Abstract
Accumulation of glomerular matrix is a hallmark of diabetic nephropathy. The serine/threonine kinase Akt mediates glucose-induced upregulation of collagen I in mesangial cells through transactivation of the EGF receptor (EGFR). In addition, in renal tubular cells, glucose-induced secretion of TGF-β requires phosphoinositide-3-OH kinase, suggesting a possible role for Akt in the modulation of TGF-β expression, but the mechanisms of Akt activation and its involvement in TGF-β regulation are unknown. Here, in primary mesangial cells, high glucose induced AktS473 phosphorylation, which correlates with its activation, in a protein kinase C β (PKC-β)-dependent manner. Glucose led to PKC-β1 membrane translocation and association with Akt, and PKC-β1 immunoprecipitated from glucose-treated cells phosphorylated recombinant Akt on S473. PKC is known to mediate glucose-induced TGF-β1 upregulation through the transcription factor AP-1; here, inhibitors of phosphoinositide-3-OH kinase, PKC-β and Akt, and dominant-negative Akt all prevented glucose-induced activation of AP-1 and upregulation of TGF-β1. Finally, pharmacologic and dominant negative inhibition of EGFR blocked glucose-induced activation of PKC-β1, phosphorylation of AktS473, activation of AP-1, and upregulation of TGF-β1. In vivo, the PKC-β inhibitor ruboxistaurin prevented Akt activation in the renal cortex of diabetic rats. In conclusion, PKC-β1 is an Akt S473 kinase in glucose-treated mesangial cells, and TGF-β1 transcriptional upregulation requires EGFR/PKC-β1/Akt signaling. New therapeutic approaches for diabetic nephropathy may result from targeting components of this pathway, particularly the initial EGFR transactivation.
The kidney is an important site of diabetic microvascular complications, and hyperglycemia is central to glomerular matrix accumulation. Although strict glucose control and inhibition of the renin-angiotensin system are effective in delaying the development of nephropathy, disease progression often occurs. The development of new treatment approaches is thus an important goal.
We have shown that collagen I induction by high glucose (HG) requires activation of the serine/threonine kinase Akt, and this depends on transactivation of the EGF receptor (EGFR).1 Akt activation requires membrane translocation and phosphorylation on two sites, S473 and T308.2 Phosphoinositide-3-OH kinase (PI3K) is an upstream mediator of Akt activation, generating phosphorylated lipid second messengers that recruit proteins with pleckstrin homology domains such as Akt to the membrane.3 At the membrane, phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates Akt at T308.3 Several S473 kinases, however, have been identified, including DNA-PK and mammalian target of rapamycin in complex with rictor and PKC.4–7 Their role in S473 phosphorylation and Akt activation may be context specific. For example, PKC-β2 was found to phosphorylate Akt S473 in response to IgE but not IL-3 in mast cells and did not function as an S473 kinase in other cells such as serum-stimulated fibroblasts.7
PKC comprises a large family of kinases, some isoforms of which interact with Akt,7,8 although their specific effect on Akt activity is isoform dependent. Conventional isoforms PKCα, -β1, and -β2 were recently demonstrated to phosphorylate Akt on S473 in in vitro assays; however, in vivo studies indicated the existence of cell and stimulus specificity, with kinase function limited to PKC-β2.7 How HG-induced Akt S473 phosphorylation occurs is not known, but PKC isoforms, key players in HG responses in mesangial cells (MCs) and in diabetic kidneys, are potential candidates. Indeed, in endothelial cells, longer term (24 h) HG-induced Akt activation and fibronectin upregulation were blocked by a general PKC inhibitor.9 PKC-β, in particular, is of interest as a possible Akt S473 kinase in the setting of HG. Its inhibition in several models of diabetes prevents the development of diabetic nephropathy, and PKC-β null mice rendered diabetic were protected from glomerular hypertrophy and matrix accumulation.10,11 We thus investigated the role of PKC as an Akt S473 kinase in HG-treated MCs.
TGF-β1 is a major mediator of matrix accumulation in diabetic kidneys and glucose-exposed MCs.12 Its upregulation by HG in MCs requires PKC.13,14 Furthermore, in renal tubular cells, HG-induced TGF-β secretion required PI3K, suggesting a possible role for Akt in TGF-β upregulation.15 The aims of this study were thus two-fold. We first sought to investigate whether PKC serves as an Akt S473 kinase in MCs in response to HG and to identify which isoform possessed this function. We further sought to identify whether PKC/Akt cross-talk is required for HG-induced TGF-β1 upregulation. Extending our previous data implicating the EGFR as a proximal initiator of HG fibrogenic responses in MCs, we also addressed the role of this receptor in these events.
RESULTS
PKC-β-Akt Signaling Is Required for Glucose-Induced TGF-β upregulation
We have shown that in MCs, Akt is S473-phosphorylated (pAktS473) in response to HG.1 We used the broad-spectrum PKC inhibitor PMA (phorbol 12-myristate 13-acetate) and the conventional PKC inhibitor Gö6976 to assess whether PKC might be an upstream mediator of pAktS473. Figure 1A shows that both prevented HG-induced pAktS473; however, phosphorylation on the PDK1-dependent site T308 in Akt was PKC independent (Figure 1B). A more specific PKC-β inhibitor also prevented HG-induced pAktS473 (Figure 1C). Because activation of PKC-β2 is not observed in MCs and PKC-β2 is undetectable in glomeruli in vivo,16,17 we took PKC-β1 as the probable relevant isoform.
Figure 1.
PKC-β is required for glucose-induced Akt S473 phosphorylation. Serum-deprived MCs were pretreated with PMA (200 nM, 24 h) or Gö6976 (2 μM, 30 min) to inhibit PKC isoforms before treatment with HG for 1 h. (A and B) Glucose-induced Akt phosphorylation at S473 but not T308 was blocked by PKC inhibitors (n = 7; #P < 0.01 HG versus others in A). (C) Pretreatment with a specific PKC-β inhibitor (100 nM, 30 min) also prevented HG-induced Akt S473 phosphorylation (n = 8; *P < 0.05 HG versus others).
PKC regulates TGF-β1 transcriptional induction by HG in MCs.13 We first conducted a time course to confirm TGF-β1 gene upregulation by HG in MCs, as shown in Figure 2A. Significant upregulation was observed at 24 h, as has been noted by others.13,18 Subsequent experiments assessing TGF-β1 upregulation thus used this time point. Figure 2B confirms that conventional PKC isoforms, inhibited by Gö6976, are required for TGF-β1 transcript upregulation by HG (24 h). The PKC-β–specific inhibitor also prevented HG-induced TGF-β1 upregulation (Figure 2C). Akt was also required for TGF-β1 upregulation, because MCs overexpressing the dominant negative form of Akt, AktAAA,19 lacked HG-induced TGF-β1 upregulation (Figure 2D). In MCs transfected with a TGF-β1 promoter driving a luciferase reporter, AktAAA also prevented HG-induced promoter activation (Figure 2E). Thus, PKC-β1-Akt signaling is required for TGF-β1 transcriptional upregulation.
Figure 2.
Glucose-induced TGF-β upregulation requires PKC-β-Akt signaling. (A) Serum-deprived MCs were treated with HG for the indicated times, and TGF-β1 transcript was assessed by Northern analysis. (B and C) MCs were then treated for 24 h, the earliest time of maximal TGF-β1 induction, and pretreated with either the conventional PKC inhibitor Gö6976 (2 μM, 30 min; B) or specific PKC-β inhibitor (100 nM, 30 min; C). TGF-β1 mRNA was assessed by Northern analysis, with actin used as a loading control. Both inhibitors prevented glucose-induced TGF-β1 transcript upregulation (n = 3; †P < 0.001 HG versus others in C). Treatment with equimolar mannitol had no effect on TGF-β upregulation. (D) MCs were stably infected with dominant negative Akt, AktAAA, or empty vector pLHCX. The HG-induced increase in TGF-β1 transcript was abrogated by AktAAA (n = 4; #P < 0.01 HG pLHCX versus all others). (E) MCs overexpressing AktAAA or empty vector were transfected with a TGF-β1 promoter-luciferase construct, and promoter activity was assessed after 24 h of HG. The increase in TGF-β1 promoter activity by HG was absent in MCs overexpressing AktAAA (n = 6; #P < 0.01 HG pLHCX versus all others).
Activation of AP-1 by Glucose Is Mediated by PKC-β-Akt
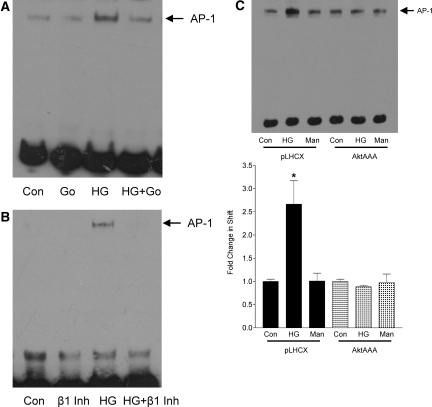
Through deletion of one or both AP-1 sites in the TGF-β1 promoter, Weigert et al.13 showed that AP-1, with JunD and c-Fos as part of the complexes, drives glucose-induced upregulation of TGF-β1 in MCs. PKC was also required.13 Some studies showed that PI3K signaling regulated AP-1 activation in some settings.20–22 We thus investigated whether PKC-β signaling through Akt mediated glucose-induced AP-1 activation. The conventional PKC inhibitor Gö6976 (Figure 3A), as well as a PKC-β–specific inhibitor (Figure 3B), completely prevented AP-1 activation in MCs treated with HG for 6 h as assessed by electrophoretic mobility shift assay (EMSA). Furthermore, in MCs overexpressing AktAAA, HG failed to activate AP-1 (Figure 3C). Conversely, we also assessed whether overexpression of constitutively active Akt, Akt-DD, could itself lead to activation of AP-1. Stable overexpression of this construct in MCs, identified by the epitope tag HA, is shown in Figure 4A. It is of interest that activation of Akt alone was able to induce AP-1 activation as assessed by EMSA (Figure 4B). Upregulation of TGF-β1 transcript was also observed in MCs overexpressing Akt-DD as compared with empty vector (Figure 4C), consistent with the role of AP-1 in mediating TGF-β1 promoter activation.13
Figure 3.
PKC-β-Akt mediate activation of AP-1 by glucose. (A and B) MCs were treated with HG for 6 h in the presence or absence of the conventional PKC inhibitor Gö6976 (2 μM, 30 min; A) or specific PKC-β inhibitor (100 nM, 30 min; B). AP-1 activation, assessed by EMSA, was abrogated by both inhibitors. (C) AP-1 activation by HG was not observed in MCs overexpressing AktAAA as compared with empty vector pLHCX (n = 2; *P < 0.05 pLHCX HG versus others).
Figure 4.
Constitutively active Akt leads to TGF-β1 upregulation. MCs were stably infected with constitutively active Akt, AktDD, or empty vector pLHCX. (A) Overexpression in MCs was demonstrated by immunoblotting for the epitope tag HA as well as for Akt. (B) AktDD overexpression induced AP-1 activation, as assessed by EMSA (n = 4; #P < 0.01 AktDD versus pLHCX). (C) TGF-β1 transcript, as assessed by Northern analysis, was also upregulated by AktDD overexpression (n = 6; #P < 0.01 AktDD versus pLHCX).
We previously showed that PI3K mediated HG-induced Akt activation in MCs.1 PI3K may also regulate the activation of conventional PKC isoforms.23,24 We next assessed the role of PI3K in PKC-β1 activation and downstream signaling by HG. Figure 5A shows that PKC-β1 membrane translocation, indicative of its activation,25,26 was induced by HG. Two PI3K inhibitors, LY294002 and wortmannin, each prevented PKC-β1 translocation. Downstream, HG-induced AP-1 activation as assessed by EMSA and TGF-β1 upregulation as assessed by Northern were also completely prevented by PI3K inhibition (Figure 5, B and C). Figure 5D shows that activation of the TGF-β1 promoter was also blocked by PI3K inhibitors, confirming that PI3K signaling regulates the transcription of TGF-β1.
Figure 5.
PI3K is required for PKC-β1 activation and downstream signaling. MCs were treated with the PI3K inhibitors wortmannin (100 nmol/L, 60 min) or LY294002 (20 μmol/L, 30 min) followed by HG. (A) Translocation of PKC-β1 to the membrane, induced by HG for 30 min, was prevented by both inhibitors (n = 3; *P < 0.05 HG versus others). Actin served as the loading control for the cytosolic portion. (B) AP-1 activation was assessed by EMSA after HG treatment for 6 h. Both PI3K inhibitors prevented activation of this transcription factor (n = 4; *P < 0.05 HG versus others). (C) Similarly, TGF-β1 transcript upregulation after HG treatment for 24 h was prevented by both PI3K inhibitors, as assessed by Northern (n = 10; #P < 0.01 HG versus others). (D) TGF-β1 promoter activation was assessed by luciferase assay. Both PI3K inhibitors prevented promoter activation after HG treatment for 24 h (n = 5; #P < 0.01 HG versus others).
EGFR Mediates Glucose-Induced PKC-β1 Activation and Downstream Signaling
We previously observed that the EGFR is transactivated by HG within 1 h in MCs, and this is required for Akt activation.1 Here, we first assessed whether longer exposure to HG would lead to EGFR transactivation, as assessed by phosphorylation on Y1068. Figure 6A demonstrates that more prolonged glucose treatment led to increasing EGFR transactivation over time, up to 24 h. Because PKC-β1 is required for Akt activation, we assessed whether EGFR is upstream of glucose-induced PKC-β1 activation. In Figure 6B, the EGFR inhibitor AG1478 prevented PKC-β1 membrane translocation in response to HG, suggesting that transactivation of this receptor is required for glucose-induced PKC-β1 activation. Downstream effects of PKC-β1 also required EGFR. In Figure 6C, AP-1 activation by HG was blocked by AG1478. A prominent role for the EGFR in AP-1 transactivation was further confirmed in MCs stably infected with the kinase-inactive EGFR-K721A (dnEGFR). We previously demonstrated overexpression of this construct in MCs.1 As seen in Figure 6D, HG was unable to induce AP-1 activation in MCs infected with dnEGFR. TGF-β1 transcript upregulation by HG, assessed by Northern, was also blocked by AG1478 and absent in MCs overexpressing dnEGFR (Figure 6, E and F). Thus, EGFR transactivation is required for PKC-β1 activation by HG and downstream signaling events.
Figure 6.
EGFR mediates glucose-induced PKC-β1 activation and TGF-β upregulation through AP-1. (A) EGFR transactivation, assessed by its phosphorylation on Y1068, was seen by 1 h and maintained to 24 h of HG exposure (n = 3 *P < 0.05 versus control). (B) MCs were treated with the EGFR inhibitor AG1478 (1 μmol/L, 30 min) before HG for 30 min. PKC-β1 membrane translocation in response to HG was prevented by AG1478 (n = 5; #P < 0.01 HG versus others). Actin served as the loading control for the cytosolic portion. (C) AP-1 activation by HG for 6 h, assessed by EMSA, was also blocked by AG1478 (n = 2; #P <0.01 HG versus others). (D) AP-1 activity was also prevented in MCs stably overexpressing the dnEGFR K721A (n = 2; *P < 0.05 HG pLHCX versus others). (E) TGF-β1 upregulation by HG for 24 h, as assessed by Northern, was prevented by AG1478 (n = 2; *P < 0.05 HG versus others). (F) Similarly, TGF-β1 upregulation by HG was prevented in MCs overexpressing dnEGFR as compared with empty vector pLHCX (n = 7; *P < 0.05 HG pLHCX versus others).
PKC-β1 Functions Downstream of EGFR-PI3K Activation as a Glucose-Induced Akt S473 Kinase
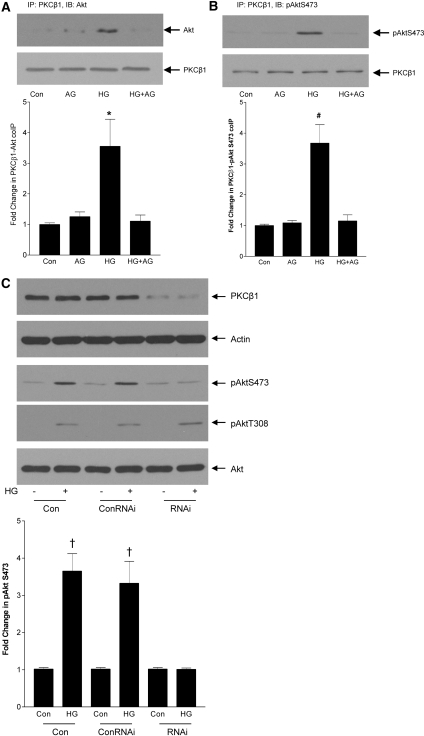
In vitro assays have demonstrated that PKC-β1 can function as an Akt S473 kinase7; however, in vivo, the requirement for PKC-β1 seems to be context and cell specific.7,27 Because, in HG, PKC-β1 mediates pAktS473 downstream of EGFR, we sought to determine whether PKC-β1 functioned as an S473 kinase. We first sought a physical association between PKC-β1 and Akt. In Figure 7A, Akt was detected in PKC-β1 immunoprecipitates after HG treatment. This association depended on EGFR transactivation because it was blocked by AG1478. Similarly, Akt phosphorylated on S473 was immunoprecipitated with PKC-β1, supporting the possibility of PKC-β1 function toward Akt as a kinase for this residue (Figure 7B). To assess more conclusively this possibility, we used RNA interference (RNAi) to downregulate PKC-β1. Figure 7C shows successful downregulation of PKC-β1 protein by RNAi. Although HG induced Akt phosphorylation at both S473 and T308, only S473 phosphorylation was abrogated by PKC-β1 downregulation (Figure 7C). This further suggests that PKC-β1 acts as an S473 kinase in response to HG in MCs.
Figure 7.
Akt S473 phosphorylation by glucose requires EGFR-dependent association with PKC-β1. (A) MCs were treated with the EGFR inhibitor AG1478 (1 μmol/L, 30 min) before HG for 1 h. After immunoprecipitation of PKC-β1, Western blotting for Akt was performed. HG led to the association of PKC-β1 and Akt, and this was dependent on EGFR transactivation (n = 6; *P < 0.05 HG versus others). (B) Similarly, HG led to the association of PKC-β1 with Akt phosphorylated on S473 (n = 4; #P < 0.01 HG versus others). (C) To downregulate the expression of PKC-β1, MCs were transfected with PKC-β1 RNAi or control RNAi (ConRNAi) as indicated. RNAi significantly attenuated PKC-β1 protein expression and prevented AktS473 phosphorylation in response to HG (n = 5; †P < 0.001 HG versus control). No effect on HG-induced phosphorylation of Akt at T308 was observed.
We next performed a PKC-β1 S473 kinase activity assay by immunoprecipitating membrane-translocated PKC-β1 and assessing its ability to phosphorylate a GST-Akt fusion protein on S473. In Figure 8A, immunoprecipitated PKC-β1 phosphorylated Akt on S473 in response to HG, which was prevented by the specific PKC-β inhibitor. No activity was observed when immunoprecipitation was performed with nonspecific IgG. We then confirmed that both EGFR and PI3K are upstream of PKC-β1 activity toward Akt. PI3K inhibitors wortmannin and LY294002, as well as the EGFR inhibitor AG1478, prevented PKC-β1 phosphorylation of GST-Akt at S473 in HG-treated MC (Figure 8, B and C). Similarly, in MCs overexpressing dnEGFR, PKC-β1 immunoprecipitated after HG treatment was unable to phosphorylate GST-Akt (Figure 8D). Hence, PKC-β1 functions downstream of EGFR/PI3K as a novel S473 kinase for Akt in HG-treated MC.
Figure 8.
PKC-β1 functions downstream of EGFR-PI3K activation as a glucose-induced Akt S473 kinase. For all experiments in this figure, PKC-β1 was immunoprecipitated from membrane fractions of MCs treated with HG for 1 h, and its kinase activity toward GST-Akt was assessed by immunoblotting for phosphorylated Akt S473. (A) PKC-β1 served as an Akt S473 kinase in HG-treated MC. Inhibition with the specific PKC-β inhibitor (100 nM, 30 min) supports a role for PKC-β1 in the observed S473 phosphorylation (n = 3; *P < 0.05 HG versus others). No phosphorylation of GST-Akt was observed when immunoprecipitation was carried out with control IgG. (B) PI3K inhibitors wortmannin (100 nmol/L, 60 min) and LY294002 (20 μmol/L, 30 min) prevented PKC-β1 kinase activity toward Akt at S473 (n = 2; *P < 0.05 HG versus others). (C) EGFR inhibition with AG1478 (1 μmol/L, 30 min) also prevented PKC-β1 kinase activity toward Akt at S473 (n = 4; #P < 0.01 HG versus others). (D) In MCs stably overexpressing dnEGFR, HG was unable to induce PKC-β1 function as an Akt S473 kinase as compared with MCs with empty vector pLHCX (n = 2; *P < 0.05 HG pLHCX versus dnEGFR).
PKC-β Inhibition In Vivo Prevents Akt S473 Phosphorylation in Diabetic Rat Kidneys
We next sought to confirm that our observed in vitro findings showing that PKC-β1 served as an Akt S473 kinase in response to glucose also occurred in vivo. Type 1 diabetes was induced in homozygous (mRen-2)27 rats by streptozotocin (STZ) injection. A group of diabetic rats was treated with the PKC-β inhibitor ruboxistaurin (LY333531) as described previously.28 This model, in which overexpression of the mouse renin gene leads to hypertension, has been shown to develop renal lesions similar to those observed in human diabetic nephropathy as early as 12 wk after STZ injection.29 Ruboxistaurin prevents ATP binding to the active site of PKC-β, thereby inhibiting its ability to phosphorylate substrates and functioning as a specific PKC-β inhibitor.30 It was shown to decrease glomerular TGF-β upregulation, glomerulosclerosis, and albuminuria in this model.31 After 12 wk, there was no difference between groups in systolic BP (control 204 ± 14 mmHg, STZ 208 ± 10 mmHg, STZ+Rub 218 ± 7 mmHg). Diabetic rats had an average plasma glucose level >25 mmol/L, unaffected by treatment with ruboxistaurin (control 5.78 ± 0.51 mmol/L, STZ 25.7 ± 1.94 mmol/L, STZ+Rub 28.5 ± 0.84 mmol/L; P < 0.01 for STZ+Rub versus Con).28 Figure 9A shows that pAktS473 was increased in the cortex of diabetic rats and that this was inhibited by ruboxistaurin. Data are quantified in Figure 9B. Ruboxistaurin had no effect on Akt T308 phosphorylation, further supporting a more specific role of PKC-β as an Akt S473 kinase in vivo. Immunofluorescence of cortical sections for pAkt S473 and Thy 1.1, an MC marker, shows an increase in glomerular staining for phosphorylated Akt in diabetic rats that is absent after ruboxistaurin treatment. Merged images demonstrate overlay (yellow), supporting localization of pAkt S473 to MCs (Figure 9C). Although we cannot exclude some degree of nonspecific binding of primary antibodies, coupled with our immunoblots in MCs, these data demonstrate that PKC-β plays a role both in vitro and in vivo in AktS473 phosphorylation and hence its activation.
Figure 9.
PKC-β inhibition prevents Akt S473 phosphorylation in diabetic kidneys. Type 1 diabetic (mRen-2)27 rat kidney cortex was analyzed 12 wk after onset of diabetes. A group was treated with the PKC-β inhibitor ruboxistaurin. (A and B) Western blots of cortical protein demonstrate a significant increase in Akt S473 phosphorylation in diabetics, prevented by ruboxistaurin (n = 8; *P < 0.05 STZ versus others). Akt T308 phosphorylation, however, was unaffected by treatment with ruboxistaurin. (C) Immunofluorescence of cortical sections demonstrates an increase in pAkt S473 (green) in glomeruli, which are identified by staining for the MC marker Thy 1.1 (red). Areas of overlap appear as yellow.
DISCUSSION
Akt has emerged as an important mediator of matrix upregulation in response to numerous stimuli including TGF-β1 and, more recently, glucose.1,9,32 We previously showed that EGFR transactivation and PI3K are upstream mediators of Akt activation.1 Although PDK1 has been well established as the T308 kinase for Akt, the identity of the S473 kinase has been more elusive and seems to be context specific. In endothelial and retinal pigment epithelial cells exposed to HG, involvement of PKC in the long-term (24 h) phosphorylation of Akt at S473 was suggested by blockade with a general PKC inhibitor.9,33 Our study explores the role of PKC as potential glucose-induced S473 kinases in MCs. This report extends the current understanding of the pathogenesis of diabetic glomerular disease in (1) identifying PKC-β1 as an Akt S473 kinase in response to HG in MCs and demonstrating relevance in vivo in diabetic kidneys, (2) showing that PKC-β1/Akt interaction is required for TGF-β upregulation, and (3) identifying EGFR transactivation as an upstream mediator of this signaling cascade. A model of this signaling cascade is presented in Figure 10.
Figure 10.
Model of the proposed signaling cascade. This summarizes our current and previous data, integrated with that of others. In MCs, HG leads to early EGFR transactivation. Downstream, activation of PI3K stimulates PDK1 activity toward T308. PI3K activation, either directly or through the action of PDK1 (as represented by dashed lines), leads to PKC-β1 activation. In turn, PKC-β1 acts as the Akt S473 kinase in this setting, with phosphorylation of both T308 and S473 leading to Akt activation. Subsequent AP-1 activation results in upregulation of TGF-β1 transcription.
PKC isoforms α, β1, β2, ɛ, θ, and ζ were shown to interact with Akt either in vitro or in vivo.7,8,34,35 Of these, PKCζ is not expressed by MCs,36 and PKCζ is inhibitory to Akt activation.35 PKCβ2 was not detected in any glomerular cell type in rats or mice17,37 and in isolated MCs was unresponsive to stimulation.16 This PKC-β isoform thus seems to have limited significance in mesangial responses. Both conventional isoforms PKCα and -β were shown to phosphorylate Akt at S473 in in vitro assays, although PKCα did not play a role in vivo in stimulated mast cells. Here, PKC-β2 served as the primary S473 kinase.7 Indeed, some studies have shown PKCα to inhibit Akt activation.38 Furthermore, in HepG2 cells, PKCδ was required for Akt S473 phosphorylation in response to an orgovanadium compound, whereas conventional PKC isoform inhibition was without effect.27 The role of specific PKC isoforms as S473 kinases is thus highly cell and stimulus specific.
Using a conventional PKC inhibitor, we demonstrated a role for PKCα and/or -β in Akt S473 phosphorylation in response to HG. Both isoforms are activated in MCs by HG and in glomeruli of diabetic rats.39 We further identified PKC-β1 as the relevant kinase through the use of a more specific inhibitor, by knockdown of PKC-β1 with small interfering RNA (siRNA), by showing that HG induces physical interaction between Akt and PKC-β1, and with an activity assay that demonstrated that PKC-β1 isolated from HG-treated MCs phosphorylated GST-Akt at S473. In thrombin-treated platelets, a conventional PKC inhibitor also prevented pAktS473, although the effect was only partial and a co-factor was probably necessary.40 Because our activity assays were performed with PKC-β1 immunoprecipitated from MC under various conditions, we cannot exclude requirement for a co-factor when PKC-β1 functions as an AktS473 kinase.
We previously showed that Akt mediates HG-induced collagen I upregulation in MCs.1 Although it is known that TGF-β can signal through PI3K/Akt, whether these kinases are involved in the upregulation of TGF-β in HG-exposed MCs is not known. Inhibitor studies suggested that PI3K mediates HG-induced TGF-β bioactivity in tubular epithelial cells.15 Our studies demonstrate, through the use of both PI3K inhibitors and dominant negative Akt, that HG-induced TGF-β1 upregulation requires PI3K/Akt signaling in MCs. We further showed that PKC-β1 was required for TGF-β1 upregulation. This is in keeping with previous studies showing a role for PKC in TGF-β promoter activation.13 The specific function of PKC-β in TGF-β upregulation demonstrated by our studies is supported by findings in selective PKC isoform knockout models. Diabetic PKC-α knockout mice had comparable upregulation of glomerular TGF-β as their wild-type counterparts, whereas TGF-β upregulation was abrogated in diabetic PKC-β knockouts.10,11,41
The TGF-β1 promoter has regulatory binding sites for the transcription factor AP-1.13 AP-1 is activated by HG in MCs and in diabetic kidneys.42,43 PI3K/Akt signaling regulates AP-1 activity in response to various stimuli,20–22 and HG-induced AP-1 activation in endothelia was blocked by Akt inhibition.9 Our experiments confirmed a role for Akt in AP-1 activation in MCs in response to HG and additionally demonstrated dependence on PKC-β1 activation. Taken together, these studies suggest that PKC-β1/Akt signaling to AP-1 is required for HG-induced TGF-β1 transcriptional upregulation in MCs. Furthermore, we have shown that transactivation of the EGFR is required as an upstream event. This may mediate the involvement of other pathways that contribute to TGF-β1 upregulation. Indeed, MEK/ERK signaling is a better known AP-1 activator,44 and we previously showed that EGFR transactivation also mediates glucose-induced ERK activation in MCs.1 Isono et al.14 showed that the MEK inhibitor PD98059 blocked glucose-induced TGF-β1 gene and protein upregulation in MCs. Hence, several pathways likely converge downstream of EGFR transactivation to effect upregulation of prosclerotic cytokines and matrix proteins.
Conventional PKC isoforms require calcium, DAG, and phosphatidylserine for their activity.36 Our studies demonstrate that EGFR transactivation and PI3K are necessary for HG-induced PKC-β1 activation and downstream events. PI3K interacts with receptor tyrosine kinases such as EGFR and then generates lipid second messengers that recruit proteins with PH domains to the membrane, enabling their activation. These include PDK1, the T308 kinase for Akt, as well as Akt itself. PDK1 may associate with PKC isoforms including PKC-β123 and activate them by phosphorylation in the activation loop.23,24,45 Thus, EGFR/PI3K-induced activation of PKC-β1 may be enabled by PDK1 downstream of PI3K. Phospholipase Cγ (PLCγ), known to interact with the EGFR and to generate DAG, may also represent an important early mediator of PKC-β1 activation in response to HG.46 Indeed, it is activated at early time points by HG in vascular smooth muscle cells, with generation of DAG observed by 30 min.47 We are currently exploring the potential role of PLCγ in mediating PKC-β1 activation downstream of the EGFR in response to HG.
PKC-β has been shown to play an important role in diabetic nephropathy in in vivo models. Both PKC-β inhibition and PKC-β deletion suppressed TGF-β1 upregulation and matrix expansion in diabetic glomeruli or kidneys.10,11,30 We previously showed pAktS473 in diabetic glomeruli.1 We now demonstrate that PKC-β inhibition with ruboxistaurin prevents Akt phosphorylation in diabetic kidneys, supporting a function for PKC-β/Akt in the TGF-β upregulation identified in our in vitro studies. The role of Akt in diabetic glomerular disease, however, likely extends beyond that of matrix accumulation. PI3K/Akt signaling mediated glucose-induced hypertrophy in MC and tubular epithelia.15,48 Glomerular hypertrophy is attenuated in diabetic PKC-β null mice,10,11 whereas PKCα deletion did not affect this parameter,41 thus supporting a role for PKC-β1/Akt signaling in mediating cell and organ size. Although a significant decrease in renal hypertrophy was not observed in type 1 diabetic rats treated with ruboxistaurin, glomerular hypertrophy was not specifically assessed.39,49
Although PKC-β inhibitors have shown promise in preclinical studies of diabetic nephropathy, analyses of diabetic knockout mice suggest that other isoforms contribute importantly to its phenotype. For example, PKCα translocation is preserved in diabetic PKC-β knockout mice, and, indeed, albuminuria in the PKCα knockout phenotype is prevented, with no significant changes observed in PKC-β knockouts.10,41 Because PDK1 and PI3K would also be expected to be upstream of other isoforms, including PKCα,23 targeting these effectors may lead to greater improvement in renal protection. In vivo studies would be required to assess this possibility, given the potential of adverse effects from inhibition of other downstream targets. Alternatively, targeting the initial signaling initiators of complex or multiple pathways, such as the EGFR, which lies upstream of both PDK1 and PI3K, is another attractive target for therapy of diabetic kidney disease.
CONCISE METHODS
Cell Culture
Sprague-Dawley primary rat MCs (passages 6 through 15) were cultured in DMEM supplemented with 20% FCS (Invitrogen, Burlington, Ontario, Canada), streptomycin (100 μg/ml), and penicillin (100 U/ml) at 37°C in 95% air/5% CO2. Medium contained 5.6 mmol/L glucose. Either 24.4 mmol/L glucose (final concentration 30 mmol/L) or mannitol was added for HG or osmotic control, respectively. MCs were made quiescent by serum deprivation for 24 h before treatment. Medium was changed every 2 d for longer duration experiments. Pharmacologic inhibitors were added before HG as follows: Gö6976 2 μM, 30 min (Calbiochem, Mississauga, Ontario, Canada); PMA 200 nM, 24 h (Sigma); PKC-β inhibitor 100 nM, 30 min (Calbiochem); wortmannin 100 nmol/L, 60 min (Sigma); LY294002 20 μmol/L, 30 min (Sigma); and AG1478 1 μmol/L, 30 min (Sigma, Oakville, Ontario, Canada).
Protein Extraction and Analysis
Cells were lysed and protein extracted as we have published previously.50 Cell lysates were centrifuged at 4°C and 14,000 rpm for 10 min to pellet cell debris. Supernatant (50 μg) was separated on SDS-PAGE, and Western blotting was performed. Antibodies were polyclonal phospho-Akt S473 (1:1000), polyclonal phospho-Akt T308 (1:1000), polyclonal Akt (1:1000), polyclonal EGFR (1:1000; all Cell Signaling, Boston, MA), polyclonal phospho-EGFR Y1068 (1:1000; Sigma), and monoclonal PKC-β1 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA).
For immunoprecipitation experiments, cells were lysed and clarified, and equal amounts of lysate were incubated overnight with 2 μg of primary antibody rotating at 4°C, followed by 25 μl of protein G-agarose slurry for 1.5 h at 4°C. Immunoprecipitates were extensively washed, resuspended in 2× sample buffer, boiled, and analyzed by immunoblotting.
For cytosol-membrane fractionation, cells were harvested in hypotonic lysis buffer (50 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 10 mM EGTA, 2 mM EDTA, and inhibitor cocktail), homogenized by 25-G needle passage, and centrifuged at 100,000 × g for 60 min. Supernatant was taken as cytosol, and the pellet was resuspended in regular lysis buffer50 with 60 mM N-octyl-glucopyranoside. After centrifugation at 100,000 × g for 60 min, the supernatant was collected as the membrane fraction.
Northern Analysis
Total RNA (20 μg), extracted using Trizol (Invitrogen), was separated on a formaldehyde-agarose gel and transferred to a nylon membrane (Hybond; Amersham Biosciences, Baie d'Urfe, Quebec, Canada). Hybridization was performed with random primed digoxigenin-11-dUTP–labeled cDNA probes prepared from TGF-β or β-actin cDNA amplified by PCR. Hybridized probes were detected using alkaline phosphatase–labeled anti-digoxigenin antibodies and CDP-star as substrate. Kits and reagents were from Roche Applied Science (Mississauga, Ontario, Canada).
MC Infection
Epitope-tagged dominant negative Akt (HA-AktAAA), constitutively active Akt (HA-AktDD), both provided by Dr. J. Woodgett51, or dnEGFR K721A (provided by Dr. S. Parsons, University of Virginia Health Services, Charlottesville, VA) were cloned into pLHCX (Clontech, Mountain View, CA) for retroviral infection and MC infected as described previously.19 Competent virus capable of single infection was generated using the vesicular stomatitis virus system (Stratagene, La Jolla, CA), and MC passages 5 through 12 were exposed to virus concentrated by centrifugation in the presence of polybrene. Seventy-two hours after infection, a 2-wk antibiotic selection period was begun. Experiments were performed using a population of pooled, stably infected MCs.
Luciferase Assay
MCs plated to 85% subconfluence were transfected with 0.5 μg of a TGF-β1 promoter-luciferase construct (provided by Dr. N. Kato52) and 0.05 μg of pCMV-β-galactosidase (β-gal; Clontech) using LipofectAMINE (Qiagen, Mississauga, Ontario, Canada). MCs were serum-deprived overnight 24 h after transfection, then exposed to glucose for 24 h. Lysis was achieved with Reporter Lysis Buffer (Promega, Madison, WI) using one freeze-thaw cycle, and luciferase and β-gal activities were measured on clarified lysate using specific kits (Promega) with a Berthold luminometer and a plate reader (420 nm), respectively. β-Gal activity was used to adjust for transfection efficiency.
EMSA
After treatment, nuclear extracts were prepared as published previously.53 Cells were lysed in hypotonic buffer, homogenized, and sedimented at 16,000 × g for 20 min at 4°C. Pelleted nuclei were resuspended in hypotonic buffer containing 0.42 M NaCl and 20% glycerol, rotated for 30 min at 4°C, and centrifuged as already described, and supernatant containing nuclear proteins was collected. Nuclear proteins (3 μg) were incubated for 5 min at room temperature with a biotin-labeled AP-1 consensus oligonucleotide (Sigma) as per the manufacturer's instructions (Pierce, Rockford, IL). Reaction mixtures were electrophoresed in a 6% polyacrylamide gel, transferred, DNA cross-linked to a nylon membrane (Amersham), and then probed with horseradish peroxidase–conjugated streptavidin antibodies (1:300; Pierce).
PKC-β1 Assay for Akt Phosphorylation
Membrane protein was isolated and PKC-β1 immunoprecipitated as described in the previous section. After washing, kinase reactions were carried out for 10 min at 30°C in kinase buffer containing 50 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 40 μM ATP, 100 nM PMA (Sigma), 15 μg of phosphatidylserine (Sigma), 0.1 μg of GST-Akt (Cell Signaling), and protease/phosphatase inhibitor cocktail per reaction. Beads were resuspended in 5× reducing sample buffer and boiled for 5 min, and the resulting supernatant was resolved on 10% SDS-PAGE. Membranes were probed for phospho-Akt S473.
RNA Interference
Rat PKC-β1 On-Target Plus Smart Pool siRNA and control nontargeting siRNA were purchased from Dharmacon (Lafayette, CO). MCs were transfected with 100 nM using GeneEraser siRNA reagent (Stratagene) at 60% confluence. After 48 h, cells were serum-deprived for 24 h and treated with HG for 1 h, and cell lysate was harvested as described already. PKC-β1 protein expression was used to assess efficacy of downregulation by RNAi.
Animal Studies
Experiments were conducted as described previously.28 Briefly, diabetes was induced in female homozygous (mRen-2)27 rats with 55 mg/kg STZ (Sigma) by tail-vein injection. Control rats received 0.1 M citrate buffer (pH 4.5). Rats were divided into control, diabetic, or diabetic treated with the selective PKC-β inhibitor ruboxistaurin mesylate 10 mg/kg per d (LY333531; Eli Lilly and Co, Indianapolis, IN) in rat chow for 12 wk (n = 8 per group). Rats were monitored weekly for weight and blood glucose and monthly for BP by tail-cuff plethysmography. For Westerns, protein was extracted as described already from cortex snap-frozen in liquid nitrogen, except that tissue was also passed through a dounce homogenizer after lysis. For immunohistochemistry, cortical sections stored in OCT were processed as described previously.1 Primary antisera used were polyclonal phospho-AktS473 (1:50; Cell Signaling) and monoclonal Thy1.1 (1:50; BD Transduction, Missisauga, Ontario, Canada).
Statistical Analysis
Statistical analyses were performed with SPSS 14 for Windows using one-way ANOVA, with Tukey HSD for post hoc analysis for experiments with more than two groups. A t test was used for analysis of two groups. P < 0.05 (two-tailed) was considered significant. Data are presented as the means ± SEM.
DISCLOSURES
None.
Acknowledgments
We are grateful for the support of the Canadian Institutes of Health Research (CIHR) and Canadian Diabetes Association (J.C.K). D.W. is a recipient of the Krescent Fellowship sponsored by the Kidney Foundation of Canada and CIHR, and F.P. is a recipient of the Father Sean O'Sullivan Research Center Fellowship. We thank Dr. J. Woodgett (Toronto, Ontario, Canada) for kindly providing pcDNA3 HA-AktAAA and pcDNA3 HA-AktDD, Dr. S. Parsons for providing pcDNA3 EGFR K721A (Charlottesville, VA), and Dr. N. Kato for providing the TGF-β1 promoter luciferase construct (Tokyo, Japan).
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Wu D, Peng F, Zhang B, Ingram AJ, Gao B, Krepinsky JC: Collagen I induction by high glucose levels is mediated by epidermal growth factor receptor and phosphoinositide 3-kinase/Akt signalling in mesangial cells. Diabetologia 50: 2008–2018, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Hanada M, Feng J, Hemmings BA: Structure, regulation and function of PKB/AKT: A major therapeutic target. Biochim Biophys Acta 1697: 3–16, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Cantley LC: The phosphoinositide 3-kinase pathway. Science 296: 1655–1657, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Feng J, Park J, Cron P, Hess D, Hemmings BA: Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem 279: 41189–41196, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Bozulic L, Surucu B, Hynx D, Hemmings BA: PKBalpha/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol Cell 30: 203–213, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM: Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Kawakami Y, Nishimoto H, Kitaura J, Maeda-Yamamoto M, Kato RM, Littman DR, Rawlings DJ, Kawakami T: Protein kinase C betaII regulates Akt phosphorylation on Ser-473 in a cell type- and stimulus-specific fashion. J Biol Chem 279: 47720–47725, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Lu D, Huang J, Basu A: Protein kinase Cepsilon activates protein kinase B/Akt via DNA-PK to protect against tumor necrosis factor-alpha-induced cell death. J Biol Chem 281: 22799–22807, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Xin X, Khan ZA, Chen S, Chakrabarti S: Glucose-induced Akt1 activation mediates fibronectin synthesis in endothelial cells. Diabetologia 48: 2428–2436, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Meier M, Park JK, Overheu D, Kirsch T, Lindschau C, Gueler F, Leitges M, Menne J, Haller H: Deletion of protein kinase C-beta isoform in vivo reduces renal hypertrophy but not albuminuria in the streptozotocin-induced diabetic mouse model. Diabetes 56: 346–354, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Ohshiro Y, Ma RC, Yasuda Y, Hiraoka-Yamamoto J, Clermont AC, Isshiki K, Yagi K, Arikawa E, Kern TS, King GL: Reduction of diabetes-induced oxidative stress, fibrotic cytokine expression, and renal dysfunction in protein kinase Cbeta-null mice. Diabetes 55: 3112–3120, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Ziyadeh FN: Mediators of diabetic renal disease: The case for TGF-beta as the major mediator. J Am Soc Nephrol 15[Suppl 1]: S55–S57, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Weigert C, Sauer U, Brodbeck K, Pfeiffer A, Haring HU, Schleicher ED: AP-1 proteins mediate hyperglycemia-induced activation of the human TGF- beta1 promoter in mesangial cells. J Am Soc Nephrol 11: 2007–2016, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Isono M, Cruz MC, Chen S, Hong SW, Ziyadeh FN: Extracellular signal-regulated kinase mediates stimulation of TGF-beta1 and matrix by high glucose in mesangial cells. J Am Soc Nephrol 11: 2222–2230, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Chuang TD, Guh JY, Chiou SJ, Chen HC, Huang JS, Yang YL, Chuang LY: Phosphoinositide 3-kinase is required for high glucose-induced hypertrophy and p21(WAF1) expression in LLC-PK1 cells. Kidney Int 71: 867–874, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Kapor-Drezgic J, Zhou X, Babazono T, Dlugosz JA, Hohman T, Whiteside C: Effect of high glucose on mesangial cell protein kinase C-delta and -epsilon is polyol pathway-dependent. J Am Soc Nephrol 10: 1193–1203, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Redling S, Pfaff IL, Leitges M, Vallon V: Immunolocalization of protein kinase C isoenzymes alpha, beta I, beta II, delta, and epsilon in mouse kidney. Am J Physiol Renal Physiol 287: F289–F298, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Hoffman BB, Sharma K, Zhu Y, Ziyadeh FN: Transcriptional activation of transforming growth factor-beta1 in mesangial cell culture by high glucose concentration. Kidney Int 54: 1107–1116, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Krepinsky JC, Ingram AJ, Tang D, Wu D, Liu L, Scholey JW: Nitric oxide inhibits stretch-induced MAPK activation in mesangial cells through RhoA inactivation. J Am Soc Nephrol 14: 2790–2800, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Fisslthaler B, Benzing T, Busse R, Fleming I: Insulin enhances the expression of the endothelial nitric oxide synthase in native endothelial cells: A dual role for Akt and AP-1. Nitric Oxide 8: 253–261, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Ma C, Wang J, Luo J: Exposure to asphalt fumes activates activator protein-1 through the phosphatidylinositol 3-kinase/Akt signaling pathway in mouse epidermal cells. J Biol Chem 278: 44265–44272, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Jhun BH, Rose DW, Seely BL, Rameh L, Cantley L, Saltiel AR, Olefsky JM: Microinjection of the SH2 domain of the 85-kilodalton subunit of phosphatidylinositol 3-kinase inhibits insulin-induced DNA synthesis and c-fos expression. Mol Cell Biol 14: 7466–7475, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ: Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281: 2042–2045, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Dutil EM, Toker A, Newton AC: Regulation of conventional protein kinase C isozymes by phosphoinositide-dependent kinase 1 (PDK-1). Curr Biol 8: 1366–1375, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Oliva JL, Griner EM, Kazanietz MG: PKC isozymes and diacylglycerol-regulated proteins as effectors of growth factor receptors. Growth Factors 23: 245–252, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Ayo SH, Radnik R, Garoni JA, Troyer DA, Kreisberg JI: High glucose increases diacylglycerol mass and activates protein kinase C in mesangial cell cultures. Am J Physiol 261: F571–F577, 1991 [DOI] [PubMed] [Google Scholar]
- 27.Mehdi MZ, Vardatsikos G, Pandey SK, Srivastava AK: Involvement of insulin-like growth factor type 1 receptor and protein kinase Cdelta in bis(maltolato)oxovanadium(IV)-induced phosphorylation of protein kinase B in HepG2 cells. Biochemistry 45: 11605–11615, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kelly DJ, Buck D, Cox AJ, Zhang Y, Gilbert RE: Effects on protein kinase C-beta inhibition on glomerular vascular endothelial growth factor expression and endothelial cells in advanced experimental diabetic nephropathy. Am J Physiol Renal Physiol 293: F565–F574, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Kelly DJ, Wilkinson-Berka JL, Allen TJ, Cooper ME, Skinner SL: A new model of diabetic nephropathy with progressive renal impairment in the transgenic (mRen-2)27 rat (TGR). Kidney Int 54: 343–352, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Anderson PW, McGill JB, Tuttle KR: Protein kinase C beta inhibition: The promise for treatment of diabetic nephropathy. Curr Opin Nephrol Hypertens 16: 397–402, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Kelly DJ, Zhang Y, Hepper C, Gow RM, Jaworski K, Kemp BE, Wilkinson-Berka JL, Gilbert RE: Protein kinase C beta inhibition attenuates the progression of experimental diabetic nephropathy in the presence of continued hypertension. Diabetes 52: 512–518, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Runyan CE, Schnaper HW, Poncelet AC: The phosphatidylinositol 3-kinase/Akt pathway enhances Smad3-stimulated mesangial cell collagen I expression in response to transforming growth factor-beta1. J Biol Chem 279: 2632–2639, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Kim DI, Lim SK, Park MJ, Han HJ, Kim GY, Park SH: The involvement of phosphatidylinositol 3-kinase/Akt signaling in high glucose-induced downregulation of GLUT-1 expression in ARPE cells. Life Sci 80: 626–632, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Bauer B, Krumbock N, Fresser F, Hochholdinger F, Spitaler M, Simm A, Uberall F, Schraven B, Baier G: Complex formation and cooperation of protein kinase C theta and Akt1/protein kinase B alpha in the NF-kappa B transactivation cascade in Jurkat T cells. J Biol Chem 276: 31627–31634, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Wen HC, Huang WC, Ali A, Woodgett JR, Lin WW: Negative regulation of phosphatidylinositol 3-kinase and Akt signalling pathway by PKC. Cell Signal 15: 37–45, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Runyan CE, Schnaper HW, Poncelet AC: Smad3 and PKCdelta mediate TGF-beta1-induced collagen I expression in human mesangial cells. Am J Physiol Renal Physiol 285: F413–F422, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Pfaff IL, Wagner HJ, Vallon V: Immunolocalization of protein kinase C isoenzymes alpha, beta1 and betaII in rat kidney. J Am Soc Nephrol 10: 1861–1873, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Byrne RD, Rosivatz E, Parsons M, Larijani B, Parker PJ, Ng T, Woscholski R: Differential activation of the PI 3-kinase effectors AKT/PKB and p70 S6 kinase by compound 48/80 is mediated by PKCalpha. Cell Signal 19: 321–329, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Koya D, Jirousek MR, Lin YW, Ishii H, Kuboki K, King GL: Characterization of protein kinase C beta isoform activation on the gene expression of transforming growth factor-beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. J Clin Invest 100: 115–126, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kroner C, Eybrechts K, Akkerman JW: Dual regulation of platelet protein kinase B. J Biol Chem 275: 27790–27798, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Menne J, Park JK, Boehne M, Elger M, Lindschau C, Kirsch T, Meier M, Gueler F, Fiebeler A, Bahlmann FH, Leitges M, Haller H: Diminished loss of proteoglycans and lack of albuminuria in protein kinase C-alpha-deficient diabetic mice. Diabetes 53: 2101–2109, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Wilmer WA, Cosio F: G.DNA binding of activator protein-1 is increased in human mesangial cells cultured in high glucose concentrations. Kidney Int 53: 1172–1181, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Chen S, Khan ZA, Cukiernik M, Chakrabarti S: Differential activation of NF-kappa B and AP-1 in increased fibronectin synthesis in target organs of diabetic complications. Am J Physiol Endocrinol Metab 284: E1089–E1097, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Karin M: The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 270: 16483–16486, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Li J, Gobe G: Protein kinase C activation and its role in kidney disease. Nephrology (Carlton) 11: 428–434, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Wang Z, Moran MF: Phospholipase C-gamma1: A phospholipase and guanine nucleotide exchange factor. Mol Interv 2: 352–5: 338, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Ramana KV, Friedrich B, Tammali R, West MB, Bhatnagar A, Srivastava SK: Requirement of aldose reductase for the hyperglycemic activation of protein kinase C and formation of diacylglycerol in vascular smooth muscle cells. Diabetes 54: 818–829, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Nagai K, Matsubara T, Mima A, Sumi E, Kanamori H, Iehara N, Fukatsu A, Yanagita M, Nakano T, Ishimoto Y, Kita T, Doi T, Arai H: Gas6 induces Akt/mTOR-mediated mesangial hypertrophy in diabetic nephropathy. Kidney Int 68: 552–561, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Kelly DJ, Chanty A, Gow RM, Zhang Y, Gilbert RE: Protein kinase Cbeta inhibition attenuates osteopontin expression, macrophage recruitment, and tubulointerstitial injury in advanced experimental diabetic nephropathy. J Am Soc Nephrol 16: 1654–1660, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Krepinsky JC, Ingram AJ, Tang D, Wu D, Liu L, Scholey JW: Nitric oxide inhibits stretch-induced MAPK activation in mesangial cells through RhoA inactivation. J Am Soc Nephrol 14: 2790–2800, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Wang Q, Somwar R, Bilan PJ, Liu Z, Jin J, Woodgett JR, Klip A: Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol 19: 4008–4018, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taniguchi H, Kato N, Otsuka M, Goto T, Yoshida H, Shiratori Y, Omata M: Hepatitis C virus core protein upregulates transforming growth factor-beta 1 transcription. J Med Virol 72: 52–59, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Krepinsky J, Ingram AJ, James L, Ly H, Thai K, Cattran DC, Miller JA, Scholey JW: 17beta-Estradiol modulates mechanical strain-induced MAPK activation in mesangial cells. J Biol Chem 277: 9387–9394, 2002 [DOI] [PubMed] [Google Scholar]