Abstract
Women have less access to kidney transplantation than men, but the contributions of age and comorbidity to this disparity are largely unknown. We conducted a national cohort study of 563,197 patients with first-onset ESRD between 2000 and 2005. We used multivariate generalized linear models to evaluate both access to transplantation (ATT), defined as either registration for the deceased-donor waiting list or receiving a live-donor transplant, and survival benefit from transplantation, defined as the relative rate of survival after transplantation compared with the rate of survival on dialysis. We compared relative risks (RRs) between women and men, stratified by age categories and the presence of common comorbidities. Overall, women had 11% less ATT than men. When the model was stratified by age, 18- to 45-yr-old women had equivalent ATT to men (RR 1.01), but with increasing age, ATT for women declined dramatically, reaching a RR of 0.41 for those who were older than 75 yr, despite equivalent survival benefits from transplantation between men and women in all age subgroups. Furthermore, ATT for women with comorbidities was lower than that for men with the same comorbidities, again despite similar survival benefits from transplantation. This study suggests that there is no disparity in ATT for women in general but rather a marked disparity in ATT for older women and women with comorbidities. These disparities exist despite similar survival benefits from transplantation for men and women regardless of age or comorbidities.
For many patients with ESRD, kidney transplantation (KT) improves both survival and quality of life1–4; however, the organ supply is limited, and careful patient selection is required to ensure equitable access to transplantation (ATT) for patients who stand to derive a survival benefit from transplantation (SBT). Studies of factors influencing ATT have identified a number of subgroup disparities that may not be explained by poor SBT, including patients of lower socioeconomic status,5,6 black patients,5,7–10 and obese patients.11
Among the most difficult findings in ATT research to explain has been that of gender disparity. More than 20 studies have shown that women with ESRD have 10 to 20% less ATT than men, even after adjustment for relevant demographic and clinical characteristics.5–7,9,10,12–27 This disparity has been shown to exist in both live- and deceased-donor transplantation. A systematic review20 determined that most studies found equivalent or better post-KT survival in women.13,23,27–37 At least one study suggested that women were less likely to be thought of as appropriate transplant candidates by their providers, despite similar medical histories26; however, no studies have attempted to dissect out the factors contributing to this disparity to elucidate a possible mechanism.
Even though all older adults are eligible for Medicare, this population is less likely to access tertiary care services such as surgical procedures.38–41 Older patients with ESRD are no exception; they comprise >50% of the ESRD population but account for <10% of all transplants.42 We hypothesized that physicians, friends and family, and the patients themselves might be more likely to perceive elderly female patients as frail and unable to withstand a surgical procedure. As such, there might be an interaction between increased age and female gender such that the gender disparity is exaggerated in older patients, yet, of >20 reports of gender disparities, a potential age interaction was suggested in only one statewide study between 1984 and 1989 of patients who were younger than 65 yr.16 In older patients, we thought that clinical decision making might be driven more by patient and provider perceptions of health status than by validated clinical metrics. As such, it is conceivable that older women might be more likely to be perceived by their providers or to perceive themselves as unable to survive an invasive surgical procedure such as transplantation. We also hypothesized that the presence of comorbidities in women might be perceived differently than the same comorbidities in their male counterparts and as such also contribute to the gender disparity. The goal of this study was to quantify the effects of age and comorbidity on the relationship among gender, ATT, and SBT in a recent national cohort that included patients who were older than 65 yr.
RESULTS
Baseline Characteristics
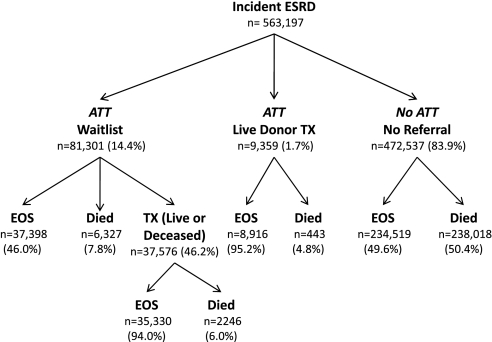
Of 563,197 incident patients who had ESRD and were eligible for this study, 81,301 (14.4%) joined the deceased-donor waiting list during the study period, 9359 (1.7%) received a transplant from a live donor without ever joining the waiting list, and 472,537 (83.9%) never joined the waiting list (Figure 1). Of those who never had ATT, 234,519 (49.6%) remained on dialysis until the end of the study, whereas 238,018 (50.4%) died during the study period.
Figure 1.
Paths of incident ESRD in our study population. EOS, end of study; TX, transplant. Percentages refer to proportion of group one level above.
Women with incident ESRD were more likely to be black (31.4 versus 25.7%), more likely to have Medicaid insurance coverage (13.7 versus 9.7%), more likely to have diabetes as the assigned cause of ESRD (48.3 versus 42.6%), and slightly more likely to have congestive heart failure (32.0 versus 29.4%) (Table 1). Men were more likely to have peripheral vascular disease (14.6 versus 12.4%) and ischemic heart disease (26.0 versus 21.8%) and were slightly more likely to be have hypertension as the assigned cause of ESRD (28.5 versus 26.3%), to have had a myocardial infarction (MI; 9.2 versus 7.1%), to smoke (5.7 versus 3.8%), and to have pulmonary disease (8.1 versus 6.7%).
Table 1.
Baseline characteristics of incident patients with ESRD, 2000 to 2005a
| Characteristic | Men | Women | % Missing |
|---|---|---|---|
| Demographics | |||
| age (yr; mean) | 62.5 | 63.9 | 0.0 |
| black | 25.7 | 31.4 | 0.0 |
| Insurance type | 7.0 | ||
| no insurance | 8.9 | 6.4 | |
| Medicaid | 9.7 | 13.7 | |
| Medicare | 45.9 | 40.1 | |
| Medicaid and Medicare | 8.9 | 17.0 | |
| private | 26.6 | 22.7 | |
| BMI | 4.0 | ||
| <18 | 4.0 | 5.9 | |
| 18 to 25 | 38.8 | 33.0 | |
| 25 to 30 | 31.7 | 26.2 | |
| 30 to 35 | 15.4 | 17.0 | |
| 35 to 40 | 6.1 | 9.4 | |
| >40 | 4.1 | 8.4 | |
| Primary cause of ESRD | 2.3 | ||
| diabetes | 42.6 | 48.3 | |
| hypertension | 28.5 | 26.3 | |
| glomerulonephritis | 9.5 | 7.3 | |
| polycystic kidney | 2.3 | 2.3 | |
| urologic | 3.2 | 2.2 | |
| other | 9.6 | 9.9 | |
| unknown | 4.3 | 3.7 | |
| Erythropoietin | 30.7 | 33.9 | 4.0 |
| Hypertension | 80.1 | 81.3 | 3.0 |
| Diabetes | 50.9 | 56.8 | 3.0 |
| Heart Disease | 3.0 | ||
| dysrhythmia | 6.6 | 5.4 | |
| MI | 9.2 | 7.1 | |
| cardiac failure | 29.4 | 32.0 | |
| Vascular disease | 3.0 | ||
| cerebrovascular | 8.7 | 9.1 | |
| peripheral vascular | 14.6 | 12.4 | |
| ischemic heart disease | 26.0 | 21.8 | |
| Other | 3.0 | ||
| smoker | 5.7 | 3.8 | |
| immobile | 3.5 | 4.5 | |
| cancer | 6.5 | 5.0 | |
| pulmonary disease | 8.1 | 6.7 |
Data are percentages unless otherwise specified. BMI, body mass index.
Association among Gender, ATT, and SBT
When all ages were analyzed together, there was a population-based association between female gender and access, with women having 11% less ATT than men (Table 2); however, when a term for the interaction between age and gender was added to the model, the gender disparity was explained by the interaction term alone, showing that women had 7% less ATT for every decade increase in age (RR 0.93; 95% confidence interval [CI] 0.92 to 0.94; P < 0.001). Older age was still associated with decreased ATT (RR 0.70; 95% CI 0.69 to 0.70; P < 0.001), indicating that age was still a predictor of ATT, in addition to the relationship between age and gender (Table 2).
Table 2.
Multivariate analysis of factors associated with ATT in patients who had ESRD and were older than 18 yr and repeated with a term for the interaction between decade of age and gender included
| Factor | Population Based | Interaction Term |
|---|---|---|
| Demographics | ||
| female | 0.89 (0.88 to 0.90) | 1.06 (1.04 to 1.08) |
| age (decade) | 0.67 (0.67 to 0.68) | 0.70 (0.69 to 0.70) |
| age and female gender | N/A | 0.93 (0.92 to 0.94) |
| black | 0.74 (0.73 to 0.75) | 0.74 (0.73 to 0.75) |
| Insurance type | ||
| no insurance | 0.52 (0.51 to 0.53) | 0.52 (0.51 to 0.53) |
| Medicaid | 0.52 (0.51 to 0.53) | 0.52 (0.51 to 0.53) |
| Medicare | 0.52 (0.51 to 0.53) | 0.52 (0.51 to 0.53) |
| Medicare and Medicaid | 0.42 (0.41 to 0.43) | 0.42 (0.41 to 0.44) |
| private | Reference | Reference |
| BMI | ||
| <18 | 0.75 (0.72 to 0.77) | 0.74 (0.72 to 0.77) |
| 18 to 25 | Reference | Reference |
| 25 to 30 | 1.19 (1.17 to 1.21) | 1.19 (1.18 to 1.21) |
| 30 to 35 | 1.22 (1.20 to 1.24) | 1.22 (1.21 to 1.24) |
| 35 to 40 | 1.05 (1.02 to 1.07) | 1.05 (1.03 to 1.07) |
| >40 | 0.70 (0.68 to 0.72) | 0.70 (0.68 to 0.72) |
| Primary cause of ESRD | ||
| diabetes | 0.87 (0.85 to 0.90) | 0.87 (0.85 to 0.90) |
| hypertension | 0.73 (0.72 to 0.74) | 0.73 (0.72 to 0.74) |
| glomerulonephritis | Reference | Reference |
| polycystic kidney | 1.43 (1.41 to 1.46) | 1.43 (1.41 to 1.46) |
| urologic | 0.80 (0.78 to 0.83) | 0.79 (0.77 to 0.82) |
| other | 0.67 (0.66 to 0.69) | 0.67 (0.65 to 0.68) |
| unknown | 0.78 (0.75 to 0.80) | 0.78 (0.75 to 0.80) |
| Erythropoietin | 1.34 (1.32 to 1.35) | 1.33 (1.32 to 1.35) |
| Hypertension | 1.21 (1.20 to 1.23) | 1.22 (1.20 to 1.23) |
| Diabetes | 0.82 (0.80 to 0.85) | 0.82 (0.80 to 0.85) |
| Heart disease | ||
| dysrhythmia | 0.76 (0.72 to 0.79) | 0.75 (0.72 to 0.79) |
| MI | 0.89 (0.85 to 0.92) | 0.88 (0.85 to 0.91) |
| cardiac failure | 0.63 (0.62 to 0.64) | 0.63 (0.62 to 0.65) |
| Vascular disease | ||
| cerebrovascular disease | 0.69 (0.67 to 0.72) | 0.69 (0.67 to 0.72) |
| peripheral vascular disease | 0.71 (0.69 to 0.73) | 0.71 (0.69 to 0.73) |
| ischemic heart disease | 0.84 (0.82 to 0.86) | 0.83 (0.81 to 0.85) |
| Other | ||
| smoker | 0.78 (0.76 to 0.80) | 0.78 (0.76 to 0.80) |
| immobile | 0.31 (0.28 to 0.33) | 0.31 (0.28 to 0.34) |
| cancer | 0.49 (0.46 to 0.51) | 0.48 (0.46 to 0.51) |
| pulmonary disease | 0.56 (0.53 to 0.58) | 0.55 (0.53 to 0.58) |
Data are RR (95% CI). All covariates are binary except age and the age/female gender interaction term, which are listed as RR per decade. P < 0.001 for all RR estimates.
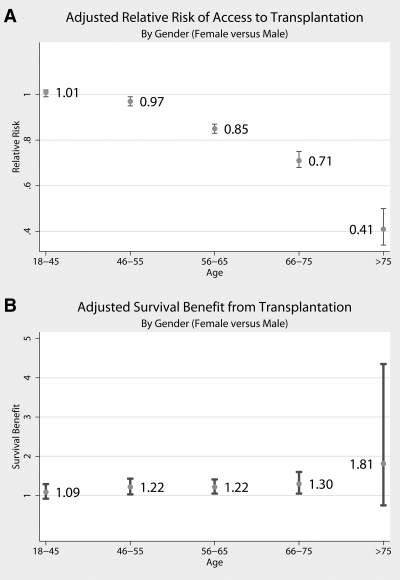
Analyses stratified by age confirmed that the disparity occurred only for older women, and, in fact, the ATT disparity for women widened exponentially with increasing age (Figure 2A). Women aged 18 to 55 had equivalent ATT; in the 56- to 65-yr age group, women had 15% less ATT (RR 0.85; 95% CI 0.83 to 0.87; P < 0.001); in the 66- to 75-yr age group, women had 29% less ATT (RR 0.71; 95% CI 0.68 to 0.75; P < 0.001); and women who were older than 75 yr had 59% less ATT than men of the same age (RR 0.41; 95% CI 0.34 to 0.50; P < 0.001). At all ages, however, women derived an SBT equivalent to or greater than that of their male counterparts (Figure 2B).
Figure 2.
(A) Adjusted access to transplantation, comparing women with men, by age category. (B) Adjusted SBT, comparing women with men, by age category.
Association of Gender with ATT by Donation Type
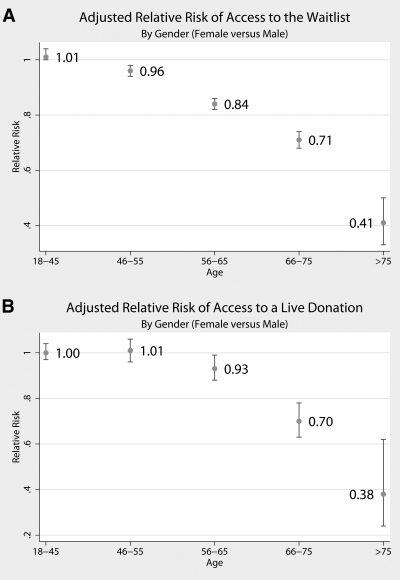
Women had less access to both the deceased-donor waiting list (Figure 3A) and live donation (Figure 3B), although the gender disparity in access to live donor transplantation seemed to emerge at an older age. Women aged 18 to 55 yr had equivalent ATT to both the deceased-donor waiting list and live donation. Women aged 56 to 65 yr had 16% less access to the waiting list (RR 0.84; 95% CI 0.82 to 0.86; P < 0.001) and 7% less access to live donation (RR 0.93; 95% CI 0.88 to 0.99; P = 0.02). Women aged 65 to 75 yr had 29% less access to the waiting list (RR 0.71; 95% CI 0.68 to 0.74; P < 0.001) and 30% less access to live donation (RR 0.70; 95% CI 0.63 to 0.78; P < 0.001). Women who were older than 75 yr had 59% less access to the waiting list (RR 0.41; 95% CI 0.33 to 0.50; P < 0.001) and 62% less access to live donation (RR 0.38; 95% CI 0.24 to 0.62; P < 0.001).
Figure 3.
(A) Adjusted access to the deceased-donor waiting list, comparing women with men, by age category. (B) Adjusted access to live-donor transplantation, comparing women with men, by age category.
Timing of Gender Disparity in Relation to Onset of ESRD
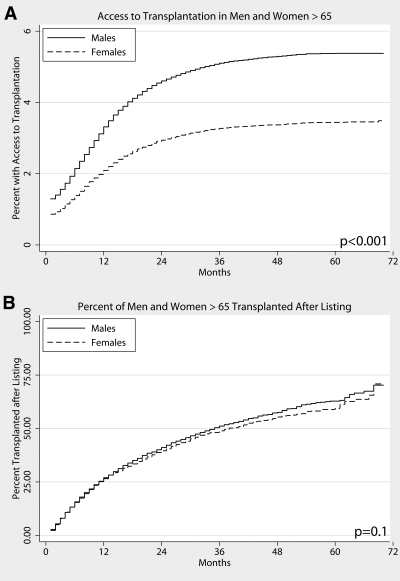
When ATT was examined as a time-dependent event, the gender disparity in older women existed almost immediately after initiation of hemodialysis and persisted throughout treatment (Figure 4A); however, of patients who eventually gained access to the deceased-donor waiting list, there was no gender disparity in the time the patient waited for a kidney transplant (Figure 4B).
Figure 4.
(A) ATT after ESRD onset, men and women older than 65 yr. (B) Transplantation rate once listed, men and women older than 65.
Association between Social Factors and Gender Disparity in Patients Who Were Older than 65
To determine how much of the observed disparity might be explained by social factors, we examined a random subset of the study population for whom social factors were measured. Although we found education to be significantly associated with ATT (RR 3.52; P < 0.001; Table 3), differences in level of education between men and women accounted for only a small part of the observed disparity. A model adjusted for only clinical and demographic factors showed that women who were older than 65 yr had 68% less ATT than men. When education was added to the model, women had 62% less ATT.
Table 3.
Role of social factors in the relationship between gender and ATT in patients who had ESRD and were older than 65 yr
| Parameter | RR for ATT for This Factor | P | RR for Women versus Men, Adjusted for This Factor and Other Clinical and Demographic Factors | P |
|---|---|---|---|---|
| No social factorsa | N/A | N/A | 0.32 (0.18 to 0.57) | <0.001 |
| Education level | 3.52 (1.97 to 6.27) | <0.001 | 0.38 (0.21 to 0.70) | 0.002 |
| Marital status | 0.94 (0.50 to 1.77) | 0.800 | 0.30 (0.16 to 0.56) | <0.001 |
| Living alone | 0.83 (0.41 to 1.68) | 0.600 | 0.33 (0.19 to 0.58) | <0.001 |
| Unable to drive | 0.63 (0.23 to 1.74) | 0.400 | 0.34 (0.19 to 0.61) | <0.001 |
| Distance to transplant center | 0.91 (0.74 to 1.11) | 0.300 | 0.33 (0.19 to 0.57) | <0.001 |
| Support level | 1.76 (0.69 to 4.47) | 0.200 | 0.27 (0.13 to 0.54) | <0.001 |
| Work (full or part time) | 2.87 (1.37 to 6.02) | 0.005 | 0.33 (0.16 to 0.72) | 0.005 |
This row represents the model adjusted for only clinical and demographic factors.
Patients who reported working full or part time were 2.87 times as likely to have ATT (P = 0.005); again, this difference accounted for only a small part of the disparity. When employment status was added to the model, women had 67% less ATT. There were no statistically significant associations between ATT and marital status, living alone, inability to drive, living >2 h from a transplant center, or level of support from family and friends in this analysis.
Association of Gender and Comorbidities with ATT
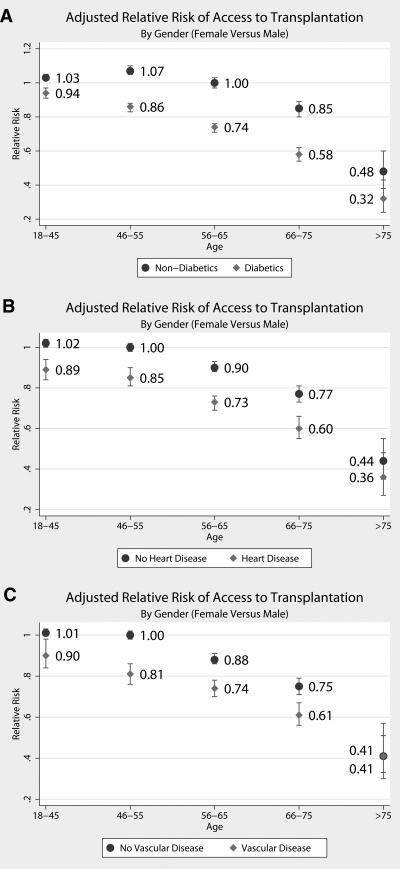
Women with comorbidities including diabetes, heart disease, or vascular disease had decreased ATT compared with men with the same comorbidities (Figure 5). Interestingly, this disparity for women with comorbidities occurred even in the younger age groups, in which in nonstratified models women had similar access as men. For example, women who were aged 18 to 45 and had diabetes had 8% less ATT than their male counterparts (RR 0.94; 95% CI 0.91 to 0.97; P < 0.001; Figure 5, top), women who were aged 18 to 45 and had heart disease had 11% less ATT than men with heart disease (RR 0.89; 95% CI 0.84 to 0.94; P < 0.001; Figure 5, middle), and women who were aged 18 to 45 and had vascular disease had 10% less ATT than men with vascular disease (RR 0.90; 95% CI 0.84 to 0.98; P < 0.001; Figure 5, bottom). Conversely, women who were aged 18 to 45 and did not have diabetes, heart disease, or vascular disease had equivalent ATT (RR 1.03, 1.02, and 1.01, respectively).
Figure 5.
Adjusted ATT, comparing women with a given comorbidity with men with that comorbidity (♦) and women without a given comorbidity with men without that comorbidity (•), by age category.
In patients who were older than 65 yr, women with or without comorbidities had decreased ATT compared with men, but the disparity was greater when a comorbidity was present. For example, in women aged 66 to 75 yr, those without diabetes had 15% less ATT than men without diabetes (95% CI 0.80 to 0.89), whereas women with diabetes had 42% less ATT than men with diabetes (95% CI 0.54 to 0.62; Figure 5, top). Similarly, women who were older than 75 yr and did not have diabetes had 52% less ATT compared with men without diabetes (95% CI 0.38 to 0.60), whereas women with diabetes had 68% less ATT compared with men with diabetes (95% CI 0.24 to 0.43; Figure 5, top).
Association of Gender and Comorbidities with SBT
In examining SBT within these strata, we found no difference in SBT between men and women, regardless of comorbidity status. Women who were aged 18 to 45 and had diabetes derived a survival benefit equivalent to men of the same age with diabetes (SBT 1.19; 95% CI 0.94 to 1.50; P = 0.20), as did women with heart disease (SBT 0.86; 95% CI 0.56 to 1.34; P = 0.50) and women with vascular disease (SBT 0.86; 95% CI 0.53 to 1.40; P = 0.50). Similarly, equivalent SBT between men and women was observed in the other age subgroups for each comorbidity (data not shown).
DISCUSSION
Almost two dozen studies have concluded that women have lower rates of ATT compared with men.5–7,9,10,12–27 In contrast to these previous studies, we showed that it is not all women but in fact only older women who experience decreased access. Furthermore, this disparity worsens with increasing age, with 29% less ATT for women aged 66 to 75 yr and 59% less ATT for women who are older than 75 yr; however, we found no difference in SBT between men and women, suggesting that a substantial number of older women who stand to benefit from transplantation lack access to it.
Several of our findings shed light on the nature of this disparity. First, we found that a disparity in access for older women occurred only at the level of joining the waiting list or receiving a transplant from a live donor and not in the time to transplantation once access to the waiting list was achieved. Such differences could be due to patient, family, or provider behaviors or attitudes. This stands in contrast to other disparities in ATT, such as those for black and for obese individuals, where patients experience both decreased likelihood of listing and decreased likelihood of receiving an organ once listed5,11; however, it seems that although older women are less likely to “get into the system,” they are equally likely to receive an organ once “in the system.” Further studies are needed to elucidate factors contributing to this phenomenon: It is not clear whether physicians are less likely to refer older female candidates, or older female candidates themselves are less likely to seek a referral.
Furthermore, our finding that the disparity is restricted to older women or women with comorbidities suggests that the gender disparity may be partly explained by differences in perceptions of women's ability to benefit from transplantation compared with men. Older and sicker women may be more likely to be perceived by their providers or to perceive themselves as frail and unable to withstand a major surgical procedure (as compared with men of the same characteristics). Frailty has recently been formally characterized as a syndrome in older adults marked by decreased physiologic reserves in multiple organ systems resulting in adverse health outcomes, independent of other disability or comorbidities.43 Despite evidence that it affects both dialysis44 and postsurgical outcomes (Makary et al., unpublished), frailty is not formally measured and used in risk prediction for patients with ESRD. Rather, we believe that both physicians and patients may use their perception of the patient's overall physiologic reserves and well-being, a so-called perceived frailty, when deciding whether to list for transplantation. It is possible that older women and sicker women are more likely to be misclassified or misclassify themselves as frail and as a result experience lower ATT than their male counterparts despite equal SBT.
Our study had several limitations. Information about the severity of comorbidities was not captured. We were able to include data only on the presence or absence of the comorbidity for each patient; therefore, even though we adjusted for all comorbidities recorded in the US Renal Data System (USRDS) in our regression models, it is possible that diabetes, heart diseases, and vascular diseases are more severe in women than in men. If this were the case; however, then we would expect women with these comorbidities to have decreased SBT when compared with men, which was not the case. Another limitation is lack of psychosocial factors such as marriage and social support network captured in registry data. Although we were able to examine these issues in a small subset of USRDS patients, detailed studies of social barriers to ATT are needed to develop effective patient- and provider-level interventions to eliminate this disparity. Unfortunately, we were unable to capture some factors that have been shown to be associated with ATT, such as cognitive decline, dementia, and medication compliance. Because women are at approximately 10% greater risk for dementia overall, in this study, they may have been more likely to have been affected by cognitive decline, dementia, and medication noncompliance, which could have contributed somewhat to their decreased ATT. We were also unable to capture factors such as dialysis facility ownership and timing of predialysis care, which are known to be associated with ATT but are unlikely to affect women differentially and as such should not confound the relationship between gender and ATT. A further limitation is that we were unable to account for panel reactive antibody (PRA) levels in our models. Because previous pregnancy can raise PRA levels, it is likely that women are more likely to have a positive cross-match with a potential live donor; however, this is unlikely to explain the disparity in listing for deceased donation, because PRA is not measured until the time of listing. Furthermore, if differences in PRA were driving the disparity, then we would expect women to wait longer for a kidney once listed, and this is not the case (Figure 4B).
In conclusion, we have identified important effect modifiers not previously accounted for in national population-based reports of a gender disparity in ATT. Our study suggests that there is no disparity in ATT for younger women but rather a marked disparity in ATT (despite similar SBT) for older women and women with comorbidities. More work is needed to understand better why this disparity is occurring to inform clinical decision making and interventions to increase ATT for women who stand to benefit from it.
CONCISE METHODS
Study Design
We performed a cohort study of 563,197 patients who were older than 18 yr and initiated hemodialysis or received a first-time preemptive KT between January 1, 2000, and September 30, 2005, as reported to the USRDS and the United Network for Organ Sharing.
Access to Transplantation
Our outcome of interest was ATT, defined as either joining the deceased-donor waiting list or receiving a transplant from a live donor. We performed separate analyses to analyze access to live donation only and access to deceased donation only. ATT was modeled in two ways: (1) As a binary outcome indicating whether an incident patient with ESRD had ATT at any time during the study period and (2) as a time-dependent event from the initial onset of ESRD. We analyzed binary outcomes using multivariate generalized linear models as described previously45; we compared coefficients with those obtained from logistic regression models to ensure that inferences were not sensitive to our modeling assumptions. We analyzed time-dependent outcomes using Kaplan-Meier survival method.
Role of Social Factors in the Gender Disparity in Patients Who Were Older Than 65
Previous studies showed ATT to be associated with education and social support; therefore, we hypothesized that differences in education level and degree of social support might contribute to the gender disparity in older patients. Because social support and education data are not captured in the USRDS main registry, we examined these associations using wave 2 of the Dialysis Morbidity And Mortality Study (DMMS-2), a substudy of patients with ESRD captured in USRDS. DMMS-2 consists of a random sample of 4024 patients who had ESRD, were older than 18 yr, and initiated dialysis for the first time between January 1, 1996, and November 1 1997. We confirmed that associations between age, gender, and ATT were similar to those found in the entire ESRD population using multivariate analyses. Because only 1426 patients who were older than 65 yr were captured in DMMS-2, we built a parsimonious model to ensure stability of estimates. We added each of the following social factors individually to the model to examine their effects on the association between gender and ATT: (1) Education level (college educated versus no college education), (2) marital status (married versus unmarried, widowed, divorced, or separated), (3) living alone (yes or no), (4) unable to drive (yes or no), (5) working full or part time (yes or no), (6) distance to a transplant center (quintile), and (7) level of support from family and friends (categorized as excellent or very good, compared with good, fair, or poor).
Survival Benefit from Transplantation
To exclude the possibility that men have more adverse events than women on dialysis and thus derive more survival benefit from transplantation, we directly compared hazard of death after transplantation with hazard of death on dialysis using a method similar to Merion et al.46 We built a time-dependent Cox proportional hazards model in which patients who received a transplant contributed time at risk to the model as dialysis patients for the time they were on dialysis and then contributed time at risk as transplant recipients after their transplant date. After adjusting for appropriate confounders, we examined the interaction term between female gender and transplantation. We performed this analysis first comparing transplant patients with all dialysis patients captured in USRDS and then repeated it comparing transplant patients with patients who were on the waiting list only.
Statistical Analysis
All clinically relevant patient characteristics captured through USRDS were included in the regression models. Lack of collinearity was confirmed by testing variance inflation factors, and the importance of each covariate in the model was confirmed by Akaike information criterion. Covariates included age; gender; ethnicity (dichotomized as black versus nonblack); insurance type (no insurance, Medicaid, Medicare, or both Medicaid and Medicare); body mass index; smoking status (current smoker versus not a current smoker), immobilized (yes or no); primary cause of renal failure (diabetes, polycystic kidney disease, urologic complications, hypertension, other, or unknown); and comorbidities including pulmonary disease, MI, ischemic heart disease, congestive heart failure, dysrhythmia, peripheral vascular disease, cerebrovascular disease, and diabetes. For analyses stratified by comorbidity, we group comorbidities as follows: Heart disease (defined as presence of MI, cardiac failure, ischemic heart disease, or dysrhythmia), vascular disease (defined as presence of ischemic heart disease, peripheral vascular disease, or cerebrovascular disease), and diabetes (defined as a diagnosis of diabetes). Forced models including all biologically relevant covariates were used for all analyses except access to live donation in patients who were older than 75 yr, for which a very small sample size of events required the creation of a parsimonious model to ensure stable estimates.
DISCLOSURES
None.
Acknowledgments
As a study of the United Network for Organ Sharing database, this work was supported in part by Health Resources and Services Administration contract 234-2005-370011C.
D.L.S. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Published online ahead of print. Publication date available at www.jasn.org.
The content of this article is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services; neither does mention of trade names, commercial products, or organizations imply endorsement by the US government.
REFERENCES
- 1.Cetingok M, Winsett RP, Hathaway DK: A comparative study of quality of life among the age groups of kidney transplant recipients. Prog Transplant 14: 33–38, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Humar A, Denny R, Matas AJ, Najarian JS: Graft and quality of life outcomes in older recipients of a kidney transplant. Exp Clin Transplant 1: 69–72, 2003 [PubMed] [Google Scholar]
- 3.Neipp M, Karavul B, Jackobs S, Meyer zu Vilsendorf A, Richter N, Becker T, Schwarz A, Klempnauer J: Quality of life in adult transplant recipients more than 15 years after kidney transplantation. Transplantation 81: 1640–1644, 2006 [DOI] [PubMed] [Google Scholar]
- 4.White MJ, Ketefian S, Starr AJ, Voepel-Lewis T: Stress, coping, and quality of life in adult kidney transplant recipients. ANNA J 17: 421–424, 431, discussion 425, 1990 [PubMed] [Google Scholar]
- 5.Alexander GC, Sehgal AR: Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA 280: 1148–1152, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Held PJ, Pauly MV, Bovbjerg RR, Newmann J, Salvatierra O Jr: Access to kidney transplantation. Has the United States eliminated income and racial differences? Arch Intern Med 148: 2594–2600, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Kjellstrand CM: Age, sex, and race inequality in renal transplantation. Arch Intern Med 148: 1305–1309, 1988 [PubMed] [Google Scholar]
- 8.Navaneethan SD, Singh S: A systematic review of barriers in access to renal transplantation among African Americans in the United States. Clin Transplant 20: 769–775, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Ojo A, Port FK: Influence of race and gender on related donor renal transplantation rates. Am J Kidney Dis 22: 835–841, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Wolfe RA, Ashby VB, Milford EL, Bloembergen WE, Agodoa LY, Held PJ, Port FK: Differences in access to cadaveric renal transplantation in the United States. Am J Kidney Dis 36: 1025–1033, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Segev DL, Simpkins CE, Thompson RE, Locke JE, Warren DS, Montgomery RA: Obesity impacts access to kidney transplantation. J Am Soc Nephrol 19: 349–355, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander GC, Sehgal AR: Variation in access to kidney transplantation across dialysis facilities: Using process of care measures for quality improvement. Am J Kidney Dis 40: 824–831, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Avula S, Sharma RK, Singh AK, Gupta A, Kumar A, Agrawal S, Bhandari M: Age and gender discrepancies in living related renal transplant donors and recipients. Transplant Proc 30: 3674, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Bayat S, Frimat L, Thilly N, Loos C, Briancon S, Kessler M: Medical and non-medical determinants of access to renal transplant waiting list in a French community-based network of care. Nephrol Dial Transplant 21: 2900–2907, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Biller-Andorno N: Gender imbalance in living organ donation. Med Health Care Philos 5: 199–204, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Bloembergen WE, Mauger EA, Wolfe RA, Port FK: Association of gender and access to cadaveric renal transplantation. Am J Kidney Dis 30: 733–738, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Bloembergen WE, Port FK, Mauger EA, Briggs JP, Leichtman AB: Gender discrepancies in living related renal transplant donors and recipients. J Am Soc Nephrol 7: 1139–1144, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Garg PP, Furth SL, Fivush BA, Powe NR: Impact of gender on access to the renal transplant waiting list for pediatric and adult patients. J Am Soc Nephrol 11: 958–964, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Gaylin DS, Held PJ, Port FK, Hunsicker LG, Wolfe RA, Kahan BD, Jones CA, Agodoa LY: The impact of comorbid and sociodemographic factors on access to renal transplantation. JAMA 269: 603–608, 1993 [PubMed] [Google Scholar]
- 20.Jindal RM, Ryan JJ, Sajjad I, Murthy MH, Baines LS: Kidney transplantation and gender disparity. Am J Nephrol 25: 474–483, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Kayler LK, Meier-Kriesche HU, Punch JD, Campbell DA Jr, Leichtman AB, Magee JC, Rudich SM, Arenas JD, Merion RM: Gender imbalance in living donor renal transplantation. Transplantation 73: 248–252, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Klassen AC, Klassen DK, Brookmeyer R, Frank RG, Marconi K: Factors influencing waiting time and successful receipt of cadaveric liver transplant in the United States. 1990 to 1992. Med Care 36: 281–294, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Koka P, Cecka JM: Sex and age effects in renal transplantation. Clin Transpl 437–446, 1990 [PubMed]
- 24.Kondo K, Shibue T, Iwaki Y, Terasaki PI: Racial effect on kidney transplants. Clin Transpl 339–349, 1987 [PubMed]
- 25.Schaubel DE, Stewart DE, Morrison HI, Zimmerman DL, Cameron JI, Jeffery JJ, Fenton SS: Sex inequality in kidney transplantation rates. Arch Intern Med 160: 2349–2354, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Soucie JM, Neylan JF, McClellan W: Race and sex differences in the identification of candidates for renal transplantation. Am J Kidney Dis 19: 414–419, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Zhou YC, Cecka JM: Effect of sex on kidney transplants. Clin Transpl 361–367, 1989 [PubMed]
- 28.Busson M, Benoit G: Is matching for sex and age beneficial to kidney graft survival? Societe Francaise de Transplantation and Association France Transplant. Clin Transplant 11: 15–18, 1997 [PubMed] [Google Scholar]
- 29.Johnson CD, Wicks MN, Milstead J, Hartwig M, Hathaway DK: Racial and gender differences in quality of life following kidney transplantation. Image J Nurs Sch 30: 125–130, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Kwon OJ, Lee HG, Kwak JY: The impact of donor and recipient age on the outcome of kidney transplantation. Transplant Proc 36: 2043–2045, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Meier-Kriesche HU, Ojo AO, Leavey SF, Hanson JA, Leichtman AB, Magee JC, Cibrik DM, Kaplan B: Gender differences in the risk for chronic renal allograft failure. Transplantation 71: 429–432, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Oh CK, Kim SJ, Kim JH, Shin GT, Kim HS: Influence of donor and recipient gender on early graft function after living donor kidney transplantation. Transplant Proc 36: 2015–2017, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Reed E, Cohen DJ, Barr ML, Ho E, Reemtsma K, Rose EA, Hardy M, Suciu-Foca N: Effect of recipient gender and race on heart and kidney allograft survival. Transplant Proc 24: 2670–2671, 1992 [PubMed] [Google Scholar]
- 34.Shibue T, Kondo K, Iwaki Y, Terasaki PI: Effect of sex on kidney transplants. Clin Transpl 351–360, 1987 [PubMed]
- 35.Vereerstraeten P, Wissing M, De Pauw L, Abramowicz D, Kinnaert P: Male recipients of kidneys from female donors are at increased risk of graft loss from both rejection and technical failure. Clinical Transplant 13: 181–186, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Kayler LK, Rasmussen CS, Dykstra DM, Ojo AO, Port FK, Wolfe RA, Merion EM: Gender imbalance and outcomes in living donor renal transplantation in the United States. Am J Transplant 3: 452–458, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Zeier M, Dohler B, Opelz G, Ritz E: The effect of donor gender on graft survival. J Am Soc Nephrol 13: 2570–2576, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Kee F, Reaney E, Savage G, O'Reilly D, Patterson C, Maxwell P, Fogarty D, Northern Ireland Targeting Social Need Renal Group: Are gatekeepers to renal services referring patients equitably? J Health Serv Res Policy 12: 36–41, 2007 [DOI] [PubMed] [Google Scholar]
- 39.O'Malley CD, Shema SJ, Clarke LS, Clarke CA, Perkins CI: Medicaid status and stage at diagnosis of cervical cancer. Am J Public Health 96: 2179–2185, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robbertz AA, Lauf RC Jr, Rupp RL, Alexander DC: A qualitative assessment of dental care access and utilization among the older adult population in the United States. Gen Dent 54: 361–365, quiz 366–368, 2006 [PubMed] [Google Scholar]
- 41.Santry HP, Lauderdale DS, Cagney KA, Rathouz PJ, Alverdy JC, Chin MH: Predictors of patient selection in bariatric surgery. Ann Surg 245: 59–67, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.United States Renal Data System: 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2007
- 43.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group: Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: M146–M156, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Johansen KL, Chertow GM, Jin C, Kutner NG: Significance of frailty among dialysis patients. J Am Soc Nephrol 18: 2960–2967, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Zou G: A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 159: 702–706, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, Ojo AO, Port FK: Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 294: 2726–2733, 2005 [DOI] [PubMed] [Google Scholar]