Abstract
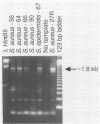
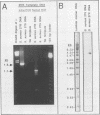
A polymerase chain reaction (PCR)-based test was developed for the detection of mecA in staphylococci. To facilitate this process, a rapid cell lysis procedure was established for the release of DNA from staphylococcal strains. Primers based on the DNA sequence of the mecA gene from Staphylococcus aureus were used in PCRs to screen for the presence of this gene in a total of 98 staphylococcal isolates. Fifty-one isolates were mecA positive (17 S. aureus strains and 34 coagulase-negative staphylococci including S. epidermidis, S. haemolyticus, and S. simulans). Results obtained with PCRs were generally consistent with those of standard microbiological assays. PCRs designed to detect the femA gene (factor essential for methicillin resistance) revealed the presence of the gene in all S. aureus strains examined regardless of the susceptibility profiles of the strains to methicillin. In contrast, femA could not be detected in coagulase-negative staphylococci by PCR with the same primers. Low-stringency hybridization suggested the presence of a gene structurally related to femA in S. epidermidis and other coagulase-negative staphylococci examined.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G. L., Pennell E. Detection of methicillin resistance in staphylococci by using a DNA probe. Antimicrob Agents Chemother. 1990 Sep;34(9):1720–1724. doi: 10.1128/aac.34.9.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N. Reliability of high-content disks and modified broth dilution tests for detecting staphylococcal resistance to the penicillinase-resistant penicillins. J Clin Microbiol. 1987 Oct;25(10):1897–1901. doi: 10.1128/jcm.25.10.1897-1901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Bächi B., Barberis-Maino L., Strässle A., Kayser F. H. FemA, a host-mediated factor essential for methicillin resistance in Staphylococcus aureus: molecular cloning and characterization. Mol Gen Genet. 1989 Oct;219(1-2):263–269. doi: 10.1007/BF00261186. [DOI] [PubMed] [Google Scholar]
- Brisson-Noël A., Gicquel B., Lecossier D., Lévy-Frébault V., Nassif X., Hance A. J. Rapid diagnosis of tuberculosis by amplification of mycobacterial DNA in clinical samples. Lancet. 1989 Nov 4;2(8671):1069–1071. doi: 10.1016/s0140-6736(89)91082-9. [DOI] [PubMed] [Google Scholar]
- Canawati H. N., Witte J. L., Sapico F. L. Temperature effect on the susceptibility of methicillin-resistant Staphylococcus aureus to four different cephalosporins. Antimicrob Agents Chemother. 1982 Jan;21(1):173–175. doi: 10.1128/aac.21.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F., Archer G., Matsuhashi M. Low-level methicillin resistance in strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1989 Apr;33(4):424–428. doi: 10.1128/aac.33.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F. Coagulase-negative staphylococci resistant to beta-lactam antibiotics in vivo produce penicillin-binding protein 2a. Antimicrob Agents Chemother. 1987 Dec;31(12):1919–1924. doi: 10.1128/aac.31.12.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F., Hackbarth C. J. Effect of NaCl and nafcillin on penicillin-binding protein 2a and heterogeneous expression of methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1987 Dec;31(12):1982–1988. doi: 10.1128/aac.31.12.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke K. G. Penicillinase production and intrinsic resistance to penicillins in methicillin-resistant cultures of Staphylococcus aureus. J Med Microbiol. 1969 Aug;2(3):261–278. doi: 10.1099/00222615-2-3-261. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I. The polymerase chain reaction. A new method of using molecular genetics for medical diagnosis. N Engl J Med. 1990 Jan 18;322(3):178–183. doi: 10.1056/NEJM199001183220307. [DOI] [PubMed] [Google Scholar]
- Fitts R., Diamond M., Hamilton C., Neri M. DNA-DNA hybridization assay for detection of Salmonella spp. in foods. Appl Environ Microbiol. 1983 Nov;46(5):1146–1151. doi: 10.1128/aem.46.5.1146-1151.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franciolli M., Bille J., Glauser M. P., Moreillon P. Beta-lactam resistance mechanisms of methicillin-resistant Staphylococcus aureus. J Infect Dis. 1991 Mar;163(3):514–523. doi: 10.1093/infdis/163.3.514. [DOI] [PubMed] [Google Scholar]
- Hackbarth C. J., Chambers H. F. Methicillin-resistant staphylococci: detection methods and treatment of infections. Antimicrob Agents Chemother. 1989 Jul;33(7):995–999. doi: 10.1128/aac.33.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackbarth C. J., Chambers H. F. Methicillin-resistant staphylococci: genetics and mechanisms of resistance. Antimicrob Agents Chemother. 1989 Jul;33(7):991–994. doi: 10.1128/aac.33.7.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski E., Moomaw E. W., Tullis R. H., Ruth J. L. Preparation of oligodeoxynucleotide-alkaline phosphatase conjugates and their use as hybridization probes. Nucleic Acids Res. 1986 Aug 11;14(15):6115–6128. doi: 10.1093/nar/14.15.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. M., Tyler S. D., Ewan E. P., Ashton F. E., Pollard D. R., Rozee K. R. Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin 1 in Staphylococcus aureus by the polymerase chain reaction. J Clin Microbiol. 1991 Mar;29(3):426–430. doi: 10.1128/jcm.29.3.426-430.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligozzi M., Rossolini G. M., Tonin E. A., Fontana R. Nonradioactive DNA probe for detection of gene for methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1991 Mar;35(3):575–578. doi: 10.1128/aac.35.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maple P. A., Hamilton-Miller J. M., Brumfitt W. World-wide antibiotic resistance in methicillin-resistant Staphylococcus aureus. Lancet. 1989 Mar 11;1(8637):537–540. doi: 10.1016/s0140-6736(89)90076-7. [DOI] [PubMed] [Google Scholar]
- McDougal L. K., Thornsberry C. The role of beta-lactamase in staphylococcal resistance to penicillinase-resistant penicillins and cephalosporins. J Clin Microbiol. 1986 May;23(5):832–839. doi: 10.1128/jcm.23.5.832-839.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Minamide W., Wada K., Nakamura E., Teraoka H., Watanabe S. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol. 1991 Oct;29(10):2240–2244. doi: 10.1128/jcm.29.10.2240-2244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive D. M. Detection of enterotoxigenic Escherichia coli after polymerase chain reaction amplification with a thermostable DNA polymerase. J Clin Microbiol. 1989 Feb;27(2):261–265. doi: 10.1128/jcm.27.2.261-265.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryffel C., Kayser F. H., Berger-Bächi B. Correlation between regulation of mecA transcription and expression of methicillin resistance in staphylococci. Antimicrob Agents Chemother. 1992 Jan;36(1):25–31. doi: 10.1128/aac.36.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryffel C., Tesch W., Birch-Machin I., Reynolds P. E., Barberis-Maino L., Kayser F. H., Berger-Bächi B. Sequence comparison of mecA genes isolated from methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. Gene. 1990 Sep 28;94(1):137–138. doi: 10.1016/0378-1119(90)90481-6. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Wallace S. J., Gerstein D. A. Suppression of intrinsic resistance to methicillin and other penicillins in Staphylococcus aureus. Antimicrob Agents Chemother. 1972 Nov;2(5):350–355. doi: 10.1128/aac.2.5.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochetman G., Ou C. Y., Jones W. K. Polymerase chain reaction. J Infect Dis. 1988 Dec;158(6):1154–1157. doi: 10.1093/infdis/158.6.1154. [DOI] [PubMed] [Google Scholar]
- Song M. D., Wachi M., Doi M., Ishino F., Matsuhashi M. Evolution of an inducible penicillin-target protein in methicillin-resistant Staphylococcus aureus by gene fusion. FEBS Lett. 1987 Aug 31;221(1):167–171. doi: 10.1016/0014-5793(87)80373-3. [DOI] [PubMed] [Google Scholar]
- Tokue Y., Shoji S., Satoh K., Watanabe A., Motomiya M. Comparison of a polymerase chain reaction assay and a conventional microbiologic method for detection of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1992 Jan;36(1):6–9. doi: 10.1128/aac.36.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Drugeon H. B., de Lencastre H. M., Jabes D., McDougall L., Bille J. New mechanism for methicillin resistance in Staphylococcus aureus: clinical isolates that lack the PBP 2a gene and contain normal penicillin-binding proteins with modified penicillin-binding capacity. Antimicrob Agents Chemother. 1989 Nov;33(11):1869–1874. doi: 10.1128/aac.33.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonin E., Tomasz A. Beta-lactam-specific resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Oct;30(4):577–583. doi: 10.1128/aac.30.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. Y., Hoskins J., Blaszczak L. C., Preston D. A., Skatrud P. L. Construction of a water-soluble form of penicillin-binding protein 2a from a methicillin-resistant Staphylococcus aureus isolate. Antimicrob Agents Chemother. 1992 Mar;36(3):533–539. doi: 10.1128/aac.36.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lencastre H., Sá Figueiredo A. M., Urban C., Rahal J., Tomasz A. Multiple mechanisms of methicillin resistance and improved methods for detection in clinical isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 1991 Apr;35(4):632–639. doi: 10.1128/aac.35.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]