Abstract
Background and objectives: In Ontario, Canada, hemodialysis services are organized in a “hub and spoke” model comprised of regional centers (hubs), satellites, and independent health facilities (IHFs; spokes). Rarely is a nephrologist on site when dialysis treatments take place at satellite units or IHFs. Situations occur that require transfer of the patient back (“fallbacks”) to the regional center that necessitate either in- or outpatient care. Growth in the satellite dialysis population has led to an increased burden on the regional centers. This study was carried out to determine the incidence, nature, and outcome of such fallbacks to aid resource planning.
Design, setting, participants, & measurements: Data were collected on 565 patients from five regional centers over 1 yr. These regional centers controlled 19 satellite dialysis centers including 7 IHFs.
Results: There were 681 fallbacks in 328 patients: 1.21 incidents per patient or 2.1 incidents per patient year. Multiple fallbacks occurred in 170 patients. Fallback episodes lasted a mean of 10.3 d, requiring 4.6 dialysis treatments. Forty-five percent of fallbacks required hospitalization with a mean stay of 16.7 d. Access-related problems (33%) and nondialysis medical causes (32%) were the major causes of fallback. Resolution of the problem occurred in 87.8%, with the patient returning to the satellite. By the end of the study 77.3% were still satellite patients, 10.8% died, 3.8% returned to the regional center, 3.4% were transplanted, and 4.7% were transferred to other treatment modalities.
Conclusions: Fallbacks are common, yet the model operates well.
The Ministry of Health and Long-Term Care of the Government of Ontario promotes a chronic kidney disease (CKD) system that includes renal insufficiency clinics, dialysis treatments, renal transplants, vascular access services, and professional health support services. CKD programs are organized as a “hub and spoke” model, compromised of regional centers (hubs), satellites, and independent health facilities (IHFs; spokes). The regional center is responsible for CKD care throughout its assigned catchment area (1). There are currently 25 regional centers within the province. A regional center multidisciplinary team oversees the care of patients receiving hemodialysis treatments at a satellite. The linkage with a regional center ensures that satellite patients have access to the full continuum of CKD services and receive the same standard of care as they would if they had remained in a regional center. Although each satellite patient will have a specified nephrologist, there usually is not a nephrologist on site at all times that dialysis treatments take place. For this reason, satellites are expected to have clear transfer protocols in place between their facility and the regional center for the transfer of dialysis patients should the need arise. There are currently 46 satellites often sited in community-based hospitals and staffed by local nurses and other support personnel. In addition to these, there are seven satellite units where services are provided by IHFs for patients of one or more regional centers. They are privately owned but publicly funded from the ministry via the participating regional center(s).
Although satellite patients usually receive all of their dialysis services at the satellite, circumstances may arise that require return of the patient to the regional center. Such an event is termed a fallback. Problems that cannot be managed at the satellite facility precipitate fallback events. The event commences with the transfer of the patient for management of the problem with back-up dialyses at the regional center on an outpatient or inpatient basis. Most fallback events end with the resolution of the underlying problem(s) and the return of the patient to the satellite.
Growth in the number of satellite centers operating in Ontario has lead to an increased burden on the regional centers providing the back-up support. The Satellite Dialysis Fallback Study in Ontario was therefore designed to determine the incidence of fallbacks, their cause and outcome, and to ascertain the resources required for their management.
Materials and Methods
Research Design
Sample.
Five regional centers in Southern Ontario were invited to participate. They were assumed by the ministry to be representative of all regional centers. A formal sample size calculation was not undertaken for this descriptive study. Data were collected on all patients and fallback events from participating satellites within a 1-yr time span.
Consent.
Participating regional centers and IHFs agreed to provide the researchers and the ministry with clinical abstractions of the patient's chart; no laboratory or other data, intervention, or investigation were carried out purely for the purpose of the study. Data were abstracted by trained chart abstractors and were in compliance with all privacy and confidentiality legislations. The local research ethics board approved the protocol for each regional center.
Data Forms.
Data required for the analysis of the study were collected on five forms. The Satellite Unit Characteristics form (Form 1) described capacity and service ability at the satellite. The Patient Characteristics form (Form 2) described the patient's baseline clinical characteristics and demographic data. The Fallback Alert (Form 3) was filled out by the satellite to alert the research team of a fallback episode. The documentation of the Fallback Event form (Form 4) described the clinical course of the fallback. The End of Study Status form (Form 5) reported on each patient's dialysis status at the end of the 12-mo study period.
Data Collection at Regional Centers.
Baseline clinical data collection began August 2001 from satellite patients' charts. These data were identified by unique study numbers, date of birth, and patient initials and sent to the Institute for Clinical Evaluative Sciences, Toronto (ICES). ICES acted as the study coordination, training, and data collection center. A registration table that linked patient names to study numbers was created and held at the regional center to ensure that when the regional center submitted a Fallback Event form (Form 4) it was accurately linked to the corresponding patient data without reporting of the patient's name. This file bearing linkable patient identifiers remained at the regional center and was not provided to ICES.
The analysis of the results was carried out at ICES under the confidentiality requirements of a general research agreement between ICES and the ministry.
Data Collection at IHFs
The data abstraction for IHF satellites was initiated in October 2001. Because ICES is not authorized to receive data directly from IHFs, a different procedure was required. The clinical records for satellite patients were maintained at the IHF; data abstraction was done there. A registration table linking patients’ names to study identifiers for each patient was prepared at the regional center and was provided to the IHF abstractors but not given to the ICES research staff. Baseline clinical data (corresponding to Form 2), bearing only study numbers as identifiers, were then sent to the ministry, which held it until the end of the study. Fallback Alert forms (Form 3) were submitted to the ICES research team directly by the regional center who completed Form 4. The End of Study Status Data (Form 5) was sent directly to the ministry by the regional center. No patient data were sent directly to the research team and ICES from an IHF. ICES subsequently obtained the data required for study analysis from the ministry.
Regional Centers, Satellites, and Patients
Five regional centers participated in the study (London, Kingston, Ottawa, Hamilton, and Toronto). These regional centers controlled a total of 19 satellite dialysis centers, including 7 IHFs. Two of the satellites each provided services to patients from two different regional centers so that, by center, there were 21 satellites and 9 IHFs.
Patient Enrollment and Follow-Up
Data were abstracted from all prevalent patients receiving their dialysis treatments at the 21 satellites at the time of study start, August 2001. These patients were then followed for a planned minimum of 12 mo. At the 12th month, patients in a fallback event were followed beyond the 12th month until the conclusion of that event. Incident satellite patients were also included and followed for the remainder of the year. The last study date was September 2002 and final form completion occurred in February 2003.
Data Analyses
Descriptive analysis was used to characterize the fallback phenomena in terms of precipitating causes, duration, and outcome. Characteristics of the satellite units (location, staffing, and laboratory facilities) and the patients (clinical and demographic factors) were examined.
Two outcomes were defined: fallback and success or failure. Fallback was defined as any fallback occurrence in the study window and was a binary outcome. Success a priori was defined as no fallbacks and remaining on dialysis at a satellite, whereas failure was defined as two or more fallback events, death, or return to in-center for dialysis. Univariate analysis of the effect of patient and satellite factors on these two binary outcomes was conducted with t test and χ2 (P < 0.05 considered significant). A multivariate Poisson regression model was constructed with the number of fallbacks as the dependent variable to examine the effect of individual patient (not satellite) factors on the risk of fallback. A logistic regression model was also constructed to examine the effect of patient (not satellite) factors on success or failure as defined above.
Results
Patient Numbers and Follow-Up
At the commencement of the study, all 427 prevalent patients receiving dialysis in the 21 satellite dialysis centers were entered. A further 138 incident patients commenced dialysis at a participating satellite, bringing the total to 565 patients (Table 1). The study follow-up of the 565 patients ranged from 7 to 403 d, with the median being 365 d.
Table 1.
Regional centers, number of satellites, and patients participating in the studya
| Regional Center | Number of Satellites (IHFs) | Number of Patients Studied
|
||
|---|---|---|---|---|
| Prevalentb | Incidentb | Total | ||
| 1 | 2 (1) | 64 | 18 | 82 |
| 2 | 5 (3) | 93 | 43 | 136 |
| 3 | 7 (0) | 148 | 53 | 201 |
| 4 | 2 (2) | 74 | 13 | 87 |
| 5 | 5 (3) | 47 | 11 | 59 |
| Total | 427 | 138 | 565 | |
IHFs, independent health facilities.
To commencement of satellite hemodialysis.
Satellite Characteristics
These are shown in Table 2.
Table 2.
Satellite characteristicsa
| Variable | Specific | Value |
|---|---|---|
| IHF, n (%) | Yes | 9 (42.9) |
| Location, n (%) | Freestanding (not in hospital) | 12 (57.1) |
| Dialysis stations, mean (range) | 8.9 (6 to 12) | |
| Nurse:patient ratio range | 1:3 to 1:5 | |
| Clinics, n (%) | On site | 9 (42.9) |
| Regional center | 9 (42.9) | |
| Nephrologist, n (%)b | On site during dialysis shifts | 3 (14.3) |
| Available by contact | 19 (90.5) | |
| Internist, n (%) | Available during dialysis shifts | 8 (38.1) |
| Family doctor, n (%) | Available during dialysis shifts | 8 (38.1) |
| Local intensive care unit, n (%) | Yes | 10 (47.6) |
| On-site lab, n (%) | Yes | 10 (47.6) |
| Local radiology | Yes | 10 (47.6) |
Other details of the satellite, not listed above, were obtained on Form 1 and were included in data analysis.
Of 21 satellites, 1 indicated nephrologist was either on site or available by contact. All satellites had access to a nephrologist at all times.
Patient/Regional Center Characteristics
Age, sex, vascular access type and other patient characteristics are shown in Table 3. Employment status and type of hemodialysis care (full care versus some form of self care) and whether or not a family practitioner was involved in the care are also shown in Table 3. Cause of CKD and comorbidities along with baseline laboratory data and use of pharmaceutical agents are shown in Table 4. There were significant center effects. Center 5 had a population that was younger (mean age 50.7 yr), showed more dependency, had a higher employment rate, used assisted self care more frequently, and had fewer comorbidities (minimum P < 0.001). Center 3 had a lower rate of arteriovenous fistula use (36%) and a higher reliance on tunneled catheters (43%; minimum P < 0.001). There were some minor center effects with regard to medication use.
Table 3.
Patient characteristics
| Variable | Specific | Value |
|---|---|---|
| Age at beginning of study, yr | Mean (range) | 62.4 (14 to 101) |
| Gender, n (%) | Female | 228 (40.4) |
| Male | 337 (59.6) | |
| Marital status, n (%) | Common law/married | 355 (62.7) |
| Widowed/single/separated/divorced | 169 (30.0) | |
| Unknown | 41 (7.3) | |
| Independence, n (%) | Independent | 108 (19.9) |
| Home care provided | 382 (67.7) | |
| Facility care: full or partial nursing support | 12 (2.2) | |
| Unknown | 70 (12.4) | |
| Employment status, n (%) | Full-time/part-time/homemaker | 114 (20.2) |
| Permanent disability | 48 (8.5) | |
| Unemployed or retired | 311 (55.0) | |
| Unknown | 92 (16.3) | |
| Satellite dialysis status, n (%) | Assisted self-care | 143 (25.3) |
| Full-care | 422 (74.7) | |
| Family physician involved, n (%) | No | 15 (2.7) |
| Unknown | 118 (20.9) | |
| Yes | 432 (76.5) | |
| Access type, n (%) | Arteriovenous fistula | 317 (56.1) |
| Graft | 98 (17.3) | |
| Temporary line | 1 (0.2) | |
| Tunneled permanent catheter | 149 (26.4) | |
| Dialysis routine, number of sessions per week | Mean (range) | 3 (2 to 4) |
| Dialysis routine, hours per session | Mean (range) | 3.9 (2.5 to 5.25) |
Fallbacks/Regional Center Characteristics
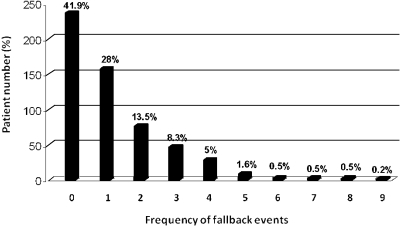
There were 681 fallback events occurring in 328 (58%) patients. This represented 1.21 fallback incidents per patient, or 2.1 incidents per patient per year. In 158 patients (28%) there was only one fallback episode. In 170 patients (30%), multiple fallbacks occurred (Figure 1). A fallback episode lasted a mean of 10.3 d. There was a center effect with Center #3 showing the shortest fallback duration (mean 6.3 d; P = 0.009). The mean number of back-up dialyses required per fallback was 4.6, with Center #3 showing fewer (3.3; P = 0.0165). Overall 45% of the fallbacks required hospitalization (Center #2 only 36%; P = 0.0005). The length of stay was a mean of 16.7 d per admission regardless of center.
Figure 1.
Distribution of frequency of fallback events.
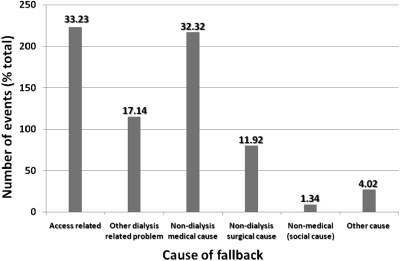
The major cause of fallback was access related (33% of events; Figure 2). A center effect was found, with a range of 26 to 45% of all fallbacks being due to vascular access issues. Nondialysis medical causes accounted for 32% of fallbacks; again a center effect was seen. Overall, the center effects were statistically significant (P < 0.001). Social causes contributed only nine fallback episodes (1.34%).
Figure 2.
Primary causes of fallbacks.
Outcomes
In 87.8% of patients, the problem for the fallback event was dealt with and the patient successfully returned to satellite dialysis treatment. Only 2.2% of fallback events led to the patient being removed from satellite dialysis and returning permanently to the regional center, and in only 5.7% did the event lead to patient death (Table 5). Examination of patient status at the end of the study showed that 77.3% of total population were still satellite patients. Only 3.8% of patients had failed, 3.4% were transplanted, 4.7% were transferred to other modalities (e.g., peritoneal dialysis), and there were 60 deaths (10.8% of population).
Table 5.
Outcomes for the fallback eventsa
| Resolution | Patient Frequency |
|---|---|
| Return to satellite | 597 (87.79) |
| Permanent transfer to in-center dialysis | 15 (2.21) |
| Permanent transfer to other program or modality | 20 (2.94) |
| Death | 39 (5.74) |
| Unresolved | 9 (1.32) |
Frequency missing = 1.
Patient Characteristics Associated with Fallbacks
Univariate analyses showed the following clinical factors were more associated with patients who experienced one or more fallbacks compared with those who did not fallback: ischemic heart disease, lung disease, peripheral vascular disease, a lower serum albumin, and a higher postdialysis weight (minimum P < 0.05). Interestingly, the presence of diabetes was not associated with fallback nor was vascular access type (P = 0.4464). In the multivariate model, fallbacks were associated with a lower serum albumin (P = 0.0002), female sex (P = 0.02), comorbid lung disease (P = 0.04), and comorbid ischemic heart disease (P = 0.01).
Patient Characteristics Associated with Failure or Success
The multivariate model showed the presence of diabetes mellitus, ischemic heart disease, lung disease, peripheral vascular disease, and other comorbidities were all significantly associated with failure (minimum P < 0.05). Patients who had no comorbidities were more likely to have successful outcomes (P = 0.0019). The univariate analyses also showed that lower serum albumin, higher serum phosphorus levels, and those on multiple medications were associated with failure (minimum P < 0.05); however, these factors were not independently significant in the multivariate model
Satellite Characteristics Associated with Fallback (by Univariate Analyses)
IHF status was not correlated with fallback, but the location was. Fallback was more likely when the satellite was in a freestanding site rather than in a hospital (P = 0.016). The number of dialysis stations, staffing ratios, type of staff, numbers of dialyses performed, and so forth were all unrelated to fallback occurrence. Having a family doctor involved in the patient's care appeared protective (P = 0.01). The presence of a local intensive care unit was associated with a reduced incidence of fallback (P = 0.03) as were local laboratories and radiology services (P = 0.035).
Satellite Characteristics Associated with Success (by Univariate Analyses)
Clinics held at the satellite were associated with success (P < 0.05). The availability of the family doctor during working shifts, although not on site, was associated more with success than failure (minimum P < 0.05). No other satellite characteristics had any association with success or failure.
Fallback Characteristics by Admission Status
Of the 681 fallback events, 376 (55.2%) were handled on an outpatient basis and 305 (44.8%) required hospitalization. The events handled by outpatient were shorter in time (4.1 versus 18 d) and required fewer back-up dialyses (2.2 versus 7.5; P < 0.0001). Of the outpatient fallbacks, 48.6% were vascular access related; 18.7% of access fallbacks required admission (P < 0.001). On the other hand, nondialysis medical causes for fallback accounted for only 26.5% of those managed as outpatients and 52.5% of those that had to be admitted (P = 0.0001). Nondialysis surgical causes accounted for 11.9% of all fallbacks, were managed as outpatients in 25% of patients (4.4% of total outpatients), and accounted for 25.2% all hospitalizations (P = 0.0001). The resolution of the fallback was different after outpatient or inpatient management: with only 2 deaths in fallbacks managed as outpatients (0.5%) whereas there were 37 (12.1%) inpatients that had to be admitted. Return to satellite occurred in 96% of outpatient-managed fallbacks and in 77.7% of those that required a hospitalization (P < 0.0001).
Discussion
The satellite program in Ontario started when regional centers identified clusters of patients who were disadvantaged by having to travel considerable distances for dialysis. Permission to set up local satellite dialysis facilities was sought. If approved by the ministry, patients conducted their own treatments with the assistance of nurses (self-care hemodialysis). Invariably, these were patients with few comorbidities managed with high patient-to-nurse staffing ratios. As regional programs grew, pressure was exerted on existing satellites to take “full care” patients with comorbidities, requiring more nursing care. The growth in satellite usage became confusing to the ministry because of variations in scope (e.g., self care versus full care) and in requested resources, including those at the regional center. The ministry's need to better understand satellite dialysis led to this study.
The study was conducted by 5 of a total of 25 regional centers within the province. They were chosen by the ministry as being representative of the renal programs in the more urban areas. The regional centers chosen were academic centers. A weakness of the study is that its results may not necessarily reflect that patterns of practice of regional centers run by community nephrologists in northern Ontario with its vast geographical areas.
The prime focus of the study was on the fallback events. The results indicate that fallbacks are common and are mainly due to vascular access or nondialysis medical problems. Center effects were noted that were case-mix related and reflect the variation in patient selection for satellite treatment. The center with the lowest use of arteriovenous fistulae and the highest use of venovenous catheters had the shortest fallback period, requiring the fewest number of back-up dialyses. The center with the highest fallback rate also had the most nondialysis-related medical causes; their patients were more likely to have ischemic heart disease, lung disease, or the other clinical characteristics associated with fallback. Failure of satellite dialysis arbitrarily defined as two or more fallbacks, death, or the need to transfer the patient back to the regional center was associated, in general, with the number of comorbidities, which is to be expected.
Despite this, most (87.8%) fallbacks are resolvable with the patient returning to satellite hemodialysis. The program is therefore successful even with patients who have comorbidities. In fact, 83.5% of the population studied did have identified comorbidities and yet the failure rate (patients returning to the regional center for dialysis) was low at only 3.8%. An objective of the study was to provide information that may help in resources planning. One obvious area is in vascular access management at or near the satellite. The study does indicate that for every 100 satellite patients, each regional center must have 3 to 4 hospital beds and 1 or more of its in-center hemodialysis stations occupied at all times to support satellite fallback patients. The appropriate resources must therefore be provided to the regional center.
A secondary focus was to examine possible satellite characteristic effects on fallback. Statistical associations were found between fallback and satellite location, hospital-based being protective. This and the other associations must be viewed with caution. The presence of local intensive care, laboratory and radiologic services, or the family doctor being involved might lessen the need to dispatch a patient to the regional center; however, in this study there was no accounting of interventions by such services. It may well be that a freestanding satellite can be very effective if it has resuscitation equipment and access to laboratory and radiologic series. The desirability of having a nephrologist on site rather than available by contact also cannot be answered, because for all patients one was always available and only three satellites reported having one on site. Since this study was carried out, the ministry has created a “Model of Care” document that defines the requirements necessary for a regional center to set up a satellite program and categorizes satellites by their ability to care for patients of varying risk and comorbidity (1). Further studies on fallbacks may now answer these questions.
Conclusions
The use of satellite units by regional centers to provide community-based dialysis has become the model for Ontario, Canada. Patient fallback events are frequent and necessitate extra resources at the regional center. Nevertheless, the model works well, because its patient failure rate is low.
Disclosures
None.
Table 4a.
Patient characteristics: Cause of renal failure, comorbidities, and pharmaceutical agent usea
| Variable | Number of Patients | Percent of Total |
|---|---|---|
| Cause of renal failure | ||
| GN | 66 | 11.7 |
| pyelonephritis | 15 | 2.7 |
| polycystic kidneys | 41 | 7.3 |
| other renal | 51 | 9 |
| diabetes mellitus | 172 | 30.4 |
| hypertension | 220 | 38.9 |
| Comorbidities | ||
| diabetes mellitus | 360 | 53.9 |
| ischemic heart disease | 109 | 19.3 |
| myocardial infarction | 39 | 6.9 |
| congestive heart failure | 47 | 8.3 |
| malignancy | 52 | 9.2 |
| lung disease | 53 | 9.49.4 |
| stroke | 56 | 9.9 |
| symptomatic PVD | 63 | 11.2 |
| visual impairment | 117 | 20.7 |
| amputation | 22 | 3.9 |
| other | 381 | 67.4 |
| none | 93 | 16.5 |
| Use of Pharmaceutical Agents | ||
| blood pressure | 393 | 61.2 |
| other cardiac | 219 | 38.8 |
| anticoagulant | 161 | 28.5 |
| antiplatelet | 186 | 33 |
| oral iron | 180 | 32.1 |
| intravenous iron | 307 | 54.8 |
| rHuEPO | 514 | 91 |
PVD, peripheral vascular disease; rHuEPO, recombinant human erythropoietin.
Table 4b.
Patient characteristics: Laboratory parameters
| Laboratory Parameters | Mean Value | Range |
|---|---|---|
| Hemoglobin, g/L | 115.3 | 90 to 170 |
| Albumin, g/L | 36 | 15 to 52 |
| Creatinine, μmol/L | 745.7 | 385 to 1632 |
| Urea, mmol/L | 20.2 | 5.5 to 37 |
| Calcium, mmol/L | 2.1 | 1.9 to 2.7 |
| Phosphorus, mmol/L | 1.7 | 1.3 to 2.7 |
| Potassium, mmol/L | 4.9 | 3.2 to 5.4 |
| Predialysis urea, mmol/L | 19.7 | 5.4 to 37.8 |
| Postdialysis urea, mmol/L | 5.5 | 3 to 16.4 |
Acknowledgments
We wish to acknowledge the support of all nurses from the regional centers that carried out data abstraction, Jiming Fang of ICES for statistical analyses, and all of the dialysis nurses in each satellite for their ongoing devotion. This study was funded by a research grant from the Ministry of Health and Long Term Care, Government of Ontario, Canada.
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Satellite Dialysis in Ontario,” on pages 523–524.
Reference
- 1.Chronic Kidney Disease Program, Ministry of Health and Long-Term Care, Ontario. Model of Care: Expectations Document. February 16, 2004. Available at: http://www.health.gov.on.ca/login/kidney03.html. Accessed July 2004.