiation and senescence of the CD8+ T cell.
Supplementary Material
Acknowledgments
The authors thank D. Winton for ROSA26EYFP mice, B. Crombrugghe for the CreERT2 construct, G. Eberl for the recombineering shuttle vector and P. Digard and E. Hutchinson for help with viruses. This work was funded by the Wellcome Trust.
References and notes
- 1.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Nature. 1999;401:708. [PubMed] [Google Scholar]
- 2.Stemberger C, et al. Immunity. 2007;27:985. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Fearon DT, Manders P, Wagner SD. Science. 2001;293:248. doi: 10.1126/science.1062589. [DOI] [PubMed] [Google Scholar]
- 4.Chang JT, et al. Science. 2007;315:1687. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 5.Wherry EJ, et al. Nat Immunol. 2003;4:225. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 6.Joshi NS, et al. Immunity. 2007;27:281. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feil R, Wagner J, Metzger D, Chambon P. Biochem Biophys Res Commun. 1997;237:752. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- 8.(Materials and Methods are available as supporting material Science online)
- 9.Srinivas S, et al. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marzo AL, et al. Nat Immunol. 2005;6:793. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacob J, Baltimore D. Nature. 1999;399:593. doi: 10.1038/21208. [DOI] [PubMed] [Google Scholar]
- 12.Hanson RD, Sclar GM, Kanagawa O, Ley TJ. J Biol Chem. 1991;266:24433. [PubMed] [Google Scholar]
- 13.Williams MA, Tyznik AJ, Bevan MJ. Nature. 2006;441:890. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Nature. 2008;452:356. doi: 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.