Abstract
Background and objectives: Long pentraxin 3 (PTX3) is a multimeric inflammatory mediator. Increased serum PTX3 levels have been reported among end-stage renal disease patients. Moreover, PTX3 has been suggested to represent a novel mortality risk factor, and elevated PTX3 levels have been shown to accompany increased albuminuria among patients with chronic kidney disease (CKD).
Design, setting, participants, & measurements: We analyzed data of 49 persons with stage 1 diabetic CKD and 32 healthy subjects in a prospective controlled trial. Endothelial dysfunction was determined by flow-mediated dilation (FMD). Serum PTX3, high-sensitivity C-reactive protein (hs-CRP) levels, and FMD were studied in baseline and after 12 wk of ramipril therapy. Stepwise multivariate regression analysis evaluated the association of FMD with clinical and serologic parameters.
Results: Serum PTX3, hsCRP, and albumin levels and proteinuria were significantly decreased, and FMD levels were significantly increased, after ramipril treatment. FMD was negatively correlated with serum PTX3, 24-h proteinuria, and hsCRP levels and positively correlated to serum albumin both at baseline and after the 12-wk treatment period. Multivariate regression analysis revealed that PTX3 levels were independently related to FMD both before and after ramipril treatment.
Conclusions: Our study shows that serum PTX3 levels are associated with endothelial dysfunction in patients with stage 1 diabetic CKD, independent of CRP. In addition, short-term ACE-inhibitor treatment significantly improves FMD and normalizes PTX3, hsCRP, and urinary protein excretion. (NCT: The study was registered in clinicaltrials.gov as NCT00674596.)
Low-grade albuminuria is the earliest clinically detectable abnormality in diabetic nephropathy, and the degree of urinary albumin loss strongly predicts the rate of progression to overt nephropathy (1). In addition, albuminuria is a strong predictor of cardiovascular disease (CVD), after adjustment for other recognized risk factors, including GFR (2,3). Although it has been hypothesized that albuminuria is a reflection of generalized vasculopathy rather than of localized renal injury (4), the putative mechanisms of this link remain poorly understood.
Although recent data suggest that albuminuria is strongly predictive of both endothelial dysfunction (ED) (5,6) and high levels of circulating inflammatory mediators (5,7,8), it is unknown whether these observations represent a spurious association or whether they indeed reflect a mechanistic link. Moreover, the role of novel, putatively more endothelium-specific inflammatory cytokines such as long pentraxin 3 (PTX3) (8–10) is also unclear. PTX3 is a recently discovered multimeric inflammatory mediator that shares structural homology with hepatic short pentraxins such as C-reactive protein (CRP) and serum amyloid P component, but, as opposed to these molecules, it is expressed by many cell types in response to stimuli (9).
We recently reported that PTX3 levels are elevated in CKD stage 5 patients (8,11), in whom they represent a risk predictor (11). Furthermore, we have shown that elevated PTX3 is associated with increased albuminuria in both CKD stages 1 and 5 and with ED in CKD stage 1 (8). In the present study, we aimed to test the hypothesis that the previously demonstrated improvement in ED after initiation of angiotensin-converting enzyme inhibitor (ACEI) (12) therapy is directly linked to the relative reduction of inflammatory markers, especially PTX3.
Materials and Methods
Patients and Controls
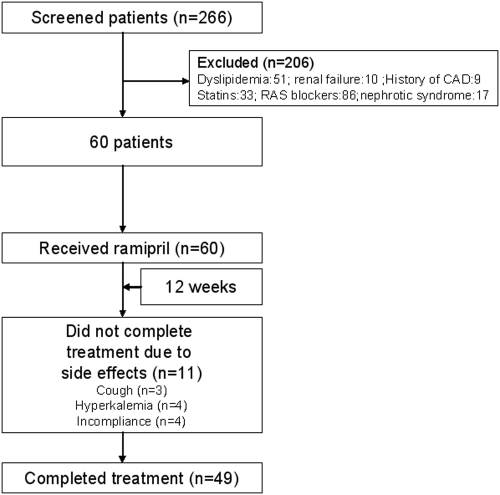
We performed a prospective study of selected patients referred to the Department of Nephrology, Gulhane School of Medicine Outpatient Clinics during the period of January 1st, 2006 to January 1st, 2008. From these referrals, after a 24-h urine collection, we selected patients with stage 1 CKD (24-h protein excretion ≥ 500 mg/d, systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg, respectively) but a normal estimated GFR (eGFR) (≥90 ml/min), who were older than 18 yr and characterized as having type 2 diabetes mellitus as the only cause of nephropathy (renal biopsy and medical history). A total of 266 patients fulfilled the above inclusion criteria, but from these, we then excluded patients who were previously treated using ACEI or angiotensin receptor blockers (ARBs, medical history); who were obesity (BMI > 30kg/m2); who had dyslipidemia (total cholesterol > 200 mg/dl, fasting triglycerides > 150 mg/dl), renal failure, (eGFR < 90 ml/min), nephrotic syndrome (urinary protein excretion > 3000 mg/d), or a history of CVD (medical history, abnormal electrocardiogram [ECG], see below); who smoked or had smoked within the last 3 mo and were taking either statins or ACEI or ARBs. The final cohort of eligible patients starting the study consisted of 60 individuals (29 men, age 47 ± 5 yr) (Figure 1). The duration of proteinuria and diabetic nephropathy in these patients before and after the initial diagnosis of diabetes was made was unknown.
Figure 1.
Flow chart of patients enrolled in the trial.
We also recruited 32 healthy subjects (16 men, age 46.9 ± 5.4 yr) to serve as controls. These individuals had no known diseases and were not currently taking any drugs. The control subjects were subject to the same inclusion and exclusion criteria as the patients. Informed consent to participate in the study was obtained from both patients and controls. The drug ethical committee of Gulhane School of Medicine approved the study, which was also registered at clinicaltrials.gov (NCT00674596: “The Effect of Renin Angiotensin System Blockage (RAS) On PTX3 Levels In Diabetic Patients With Proteinuria”).
Baseline Characterization
Recruited patients were evaluated by standard physical examination; chest x-ray; baseline ECG; two-dimensional echocardiography; and routine clinical laboratory tests, including liver and kidney function tests and 24-h urinary protein measurements. Arterial BP was measured in the right arm by mercury sphygmomanometer three times in a resting condition in the morning, and mean values were calculated for diastolic and systolic pressures. The exclusion criteria were as follows: nephrotic syndrome, coronary heart disease (patients with ischemic ST-T wave changes in electrocardiogram alterations and voltage criteria for LVH and a history of revascularization or myocardial infarction), elevated liver enzymes (aspartate amino transferase or alanine amino transferase levels ≥ 40 U/L), and renal failure (serum creatinine levels > 1.3 mg/dl).
Intervention and Follow-Up Measurements
In an open-label trial, patients were given an ACE inhibitor (ramipril, 5 mg once per day) for 12 wk immediately after baseline measurements. During the study period, serum creatinine and potassium concentrations were measured every 2 wk, and the dose of ramipril was titrated to achieve a serum potassium concentration of <5.5 mEq/L. The average dose of ramipril treatment was one tablet a day (5 mg/d). The medications were given with meals, and the doses were increased as needed to a maximum of 10 mg/d. After this period, blood samples were obtained for measurements as shown below. Urine samples were also collected over 24 h to determine the degree of proteinuria.
Blood Chemistry
Morning blood samples were collected from patients and control subjects after 12 h of fasting. Subjects were asked to refrain from physical activity for at least 30 min before the blood draw. In addition to routine clinical laboratory tests, serum PTX3 concentrations and basal insulin levels were analyzed from all patients. After the intervention period, blood samples were obtained for the measurement of serum PTX3 concentration, high-sensitivity C-reactive protein (hsCRP) levels, and insulin levels, as well as for determining the insulin resistance index. The measurement of total cholesterol (TC), triglyceride (TG), HDL cholesterol, and fasting plasma glucose (FPG) was performed by the enzymatic colorimetric method with an Olympus AU 600 auto analyzer, using reagents from Olympus Diagnostics, GmbH (Hamburg, Germany). LDL cholesterol was calculated by Friedewald's formula (13). The serum basal insulin value was determined by the coated tube method. In particular, the insulin resistance index homeostasis model assessment-insulin resistance (HOMA-IR) was computed with the formula: HOMA-IR = fasting plasma glucose(mg/dl) × immunoreactive insulin (μIU/ml)/405 (14). All samples were run in triplicate.
Plasma PTX-3 Measurements
In both patients and controls, plasma PTX-3 concentration was measured a posteriori from frozen samples by using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Perseus Proteomics Inc, Japan).
hsCRP Assessment
Serum samples were diluted at a ratio of 1:101 with the diluent solution. Calibrators, kit controls, and serum samples were all added on each micro well with an incubation period of 30 min. After three washing intervals, 100 μl enzyme conjugate (peroxidase-labeled anti-CRP) was added on each micro well for additional 15 min incubation at room temperature in the dark. The reaction was stopped with a stop solution, and photometric measurement was performed at the 450-nm wavelength. The amount of serum samples was calculated in milligrams per liter with a graphic that was made by noting the absorbance levels of the calibrators.
GFR Assessment
GFR was calculated according to the simplified version of the Modification of Diet in Renal Disease (MDRD) Study prediction equation formula, GFR = 186 × PCR−1.154 × age−0.203 × 1.212 (if black) × 0.742 (if female), as defined by Levey (15).
Assessment of Endothelial Dysfunction
The determination of endothelial dysfunction was performed according to the method described by Celemajer et al. (16). Measurements were made by a single observer using an ATL 5000 ultrasound system (Advanced Technology Laboratories Inc., Bothell, WA) with a 12-Mhz probe. All vasoactive medications were withheld for 24 h before the procedure. The subjects remained at rest in a supine position for at least 15 min before the examination started. The subject's right arm was comfortably immobilized in an extended position to allow consistent recording of the brachial artery 2 to 4 cm above the antecubital fossa. Three adjacent measurements of end-diastolic brachial artery diameter were made from single two-dimensional frames. All ultrasound images were recorded on S-VHS videotape for subsequent blinded analysis. The maximum FMD diameters were calculated as the average of the three consecutive maximum diameter measurements after hyperemia and nitroglycerin, respectively. The FMD levels were then calculated as the percent change in diameter compared with baseline resting diameters.
Statistical Analyses
All of the statistical analyses were performed by using SPSS 11.0 (SPSS Inc., Chicago, IL) statistical package. Non-normally distributed variables were expressed as median (range) and normally distributed variables were as mean ± SD as appropriate. A P value of <0.05 was considered to be statistically significant. One-sample Kolmogorov-Smirnov test was used for analysis distribution of data. One-way ANOVA, t test, and paired-sample t test were used to compare numeric data. Spearman's rank correlation was used to determine correlations with continuous variables. Stepwise multivariate regression analysis was used to assess the predictors for flow-mediated dilation levels.
Results
Baseline Characteristics
Baseline clinical and laboratory characteristic and vascular measurements for the study population are shown in Table 1. There were no differences between diabetic patients and the control group with respect to age, sex, eGFR, and BMI. As expected, serum PTX3, 24-h proteinuria, HbA1c, serum albumin, HOMA, SBP, DBP, and hs-CRP levels were higher in diabetic patients. Moreover, FMD levels were lower in diabetic patients than in controls (Table 1).
Table 1.
Baseline clinical and laboratory characteristics of 49 diabetic patients with proteinuria and controls, and longitudinal changes after 12 wk of ramipril therapy
| Characteristic | Controls (n = 32) | Ramipril (n = 49) Baseline | Ramipril (n = 49) Follow-Up |
|---|---|---|---|
| Age (years) | 46 ± 5 | 47 ± 6 | 47 ± 6 |
| Gender (M/F) | 19/13 | 23/26 | |
| BMI (kg/m2) | 24.1 ± 2.1 | 25.6 ± 2.9 | 25.0 ± 2.9 |
| Total Cholesterol (mg/dl) | 140.3 ± 23.4 | 174.8 ± 25.8a | 168.4 ± 28.1 |
| Triglycerides (mg/dl) | 136.7 ± 13.1 | 146.4 ± 23.1a | 148.5 ± 27.6 |
| LDL-cholesterol (mg/dl) | 89 (47–121) | 100 (46–166)a | 101 (55–127) |
| HDL-cholesterol (mg/dl) | 47.3 ± 4.5 | 37.3 ± 6.6a | 41.8 ± 6.1 |
| Systolic BP (mmHg) | 116 ± 10 | 135 ± 5a | 128 ± 8b |
| Diastolic BP (mmHg) | 81 (80–84) | 85 (75–92)a | 80 (70–91)b |
| eGFR (ml/min) | 114 (95–126) | 115 (95–127)a | 105 (82–127)b |
| HOMA-IR | 1.01 ± 0.19 | 1.76 ± 0.41a | 1.44 ± 0.36b |
| Serum albumin (g/dl) | 4.2 ± 0.3 | 3.8 ± 0.3a | 4.0 ± 0.3b |
| HbA1c (%) | 4.1 ± 0.1 | 8.1 ± 1.1a | 7.3 ± 1.1b |
| Duration (months) | — | 50 (35–73) | — |
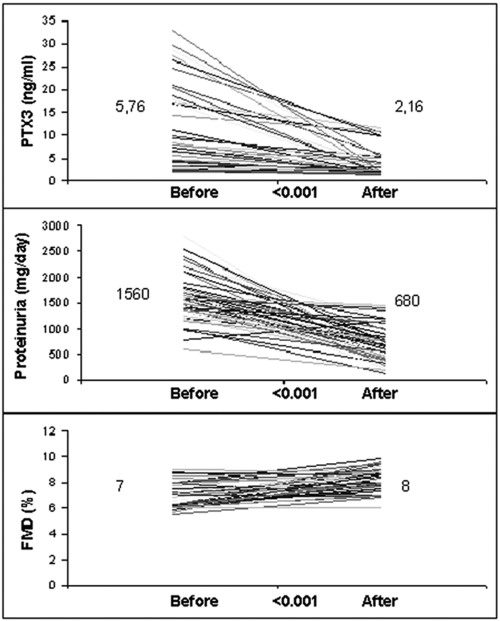
| 24-h proteinuria (mg/day) | 40 (10–90) | 1560 (600–2800)a | 680 (130–1450)b |
| hs-CRP (mg/l) | 2 (1–4) | 11 (5–24)a | 7 (2–16)b |
| FMD (%) | 8.8 (7.6–12.4) | 7.0 (5.5–9.0)a | 8.0 (6.0–9.8)b |
| PTX3 (ng/ml) | 1.32 (0.14–2.75) | 5.76 (1.80–32.94)a | 2.16 (1.15–11.58)b |
Data are means ± SD and median. eGFR, estimated glomerular filtration rate; HOMA, homeostasis model assessment; hs-CRP, high-sensitivity C-reactive protein; FMD, flow-mediated dilation (endothelium-dependent vasodilatation); PTX3, pentraxin 3.
Student t test, statistically significant (P < 0.001) compared with control group (baseline vs controls)
Paired samples t test, statistically significant (P < 0.05) compared with treatment group (before and after treatment)
Effect of ACEI Treatment
Table 1 shows the longitudinal changes of selected parameters in the 49 patients who completed the study. After ACEI treatment, PTX3, HOMA index, hsCRP, eGFR, SBP, DBP, HbA1c, serum albumin, and proteinuria levels were significantly decreased, and FMD levels were significantly increased (Figure 2). However, the lipid parameters and BMIs of the patients did not change significantly during the study period. During the study period, 11 patients were excluded because of adverse drug reactions, including cough (n = 3) and hyperkalemia (n = 4), or because of noncompliance with the study protocol (n = 4).
Figure 2.
Long pentraxin 3 (PTX3), proteinuria, and flow-mediated dilation (FMD) levels in the group receiving ramipril before and after 12 wk of treatment.
Univariate Correlations
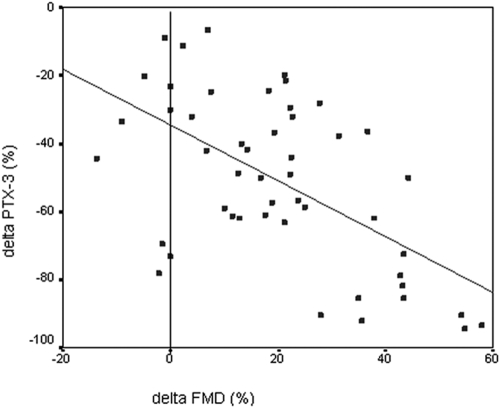
At baseline, FMD was negatively correlated with serum PTX3, 24-h protein excretion, and hsCRP levels and positively correlated serum albumin and eGFR levels. The negative correlations between FMD levels and PTX3, 24-h proteinuria, serum albumin, and hsCRP were present after the 12-wk treatment period as well (Table 2). PTX3 levels were positively correlated with HOMA, 24-h proteinuria, hsCRP, and duration of diabetes and negatively correlated with FMD and HbA1c levels before the treatment period. After the treatment period, PTX3 levels were positively correlated with 24-h proteinuria and hsCRP levels, whereas PTX3 levels were negatively correlated with serum albumin and FMD levels. The percent increase in FMD was negatively correlated with the percent reduction in serum PTX3 concentration (ρ = −0.51, P < 0.001) (Figure 3). In parallel, the reduction in hs-CRP levels correlated with the percent reduction in serum PTX3 concentration (ρ = 0.35, P = 0.015).
Table 2.
Analysis of association between FMD and some relevant parameters by univariate and multivariate linear regression at baseline and after 12 wk of ramipril therapy
| Parameter | Univariate ρ (P) | Multivariate β (P) |
|---|---|---|
| Baseline data (r2 = 0.46) | ||
| PTX3 (ng/ml) | −0.52 (<0.001) | −0.31 (0.02) |
| eGFR (ml/min) | 0.53 (<0.001) | 0.38 (0.01) |
| 24-h proteinuria (mg/day) | −0.44 (0.001) | NS |
| hs-CRP (mg/l) | −0.32 (0.02) | NS |
| Serum albumin (g/dl) | 0.29 (0.04) | NS |
| HOMA-IR | −0.18 (0.22) | NS |
| HbA1c (%) | 0.17 (0.26) | NS |
| After 12 wk of ramipril therapy (r2 = 0.25) | ||
| PTX3 (ng/ml) | −0.45 (0.001) | −0.27 (0.03) |
| 24-h proteinuria (mg/day) | −0.41 (0.04) | −0.41 (0.002) |
| hs-CRP (mg/l) | −0.29 (0.03) | NS |
| Serum albumin (g/dl) | 0.31 (0.03) | NS |
| HOMA-IR | −0.16 (0.29) | NS |
| HbA1c (%) | −0.07 (0.62) | NS |
PTX3, pentraxin 3; eGFR, estimated glomerular filtration rate; hs-CRP, high-sensitivity C-reactive protein; HOMA, homeostasis model assessment; FMD, flow-mediated dilation (endothelium-dependent vasodilatation); NS, nonsignificant.
Figure 3.
Scatter plot showing the significant negative relationship between the percent changes in long pentraxin 3 (PTX3) and flow-mediated dilation (FMD) during the 12-wk ramipril intervention.
Multivariate Regression Analysis
We next investigated the independence of the observed correlations (Table 2) with FMD using a multiple regression model incorporating sex and age, as well as variables significantly associated with FMD at basal (sex, age, 24-h proteinuria, eGFR, PTX3, serum albumin, HOMA-IR, HbA1c, and hs-CRP). Briefly, PTX3 levels were independently related to FMD both before (P = 0.02) and after (P = 0.03) ACEI treatment (Table 2). In addition, in a third and fourth model, we investigated independent predictors of the change in PTX3 and FMD after ACEI therapy (Table 3). The models included changes in 24-h protein excretion, eGFR, SBP, DBP, serum albumin, and hs-CRP, as well as change in FMD or PTX3, respectively. Briefly, change in serum PTX3 levels was independently related only to changes in proteinuria (P < 0.001) and FMD (P = 0.009). Changes in FMD were independently related to changes in serum PTX3 (P = 0.002), hsCRP (P = 0.02), and serum albumin (P = 0.04).
Table 3.
Analysis of association between change (Δ) in PTX3 and change in FMD and relevant parameters by univariate and multivariate linear regression analysis
| Parameter | Univariate ρ (P) | Multivariate β (P) |
|---|---|---|
| Δ PTX3 (ng/ml) (r2 = 0.52) | ||
| Change in FMD (%) | −0.42 (0.003) | −0.32 (0.009) |
| Change in 24-h proteinuria (mg/day) | 0.41 (0.003) | 0.51 (<0.001) |
| Change in hsCRP (mg/l) | 0.30 (0.045) | NS |
| Δ FMD (%) (r2 = 0.45) | ||
| Change in PTX3 (ng/ml) | −0.42 (0.003) | −0.39 (0.002) |
| Change in 24-h proteinuria (mg/day) | −0.52 (<0.001) | NS |
| Change in hsCRP (mg/l) | −0.51 (<0.001) | −0.30 (0.02) |
| Change in serum albumin (g/dl) | 0.35 (0.014) | 0.24 (0.04) |
PTX3, pentraxin 3; FMD, flow-mediated dilation (endothelium-dependent vasodilatation); hs-CRP, high-sensitivity C-reactive protein; NS, nonsignificant.
Discussion
In the present study, we report the results of an open-label, 12-wk trial investigating the impact of initiation of ramipril therapy in ACEI-naive diabetics with proteinuria but normal GFR. We found that the expected reduction in proteinuria was accompanied by a significant reduction in the inflammatory markers hsCRP and PTX3, the changes in which correlated independently with an observed improvement in ultrasonographically measured FMD.
PTX3 is a recently described multimeric inflammatory mediator structurally linked to the short pentraxins, which include CRP (9). However, although CRP is exclusively derived from hepatocytes, PTX3 seems to be synthesized by a variety of tissues and cells, including vascular endothelial cells, macrophages (17), and fat tissue (18). Thus, PTX3 may be a better marker of local tissue inflammatory activity than CRP. In CKD stage 5, we have shown that PTX3 levels are elevated (8,11), and in CKD stage 1 patients, closely related to FMD (8). Indeed, in both incident (11) and prevalent dialysis patients (19), PTX3 was shown to predict mortality, independently of traditional risk factors and CRP (11). In type 2 diabetic kidney disease, we have previously reported that PTX3 levels are significantly associated with the severity of proteinuria and impairment of FMD in cross-sectional analysis (8). The present interventional study extends these findings by showing that a decrease in proteinuria correlates significantly and independently with both a reduction of pro-inflammatory PTX3 signaling and an improvement in FMD. Although our study was not designed to explain the exact mechanisms behind these associations, proteinuria is a well-established risk marker for CVD (20). Multiple pathways have been demonstrated to link CKD to inflammation and endothelial damage (21), including, but not limited to, a decreased clearance of proinflammatory cytokines (22), increased asymmetric dimethylarginine levels (6), and an increased oxidative stress (23). A previously reported accelerated development of atherosclerotic CVD (24) and vascular calcification (24) in uremia may also contribute. The target of ACE inhibition, angiotensin II, is itself an important inducer of vascular injury in several inflammatory settings other than uremia (25), where it induces endothelial dysfunction (25) and CRP generation (26), enhances vascular remodeling (25), and accelerates the progression of atherosclerosis (25). Finally, a reverse causality, whereby lower BP and/or normalization of hyperglycemia in ACEI-treated patients leads to lower levels of systemic inflammation cannot be excluded. Indeed, hypertensive vascular damage is associated with upregulation of endothelial expression of inflammatory cytokines (27), whereas ACEI has been reported to lead to lower systemic (28) and vascular inflammation (26).
Interestingly, in this study, changes in PTX3 correlated better with both improvement in FMD and reduction in proteinuria than did CRP. While CRP is a well-established risk factor for CVD in epidemiologic studies of both the general population (29) and CKD patients (21), it does not appear to be causally linked to vascular damage (30,31). While CRP is derived from hepatocytes, PTX3 is synthesized in several cell types found in atherosclerotic lesions (8–10). Thus, in theory, it is at least possible that PTX3 better reflects changes in local vascular health better than does CRP. Clearly this hypothesis will have to be tested in future studies.
Limitations of our study deserve mentioning. First, the number of the patients was small, which limits the power to detect changes in a marker as variable as PTX3. Moreover, factors other than PTX3 that were not controlled for, such as glycemic control, could reasonably also affect endothelial function and proteinuria. Finally, because we evaluated a specifically selected group of comparatively healthy type 2 diabetic CKD 1 patients who not represent the heterogeneous CKD patient population at large, these results need confirmation in other studies.
In conclusion, we found that 12 wk of ACEI treatment significantly reduced serum PTX3 levels in direct proportion to the reduction in proteinuria and an observed improvement in endothelial functions in ACEI-naive type 2 diabetic patients. Thus, in addition to supporting previous data linking ACEI treatment to beneficial vascular and anti-inflammatory effects in diabetic kidney disease, this study suggests that ACEI decreases circulating PTX3 levels in pathways that operate independently of CRP.
Disclosures
None.
Acknowledgments
The authors were supported by grants from the European Dialysis and Transplant Association, the Swedish Society for Medical Research, the Heart and Lung Foundation, and Spanish Ministry of Education and Science (EX2006–1670). This study was supported by the Gulhane School of Medicine Research Center (2008/31). Bengt Lindholm is an employee of Baxter Healthcare Inc. Peter Stenvinkel is a member of the scientific advisory board of Gambro AB.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Gall MA, Hougaard P, Borch-Johnsen K, Parving HH: Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: Prospective, observational study. BMJ 314: 783–788, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J: Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: The HUNT II Study. Arch Intern Med 167: 2490–2496, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE; Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group: Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106: 1777–1782, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Deckert T, Feldt-Rasmussen B, Borch-Jonsen K, Deckert M, Jensen G, Kofoed-Enevoldsen A: Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 32: 219–226, 1989 [DOI] [PubMed] [Google Scholar]
- 5.Yilmaz MI, Saglam M, Carrero JJ, Qureshi AR, Caglar K, Eyileten T, Sonmez A, Cakir E, Yenicesu M, Lindholm B, Stenvinkel P, Axelsson J: Serum visfatin concentration and endothelial dysfunction in chronic kidney disease. Nephrol Dial Transplant 23: 959–965, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Yilmaz MI, Sonmez A, Saglam M, Qureshi AR, Carrero JJ, Caglar K, Eyileten T, Cakir E, Oguz Y, Vural A, Yenicesu M, Lindholm B, Stenvinkel P, Axelsson J: ADMA levels correlate with proteinuria, secondary amyloidosis, and endothelial dysfunction. J Am Soc Nephrol 19: 388–395, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kshirsagar AV, Bomback AS, Bang H, Gerber LM, Vupputuri S, Shoham DA, Mazumdar M, Ballantyne CM, Paparello JJ, Klemmer PJ: Association of C-reactive protein and microalbuminuria (from the National Health and Nutrition Examination Surveys, 1999 to 2004). Am J Cardiol 101: 401–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suliman ME, Yilmaz MI, Carrero JJ, Qureshi AR, Saglam M, Ipcioglu OM, Yenicesu M, Tong M, Heimbürger O, Barany P, Alvestrand A, Lindholm B, Stenvinkel P: Novel links between the long pentraxin 3, endothelial dysfunction, and albuminuria in early and advanced chronic kidney disease. Clin J Am Soc Nephrol 3: 976–985, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bottazzi B, Vouret-Craviari V, Bastone A, De Gioia L, Matteucci C, Peri G, Spreafico F, Pausa M, D'Ettorre C, Gianazza E, Tagliabue A, Salmona M, Tedesco F, Introna M, Mantovani A: Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component. J Biol Chem 272: 32817–32823, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Savchenko A, Imamura M, Ohashi R, Jiang S, Kawasaki T, Hasegawa G, Emura I, Iwanari H, Sagara M, Tanaka T, Hamakubo T, Kodama T, Naito M: Expression of pentraxin 3 (PTX3) in human atherosclerotic lesions. J Pathol 215: 48–55, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Tong M, Carrero JJ, Qureshi AR, Anderstam B, Heimbürger O, Bárány P, Axelsson J, Alvestrand A, Stenvinkel P, Lindholm B, Suliman ME: Plasma pentraxin 3 in patients with chronic kidney disease: Associations with renal function, protein-energy wasting, cardiovascular disease, and mortality. Clin J Am Soc Nephrol 2: 889–897, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Yilmaz MI, Saglam M, Sonmez A, Caglar K, Cakir E, Kurt Y, Eyileten T, Tasar M, Acikel C, Oguz Y, Vural A, Yenicesu M: Improving proteinuria, endothelial functions and asymmetric dimethylarginine levels in chronic kidney disease: Ramipril versus valsartan. Blood Purif 25: 327–335, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972 [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Berg RL, Gassman JJ, Hall PM, Walker WG: Creatinine filtration, secretion and excretion during progressive renal disease. Modification of Diet in Renal Disease (MDRD) Study Group. Kidney Int 27: S73–80, 1989. [suppl] [PubMed] [Google Scholar]
- 16.Celermajer DS, Sorensen K, Ryalls M, Robinson J, Thomas O, Leonard JV, Deanfield JE: Impaired endothelial function occurs in the systemic arteries of children with homozygous homocystinuria but not in their heterozygous parents. J Am Coll Cardiol 22: 854–858, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Garlanda C, Bottazzi B, Bastone A, Mantovani A: Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol 23: 337–366, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Alberti L, Gilardini L, Zulian A, Micheletto G, Peri G, Doni A, Mantovani A, Invitti C: Expression of long pentraxin in human adipose tissue and its relation with cardiovascular risk factors. Atherosclerosis 2008, [Epub ahead of print]. [DOI] [PubMed]
- 19.Suliman ME, Qureshi AR, Carrero JJ, Bárány P, Yilmaz MI, Snaedal-Jonsdottir S, Alvestrand A, Heimbürger O, Lindholm B, Stenvinkel P: The long pentraxin PTX-3 in prevalent hemodialysis patients: Associations with comorbidities and mortality. QJM 101: 397–405, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Klausen KP, Parving HH, Scharling H, Jensen JS: The association between metabolic syndrome, microalbuminuria and impaired renal function in the general population: Impact on cardiovascular disease and mortality. J Intern Med 262: 470–478, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimbürger O, Massy Z: Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 3: 505–521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pecoits-Filho R, Heimbürger O, Bárány P, Suliman M, Fehrman-Ekholm I, Lindholm B, Stenvinkel P: Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis 41: 1212–1218, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Rabbani N, Sebekova K, Sebekova K Jr., Heidland A, Thornalley PJ: Accumulation of free adduct glycation, oxidation, and nitration products follows acute loss of renal function. Kidney Int 72: 1113–1121, 2007 [DOI] [PubMed] [Google Scholar]
- 24.McCullough PA, Agrawal V, Danielewicz E, Abela GS: Accelerated atherosclerotic calcification and Monckeberg's sclerosis: A continuum of advanced vascular pathology in chronic kidney disease. Clin J Am Soc Nephrol 3(6): 1585–1598, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Ferrario CM, Strawn WB: Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol 98: 121–128, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Peng N, Liu JT, Gao DF, Lin R, Li R: Angiotensin II-induced C-reactive protein generation: Inflammatory role of vascular smooth muscle cells in atherosclerosis Atherosclerosis 193: 292–298, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Casey DP, Conti CR, Nichols WW, Choi CY, Khuddus MA, Braith RW: Effect of enhanced external counterpulsation on inflammatory cytokines and adhesion molecules in patients with angina pectoris and angiographic coronary artery disease. Am J Cardiol 101: 300–302, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schieffer B, Bünte C, Witte J, Hoeper K, Böger RH, Schwedhelm E, Drexler H: Comparative effects of AT1-antagonism and angiotensin-converting enzyme inhibition on markers of inflammation and platelet aggregation in patients with coronary artery disease. J Am Coll Cardiol 44: 362–368, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Lloyd-Jones DM, Liu K, Tian L, Grenland P: Narrative review: Assessment of C-reactive protein in risk prediction for cardiovascular disease. Ann Intern Med 145: 35–42, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Pai JK, Mukamal KJ, Rexrode KM, Rim EB: C-reactive protein (CRP) gene polymorphisms, CRP levels, and risk of incident coronary heart disease in two nested case-control studies. PLoS ONE 3: e1395, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tennent GA, Hutchinson WL, Kahan MC, Hirschfield GM, Gallimore JR, Lewin J, Sabin CA, Dhillon AP, Pepys MB: Transgenic human CRP is not pro-atherogenic, pro-atherothrombotic or pro-inflammatory in apoE-/- mice. Atherosclerosis 196: 248–255, 2008 [DOI] [PubMed] [Google Scholar]