Abstract
Little is known about heart tissue/donor dendritic cells, which play a key role in mounting alloimmune responses. In this report, we focus on three primary features of donor dendritic cells: their generation, their trafficking after transplantation, and their role in regulating tolerance versus rejection. Using transgenic mice as donors of heart allografts enabled us to monitor trafficking of donor dendritic cells after transplantation. Donor dendritic cells rapidly migrated into secondary lymphoid tissues within 3 h of transplantation. We found that the chemokine receptor CX3CR1 regulates the generation of heart tissue dendritic cells constitutively. Compared with wild-type hearts, CX3CR1−/− hearts contained fewer dendritic cells, and heart allografts from CX3CR1−/− donors survived significantly longer without immunosuppression. Unexpectedly, though, co-stimulatory blockade with anti-CD154 or CTLA4-Ig induced long-term survival for wild-type heart allografts but not for CX3CR1−/− heart allografts. Increasing the dendritic cell frequency in CX3CR1−/− hearts by treatment with Flt3L restored the anti-CD154–induced prolongation of CX3CR1−/− heart allograft survival. Compared with wild-type donors, depleting transgenic donors of dendritic cells before heart transplantation also markedly worsened chronic rejection under anti-CD154 treatment. These data indicate the importance of the CX3CR1 pathway in the generation of heart tissue dendritic cells and the divergent role of tissue/dendritic cells in rejection versus tolerance.
It is widely known that resident dendritic cells (DC) in tissue, or donor DC (dDC), are able to traffic to the secondary lymphoid tissues of recipients, where they present alloantigens to recipient T cells.1–3 This event is the basis for the process of direct allorecognition, in which recipient T cells directly recognize intact allo-MHC molecules on tissue-resident DC. Despite ample evidence demonstrating the central role of tissue/dDC in alloimmune responses, studying dDC in nonattenuated models has not been rigorously examined, which may be related to the lack of animal models in which dDC can be easily monitored. Thus, characterization of dDC and their specific contributions to transplant rejection versus tolerance remain poorly described. Such data are necessary to better understand dDC and to formulate tolerance induction strategies, which could be regulated by dDC to a great extent. In this report, we used B6.FVB-Tg(Itgax-DTR/GFP)57Lan (DTR-GFP-DC) mice, which have a green fluorescent protein (GFP) linked to the CD11c promoter. Using these mice as donors in heart allograft transplantation enabled us to study dDC trafficking after transplantation. The other important feature of our study was to investigate the role of the CX3CR1 pathway in the constitutive formation of heart tissue DC. Recent studies have demonstrated the importance of the CX3CR1 pathway in regulating the influx of monocytes to the lymphoid tissue and their differentiation into DC.4–8 No data are yet available on the importance of chemokine pathways in regulating generation of heart tissue DC. Such data could support a rationale for the future use of novel protocols to reduce the immunogenicity of allografts by manipulating the donor chemokine system.
RESULTS
Monitoring dDC after Heart Allograft Transplantation
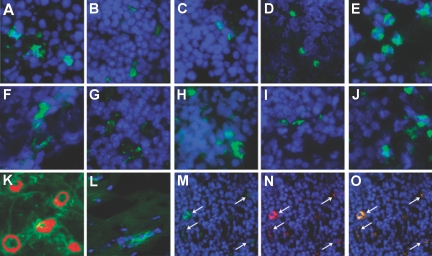
Although dDC trafficking to lymphoid tissue has been agreed upon universally to be the central step in the process of the alloimmune response, because of a difficulty in accurately monitoring dDC, the process of trafficking has not rigorously been examined. We recently published our data using the DTR-GFP-DC mouse to monitor dDC in a model of islet cell transplantation.9 These data demonstrated that using DTR-GFP-DC is a feasible model to study dDC in transplantation. To monitor dDC trafficking, we transplanted heart allografts from DTR-GFP-DC mice (on a C57BL/6 background) intra-abdominally into BALB/c mice. We examined the spleen and lymph nodes (LN; para-aortic and mesenteric) recovered from recipients at 3 h and at days 1, 3, 5, and 7 after transplantation in our immunohistologic analysis. Histologic examination of recipient spleens and LN revealed the presence of heart dDC as early as 3 h after transplantation (Figure 1). That dDC are present in the spleen at such an early time point raises the possibility that trafficking of dDC to the spleen must have occurred through direct and rapid migration of DC into the systemic circulation. As shown in Figure 1, sections of naive hearts of DTR-GFP-DC mice (used as donors in heart transplantation studies) were stained for anti-CD31 (an endothelial cell marker). Most GFP+ dDC cluster near the vessels of the heart, which presumably allows them to migrate efficiently to the blood circulation. To ensure that the GFP+ dDC were indeed live cells and were not simply phagocytosed protein in recipient DC or macrophages, we stained sections of LN recovered from heart allograft recipients with class I antibody. dDC are identified by anti-GFP; we also show class I staining and co-staining for class I and GFP, demonstrating that the dDC are indeed alive and intact (Figure 1). DAPI was used to stain cell nucleoli.
Figure 1.
dDC Trafficking. LN (A through E) and spleens (F through J) of recipients of DTR-GFP-DC hearts were recovered at 3 h and at days 1, 3, 5, and 7, respectively, and were examined for the presence of GFP+ cells by anti-GFP staining (green); DAPI (blue) was used to counterstain nuclei. (K) Naive DTR-GFP-DC hearts stained for capillaries by anti-CD31 (Cy3, red) showed that DC cluster proximal to the vasculature; the endogenous GFP+ cells (green) are shown within the vicinity of the capillaries. (L) Heart samples from GFP+ donors were also stained with anti-GFP primary antibody (green) and DAPI to reveal cell nuclei (blue), and double-stained cells were found in these hearts, thereby demonstrating their presence in donor organs before transplantation. Sections of LN from day 5 after transplantation reveal that dDC, stained with anti-GFP (M, green), express donor MHC class I (N, using anti-H2b class I, red) and that cells coexpress GFP and class I (O, orange-yellow). DAPI was used to reveal cell nuclei (blue), ruling out the possibility that dDC were phagocytosed by recipient macrophages or DC. Magnifications: ×40 in A through L; ×20 in M through O.
Generation of Heart Tissue DC Depends on the CX3CR1 Pathway
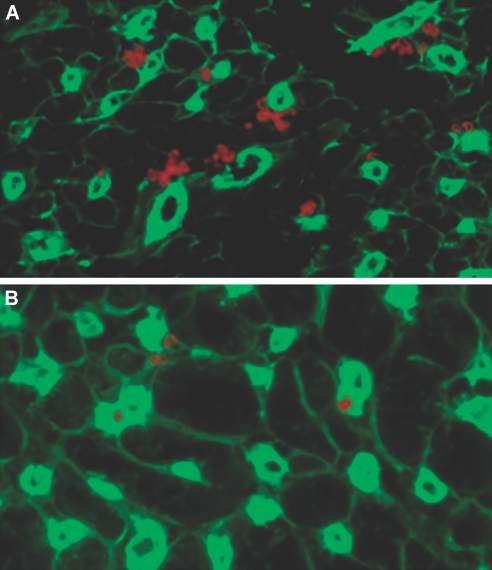
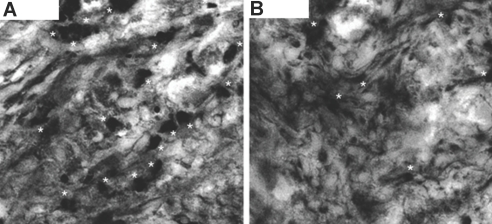
Although CX3CR1 plays a role in the migration of monocytes into peripheral tissue and promotes monocyte transformation into DC, no data are yet available on the role of CX3CR1 in the formation of heart tissue DC. We first examined the DC content of hearts recovered from naive untreated wild-type (WT) and CX3CR1−/− mice. As shown in Figure 2, CX3CR1−/− hearts (Figure 2B) show considerably less CD11c+ expression by immunostaining as compared with WT hearts (Figure 2A). Also refer to our supplemental data in which we use pertussis toxin to disrupt the function of CX3CR1 to examine its effect in the generation of heart tissue DC (Supplemental Figure S2).
Figure 2.
The CX3CR1 pathway and formation of heart tissue DC. (A and B) Compared with CX3CR1−/− hearts (B), naive WT hearts (A) contain a much greater percentage and number of DC. DC were counted per 20 sections of naive heart tissue and were 75 ± 5 and 43 ± 4 for WT and CX3CR1−/−, respectively (n = 3 mice/group; P < 0.02). Sections of naive hearts were immunostained for CD11c. CD11c+ cells (red) co-localized with endothelial cells (stained with anti-CD31 FITC, green) were identified in WT and CX3CR1−/− hearts.
Prolongation of Heart Allograft Survival Using CX3CR1−/− Donor Hearts
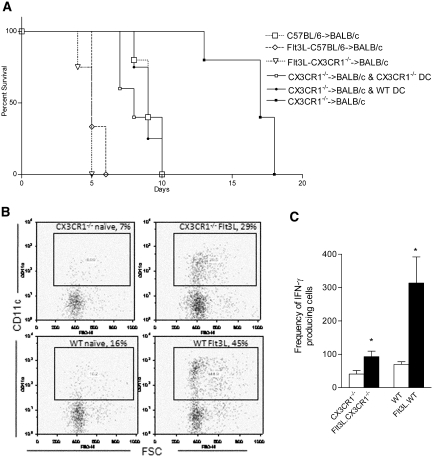
We transplanted WT or CX3CR1−/− hearts into complete MHC-mismatched BALB/c recipients. As shown in Figure 3A, compared with WT hearts, a significant prolongation of heart allograft survival was noted when using CX3CR1−/− mice as heart donors (median survival time [MST] of 8 and 17 d, respectively; n = 6 mice/group; P < 0.01). Transplanting BALB/c allogeneic hearts into CX3CR1−/− and WT recipients was not associated with any prolongation (MST of 8 and 9 d, respectively; n = 7 mice/group). Compared with the CX3CR1−/− group, WT allografts showed massive infiltrates with muscle necrosis (Supplemental Figure S1). This is the first evidence to show that manipulating the dDC/chemokine system prolongs heart allograft survival in recipients with no immunosuppression. No difference was found in T regulatory cells (Treg) or effector cells between recipients in response to WT or CX3CR1−/− heart transplantation in the periphery (see supplemental data).
Figure 3.
CX3CR1−/− donors and allograft survival. (A) Prolongation of heart allograft survival using CX3CR1−/− donors compared with WT donors (MST 17 and 8 d, respectively; n = 6 mice/group; P < 0.01). Transplanting hearts from Flt3L-treated CX3CR1−/− donors results in a similar tempo of rejection as compared with using Flt3L-treated WT donors but an accelerated rejection compared with when untreated CX3CR1−/− donors are used, highlighting the importance of reduced dDC numbers and the consequent prolongation of CX3CR1−/− heart allograft survival (n = 3 to 4 mice/group; P < 0.01). (B) Flt3L enhances heart DC numbers in both CX3CR1−/− and WT mice (representing two separate experiments; n = 4/group; P < 0.03). (C) Results of our ELISpot assay show that IFN-γ production is markedly higher in recipients of WT allografts (versus CX3CR1−/−) after in vitro stimulation with C57BL/6 antigen. In both groups, treatment with Flt3L significantly increased IFN-γ production (n = 3 to 4 mice/group; *P < 0.03).
To determine whether the observed prolongation was due to lesser numbers of dDC present in CX3CR1−/− heart grafts, we transplanted hearts from CX3CR1−/− donors in which Flt3L hybridoma cells had been implanted for 2 wk. As was shown previously, stimulation of the Flt3 receptor tyrosine kinase through its ligand increases the population of DC in lymphoid tissue.10,11 As shown in Figure 3B, although naive heart DC were much fewer in number in CX3CR1−/− hearts than in WT (corroborating the immunohistologic data), implantation of the Flt3L-producing hybridoma increased the percentage of heart DC significantly in both WT and CX3CR1−/− hearts (n = 4 mice/group; P < 0.03). Transplanting hearts from Flt3L-treated CX3CR1−/− donors resulted in an accelerated rejection as compared with when untreated CX3CR1−/− donors were used, underscoring the functional importance of reduced dDC numbers and the consequent prolongation of CX3CR1−/− heart allograft survival (n = 3 to 4 mice/group; P < 0.01; Figure 3A). We also adoptively transferred bone marrow DC (BMDC) to examine whether supplementing dDC would abrogate the prolongation of heart allograft survival when using CX3CR1−/− donors. We injected 5 × 105 CX3CR1−/− or WT BMDC intravenously at the time of transplantation. Adoptive transfer of WT or CX3CR1−/− dDC resulted in restoration of allograft rejection, again confirming the functional effect of reduced dDC that results in suboptimal priming of recipient T cells and therefore prolongs heart graft survival (n = 5 mice/group; P < 0.01; Figure 3A). Pathology of rejecting heart allografts after CX3CR1−/−, and WT BMDC injection showed cellular rejection with no marked differences (data not shown).
Measurement of T Cell Priming and Alloantibodies
We performed an ELISpot assay at day 7 after transplantation using naive C57BL/6 splenocytes to stimulate splenocytes isolated from recipients of CX3CR1−/− and WT heart donors. The number of IFN-γ–producing cells was assessed as an indicator of alloreactivity as described previously.12,13 We performed an ELISpot as already described using responder splenocytes of recipients of CX3CR1−/− and WT hearts. Our ELISpot data show that the frequency of IFN-γ–producing cells was significantly higher when WT versus CX3CR1−/− hearts were transplanted (70 ± 8 versus 41 ± 10 cells, respectively; P < 0.02; Figure 3C). We also examined whether Flt3L further enhanced this response by magnifying the effect of direct allorecognition. As shown in Figure 3C, an increase in the number of IFN-γ–producing cells was noted in both groups, although this increased response was more enhanced in the WT group, which could be due to a higher number of dDC in Flt3L-treated WT hearts, as was evident after Flt3L treatment of heart DC (Figure 3B). We also measured alloantibody levels, which have been shown to represent the overall activity of indirect allorecognition (see supplemental data).14
Requirement of Heart Tissue DC for Long-Term Survival Induced by Co-stimulatory Blockade
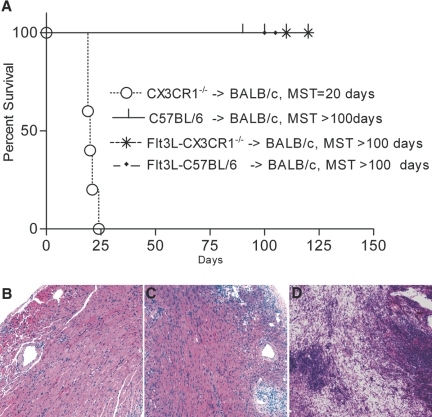
We used an established model of anti-CD154 blockade (MR1) in which multiple doses of MR1 are administered (500 μg at day 0 and 250 μg at days 2, 4, and 6) to test whether providing additional immunosuppression would further enhance the prolongation of CX3CR1−/− heart allografts.15 Interestingly, whereas treating BALB/c recipients of WT heart allografts with MR1 resulted in indefinite prolongation of heart allograft survival (MST >100 d; n = 5 mice), MR1 treatment failed to prolong heart allograft survival when using CX3CR1−/− donors (MST = 20 d; n = 5 mice; P < 0.01; Figure 4A). We then tested our hypothesis that providing WT DC would enhance the effect of MR1 when CX3CR1−/− mice were used as donors. To do so, we transplanted hearts from CX3CR1−/− donors previously implanted with the Flt3L hybridoma. All transplant combinations shown were treated with multiple doses of MR1. As shown in Figure 4A, as compared with CX3CR1−/− heart allografts, heart allografts from CX3CR1−/− donors treated with the Flt3L hybridoma showed marked increased prolongation of graft survival in the presence of MR1 (MST 20 versus >100 d, respectively; n = 5; P < 0.01). Of note, transplanting hearts from WT donors treated with the Flt3L hybridoma had no effect on the course of MR1 treatment, as recipients sustained prolongation of graft survival (MST >100 d; n = 5). Co-transplantation of WT and CX3CR1−/− heart allografts under MR1 led to a protection against rejection of the CX3CR1−/− heart, indicating the necessity of tissue/dDC for the development of MR1-induced tolerance (Figure 4, B through D). The MST of MR1-treated recipients of CX3CR1−/− and WT hearts was significantly higher than the MST of MR1-treated recipients of CX3CR1−/− hearts alone (MST >60 and 25 d, respectively; n = 3 to 4/group; P = 0.001). To test whether the importance of tissue-derived DC is unique to MR1, we transplanted heart allografts from WT and CX3CR1−/− donors into BALB/c recipients under administration of CTLA4-Ig using an established protocol. Whereas WT allografts were accepted long term (with an MST >100 d), all CX3CR1−/− allografts were rejected at approximately day 30 after transplantation (n = 5 mice/group; P < 0.01). Mice that underwent transplantation were also treated with low-dosage rapamycin to investigate whether the refractoriness observed is exclusive to co-stimulatory blockade. Rapamycin was administered intraperitoneally daily to the recipients of CX3CR1−/− and WT hearts for 3 d after transplantation at a dosage of 0.3 mg/kg. WT and CX3CR1−/− allografts were rejected with an MST of 18 and 40 d, respectively (n = 5; P < 0.01). Taken together, these data suggest that the refractoriness of CX3CR1−/− allografts is not limited to MR1 treatment but applies to other co-stimulatory molecule blockade and, furthermore, that conventional immunosuppression and low numbers of tissue DC could synergistically prolong allograft survival. We also examined the differential presence of Treg and found that the number of Foxp3+ cells was significantly higher in hearts recovered from WT (Figure 5A) compared with CX3CR1−/− donors (Figure 5B).
Figure 4.
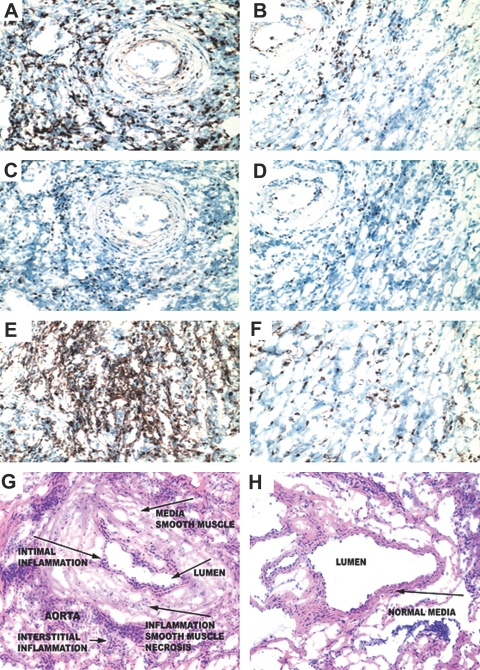
dDC and their importance in the induction of tolerance. (A) Whereas recipients of CX3CR1−/− hearts exhibited refractoriness to the effect of MR1, hearts from WT donors exhibited long-term allograft survival (MST of 20 and >100 d, respectively; n = 5 mice/group; P < 0.01). Hearts from CX3CR1−/− implanted with the Flt3L hybridoma exhibited restoration of prolongation of heart allograft survival by MR1 (MST >100; n = 5 mice; P < 0.1). Compared with WT donors treated with MR1, treatment of WT donors with Flt3L had no effect on the course of MR1 treatment (MST >100 d in both groups). To provide a suggestive mechanism for the necessity of tissue DC, we also co-transplanted hearts from WT and CX3CR1−/− mice into BALB/c recipients treated with MR1. (B and C) Both WT (B) and CX3CR1−/− (C) heart allografts showed preserved muscles with mild cellular infiltrates, although CX3CR1−/− hearts seem to have more dense infiltration (C). (D) Of note, single CX3CR1−/− heart allografts under MR1 showed massive infiltration with muscle necrosis, whereas WT allografts showed little inflammation and intact myocytes (data not shown).
Figure 5.
dDC and Treg in allografts. (A and B) Compared with WT donor hearts (A) (recovered from allograft recipients under MR1 at day 14 after transplantation), CX3CR1−/− donor hearts (B) showed a much lower number of Foxp3-stained cells. Foxp3+ cells were counted per 20 sections of heart tissue, which were 98 ± 5 and 12 ± 5, for WT and CX3CR1−/− allografts, respectively (n = 4 mice/group; P < 0.01).
Lack of Tissue/dDC and Its Association with Chronic Rejection
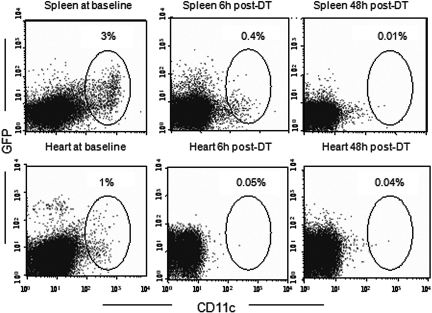
To support further the importance of tissue/dDC in the promotion of hyporesponsiveness and specifically in reducing chronic rejection, we depleted heart DC with diphtheria toxin (DT) in GFP+ donor mice and transplanted the hearts into WT recipients under MR1 (500 μg at day 0 and 250 μg at days 2, 4, and 6).15 As shown in Figure 6, compared with untreated mice, DT efficiently depleted GFP+ cells in both heart and spleen. WT mice that received DT and MR1 were used as controls. GFP+ and WT heart allografts were recovered at day 100 after transplantation for histologic examination. As shown in Figure 7, compared with untreated WT donors (Figure 7, B, D, F, and H), heart allografts from donors treated with DT showed a substantial number of infiltrates and severe chronic rejection manifested by interstitial infiltration, smooth muscle necrosis/inflammation, and intimal thickening (Figure 7, A, C, E, and G).
Figure 6.
dDC depletion with DT in DTR-GFP-DC mice. An advantage of using the DTR-GFP-DC mouse was that DC in this model could efficiently be depleted by DT. Because of the low affinity of DT for the rodent heparin-binding EGF-like growth factor, murine cells, unlike primate cells, are resistant to killing by DT. Transfer of a primate DTR into mice via transgenesis confers DT sensitivity to murine cells. Naive DTR-GFP-DC mice were administered an injection of 250 ng of DT intraperitoneally. Cells extracted from hearts and spleens of treated mice were subjected to our FACS analysis, and cells were gated on CD11c and GFP. Compared with untreated mice, DT efficiently depleted GFP+ cells in both heart and spleen.
Figure 7.
Lack of dDC and worsening of chronic rejection. WT mice that received DT and MR1 were used as controls. Mice from both groups were sacrificed at day 100 after transplantation, and heart allografts were examined for cellular infiltrates; CD4+, CD8+, and Foxp3+ cells; and the severity of chronic rejection. Samples shown were recovered from DTR-GFP-DC donors treated with DT and MR1 (right) as well as from WT donors treated with DT and MR1 (left). (A, B, E, and F) The percentage of CD4+ and CD8+ infiltrating lymphocytes in the DTR-GFP-DC donors treated with DT (A and E, respectively) was significantly higher than those of WT donors (B and F). (A through D) Examination of the localization of Foxp3 expression revealed that whereas most infiltrating CD4+ cells overlap with Foxp3+ cells (B and D) in the WT donors, infiltrating CD4+ cells in DTR-GFP-DC donors treated with DT are predominantly non-Foxp3+ cells (A and C). (G and H) Interestingly, compared with WT donors (H), DTR-GFP-DC donors treated with DT showed substantially more severe chronic rejection, as is shown by increased cell numbers and a greater degree of smooth muscle necrosis/inflammation, and intimal thickening (G). Quantitative analysis of graft arteriolosclerosis demonstrated that luminal stenosis of coronary arteries in the DT-treated group (G) was significantly higher as compared with that of the WT group (H). The percentage of luminal stenosis for DT-treated and WT grafts was 85.0 ± 4.0 and 18.0 ± 3.5, respectively (n = 3 to 4 mice/group, P < 0.002).
DISCUSSION
Despite widespread agreement as to the central importance of dDC in alloimmune responses, dDC remain largely unexplored and uncharacterized.16,17 Our data examining the secondary lymphoid tissues of recipients of GFP+ donors indicate that dDC migrate to the secondary lymphoid tissue of the recipients as early as 3 h after transplantation. Our data showing that dDC are within the vicinity of capillaries also support the notion that dDC traffic through the systemic circulation. The route by which dDC may traffic is certainly of interest and is the subject of our future studies. Ochando et al.18 examined the trafficking of dDC into lymphoid tissue of the recipients after heart allograft transplantation as well and demonstrated early trafficking at day 1 after transplantation. It should be noted that using their GFP marker, only 8.9% of CD11c+ cells from their donor hearts were identified as GFP+ DC and were subsequently monitored for trafficking after transplantation.
Chemokines tightly control migration of monocytes and hence their transformation to DC. The CX3CR1 pathway was previously demonstrated to be important in the generation of lymphoid tissue DC5–8,19 and also was shown to regulate the formation of parenchymal tissues such as the small intestine.7 Lack of CX3CR1 signaling additionally interferes with the accumulation of DC in the arterial intima, thereby reducing atherosclerotic lesions.20 We are first to show the importance of the CX3CR1 pathway in the generation of heart DC. Unexpectedly, administration of MR1 or CTLA4-Ig did not improve the engraftment of CX3CR1−/− donor hearts. Whereas WT hearts survived >100 d with either MR1 or CTLA4-Ig treatment, CX3CR1−/− donor hearts were found to be resistant to the tolerogenic effects of these agents. Given the observed further prolongation of CX3CR1−/− heart allografts by low-dosage rapamycin, abrogation of direct allorecognition and use of conventional immunosuppressive agents may synergistically promote engraftment. A significantly higher incidence of Treg in WT heart allografts under coverage of MR1 as compared with CX3CR1−/− grafts could suggest that Treg generation under MR1 may require the presence of functionally competent allogeneic donor/tissue DC and is in accordance with previous reports using Treg-dependent tolerance models that highlighted the importance of intragraft Treg.21–23 It can be postulated that WT heart DC (versus CX3CR1−/− heart DC) produce different types of chemokines, thereby recruiting more Treg to the heart allograft under MR1. Interestingly, Louvet et al.24 demonstrated that CX3CR1-CX3CL1 (fractalkine) is highly expressed by the non–T cell compartment of graft-infiltrating cells in the tolerant heart induced by donor-specific transfusion. The Louvet group posited that CX3CR1+ cells represent DC precursors that play an immunomodulatory role in the induction of tolerance.
It is important to note that a variety of sources of exogenous donor antigens (i.e., non-heart DC)—from donor-specific transfusion to exogenous DC from bone marrow and spleen—have been used to enhance further the tolerogenic effect of co-stimulatory blockade.25,26 Although these studies demonstrate the potential application for the use of exogenous dDC to promote engraftment, these strategies do not consistently induce long-term allograft acceptance and/or are difficult to apply clinically.11,27 This could be due to in vitro manipulation or, more importantly, could be because DC from different sources likely possess inherently very different immunologic properties.11,28 We have therefore primarily focused on heart tissue DC.
Traditionally, a widespread approach has been used when anti-dDC strategies are used in transplantation to promote engraftment29–33; however, studies using donor-specific transfusion have demonstrated the importance of graft antigen-presenting cells in the establishment of tolerance.34,35 These studies are limited by the fact that depleting resident antigen-presenting cells using immunosuppression or manipulations, such as “passive enhancement,” may affect the antigenicity of the allograft. Our data indicate that specifically tissue/dDC (not antigen-presenting cells as a whole) in the graft are actually instrumental to the establishment of tolerance by co-stimulatory blockade. Direct and indirect allorecognition are reportedly the two primary pathways that instigate the immune response. The current understanding is that direct allorecognition is a short-lived process initiating early acute rejection, which is thought to be due to the short life span of dDC in the recipient, whereas the indirect pathway is responsible for late acute/chronic rejection.29,36–42 Nonetheless, it is important to note that our novel data do indicate that in contrast to the general opinion, dDC are not as short-lived in the host as has been thought; they could thus have a major impact on the later development of chronic rejection.14,43,44 By specifically examining dDC, we are introducing the notion that a fraction of dDC may survive longer than expected, because they may possess or acquire protective mechanisms to escape recipient immunosurveillance. Therefore, a pretransplantation short-term chemokine-based strategy constitutes a novel approach to reduce graft immunogenicity by instituting chemokine antagonists in the donors with the goal of altering DC numbers or the DC population (toward more tolerogenic DC) of organs. Furthermore, our data revealing the divergent role of tissue/dDC in rejection versus tolerance support the need for better characterization of dDC survival and a better understanding of the protective mechanisms by which dDC escape immunosurveillance in the host.
CONCISE METHODS
Mice
CX3CR1−/− mice (courtesy of Dr. Ji-Liang Gao's Laboratory) were on a fully C57BL/6 (H-2b) background. B6.FVB-Tg(Itgax-DTR/EGFP)57Lan/J (DTR-GFP-DC) as well as C57BL/6 and BALB/c (H-2d) (WT) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All experiments were in accordance with animal care and use guidelines.
Heterotopic Heart Transplantation
Vascularized heart allografts were transplanted intra-abdominally using microsurgical techniques as described by Corry et al.45 When performing co-transplantation, the second heart was anastomosed to the femoral artery. Rejection was determined as complete cessation of cardiac contractility and was confirmed by direct visualization. Graft survival is shown as MST in days.
Histologic Analysis
Cardiac allografts were removed from recipients at several time points after operation to evaluate acute rejection. Samples were fixed in 10% buffered formalin. Ventricular short-axis sections were cut and stained with hematoxylin and eosin. The International Society for Heart and Lung Transplantation nomenclature system was used to evaluate and score acute rejection of cardiac allografts as well as the vasculopathy of chronic rejection.46,47
DT Treatment
Given that DTR-GFP-DC mice express the simian DT receptor (DTR) proximal to the CD11c promoter, administering DT to these mice was shown to deplete DC.48 DTR-GFP-DC and WT donors were administered an intraperitoneal injection of 250 ng of DT (Sigma Aldrich, St. Louis, MO) 24 h before transplantation. Hearts from these donors were then transplanted into recipients.
Immunohistology
Heart allograft recipients were sacrificed at various time points after transplantation. Heart allografts, spleen, and para-aortic and mesenteric LN were embedded in OCT and frozen, and tissues were cut into 5-μm sections that were incubated in 100 μg/ml avidin D (Vector Laboratories, Burlingame, CA) to block endogenous biotin. For CD11c staining, sections were incubated with monoclonal hamster anti-CD11c (1:50), biotinylated mouse anti-hamster IgG (1:100; Vector Laboratories), and PE-streptavidin (1:50; Biomeda, Foster City, CA).49 Endothelial cells were visualized with FITC anti-CD31 (1:50). For Treg staining, frozen tissue sections were incubated with anti-Foxp3 antibody, anti-rat biotinylated antibody for 40 min at room temperature, and avidin-conjugated peroxidase (Vectastain ABC Vector Elite Kit) and then were developed with 3,3′-diaminobenzidine.50 For GFP staining, sections were incubated for 1 h at room temperature with polyclonal rabbit anti-GFP primary antibody (1:200; Vector Laboratories). Sections were washed and incubated with TRITC-labeled goat anti-rabbit secondary antibody (Dako, Carpinteria, CA) for 30 min at room temperature (1:300), and slides were counterstained with DAPI to reveal cell nuclei. For CD4 and CD8 staining, purified anti-CD4 and anti-CD8 (1:50; BD Biosciences, San Jose, CA) were followed sequentially by biotinylated anti-rat, horseradish peroxidase–streptavidin, and 3,3′-diaminobenzidine development.
In Vitro Generation of BMDC
To generate DC from BM, we flushed the femurs and tibiae of mice, and cultured BM cells in RPMI containing 10% FCS, 1% L-glutamine, 1% penicillin-streptomycin with 20 ng/ml recombinant murine granulocyte monocyte-colony stimulating factor (R&D Systems, Minneapolis, MN) at days 0 and 3, whereas at both days 6 and 8, we added 10 ng/ml recombinant murine granulocyte monocyte-colony stimulating factor.51 Nonadherent cells were harvested for flow cytometric analysis and functional assays on day 9.
Mixed Lymphocyte Reaction
We measured DC generated in vitro from WT or CX3CR1−/− mice for their allostimulatory capacity in an mixed lymphocyte reaction assay. We used cultured DC after irradiation with 3000 rads to stimulate CD4+ BALB/c cells isolated from splenocytes by magnetic bead separation (Miltenyi Biotec, Auburn, CA) at a ratio of 1:1 DC-CD4+ cells. Splenocytes from WT and CX3CR1−/− heart allograft recipients at days 5 and 14 were also used as responders, and irradiated C57BL/6 splenocytes were used as stimulators at a ratio of 1:1. In both assays, we measured proliferation at day 3 after pulsing with [3H]TdR (Perkin Elmer, Wellesley, MA) using a liquid scintillation counter. We also stimulated splenocytes with con A as a positive control.
ELISpot Assay
We performed an ELISpot assay using recipient splenocytes as responders and naive C57BL/6 splenocytes as stimulators. Briefly, Millipore immunospot plates (Millipore Corp., Bedford, MA) were coated overnight with a capture antibody conjugated to murine IFN-γ (BD Biosciences, San Jose, CA). A total of 5 × 105 cells each of responders and irradiated stimulators were added to wells. After 24 h of incubation at 37°C and 5% CO2, plates were washed, and a biotinylated antibody specific for IFN-γ was added and incubated at 4°C for 24 h. Spots were developed after incubation with horseradish peroxidase and addition of aminoethyl carbazole.
Measurement of Alloantibody
We performed serum alloantibody measurements after isolation of serum from the peripheral blood of recipients of CX3CR1−/− and WT heart allografts treated or untreated with Flt3L. We incubated serial dilutions of each serum sample for 30 min with naive C57BL/6 splenocytes and washed them and added FITC anti-mouse IgG1 or IgG2a for an additional 30 min. We ran cells on a FACSCalibur (Becton Dickinson, San Jose, CA) and used naive C57BL/6 serum as a negative control.
FACS Analysis
We assessed the presence of leukocytes and and/or DC by staining with FITC rat anti-mouse CD45 and APC anti-mouse CD11c. We ran cells on a FACSCalibur and analyzed data using FlowJo 6.3.2 software (Treestar, Ashland, OR). To determine the presence of Treg, we stained single-cell suspensions obtained from spleens or LN for FITC anti-mouse CD4, PE anti-mouse CD25, and APC anti-mouse Foxp3 (eBioscience, San Diego, CA). We assessed CD4 and CD8 effectors using FITC anti-mouse CD4 or CD8, PE anti-mouse CD44, and APC anti-mouse CD62L. We identified plasmacytoid DC as CD11c+CD11b−Gr-1+B220+ (using APC anti-mouse CD11c, PE anti-mouse CD11b, FITC anti-mouse Gr-1/Ly-6G, and PerCP anti-mouse CD45R/B220). We purchased antibodies from BD Biosciences unless otherwise indicated.
Injection of Pertussis Toxin and Enumeration of Heart DC
We injected 500 ng of pertussis toxin (Sigma Aldrich) into CX3CR1−/− and WT mice with. We used naive untreated hearts of both CX3CR1−/− and B6 mice as controls. After 24 h, we perfused hearts with saline, cut them into 1-mm2 pieces, incubated them at 37°C in RPMI with 1 mg/ml collagenase D (Roche Diagnostics, Indianapolis, IN) for 60 min, and homogenized them through a 70-μm cell strainer. We then centrifuged cells on a Percoll gradient, after which we aspirated leukocytes and subjected them to FACS analysis.
Implantation of Flt3L Hybridoma
The Flt3L-producing hybridoma cell line (derived from the C57BL/6 murine melanoma) was provided by Dr. Ulrich von Andrian.10 Briefly, hybridoma cells were cultured in RPMI containing 10% FCS, 1% penicillin-streptomycin, and 1% l-glutamine for 2 to 3 d until confluence was reached. Monolayers were trypsinized and counted, and 10 × 106 cells in 100 μl of PBS were injected subcutaneously into the back of mice. When the tumor reached 2 cm in diameter at approximately 10 to 14 d after injection, mice were used as donors of heart transplants.
Statistical Analysis
We constructed Kaplan-Meier survival graphs and used log-rank comparisons of the groups to calculate P values for survival comparisons between groups. We used t test for comparison of means between experimental groups examined in our in vitro assays. We reported differences to be significant when at P < 0.05.
DISCLOSURES
None.
Acknowledgments
This work was supported by a National Kidney Foundation clinical scientist award, a Roche Organ Transplantation Research Foundation grant, the Juvenile Diabetes Research Foundation regular grant, and a John Merrill Transplant Scholar grant (to R.A.).
Published online ahead of print. Publication date available at www.jasn.org.
T.U. and K.T. contributed equally to this work and should be considered co-first authors.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Larsen CP, Morris PJ, Austyn JM: Migration of dendritic leukocytes from cardiac allografts into host spleens: A novel pathway for initiation of rejection. J Exp Med 171: 307–314, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker CF, Billingham RE: The role of afferent lymphatics in the rejection of skin homografts. J Exp Med 128: 197–221, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen NC, Morris B: The role of the lymphatic system in the rejection of homografts: A study of lymph from renal transplants. J Exp Med 131: 936–969, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imhof BA, Aurrand-Lions M: Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol 4: 432–444, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Geissmann F, Jung S, Littman DR: Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19: 71–82, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Vallon-Eberhard A, Landsman L, Yogev N, Verrier B, Jung S: Transepithelial pathogen uptake into the small intestinal lamina propria. J Immunol 176: 2465–2469, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC: CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307: 254–258, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Maus UA, Wellmann S, Hampl C, Kuziel WA, Srivastava M, Mack M, Everhart MB, Blackwell TS, Christman JW, Schlöndorff D, Bohle RM, Seeger W, Lohmeyer J: CCR2-positive monocytes recruited to inflamed lungs downregulate local CCL2 chemokine levels. Am J Physiol Lung Cell Mol Physiol 288: L350–L358, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Fiorina P, Jurewicz M, Tanaka K, Behazin N, Augello A, Vergani A, von Andrian UH, Smith NR, Sayegh MH, Abdi R: Characterization of donor dendritic cells and enhancement of dendritic cell efflux with CC-chemokine ligand 21: A novel strategy to prolong islet allograft survival. Diabetes 56: 912–920, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Mempel TR, Henrickson SE, Von Andrian UH: T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427: 154–159, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Emmanouilidis N, Guo Z, Dong Y, Newton-West M, Adams AB, Lee ED, Wang J, Pearson TC, Larsen CP, Newell KA: Immunosuppressive and trafficking properties of donor splenic and bone marrow dendritic cells. Transplantation 81: 455–462, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Kishimoto K, Sandner S, Imitola J, Sho M, Li Y, Langmuir PB, Rothstein DM, Strom TB, Turka LA, Sayegh MH: Th1 cytokines, programmed cell death, and alloreactive T cell clone size in transplant tolerance. J Clin Invest 109: 1471–1479, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishimoto K, Dong VM, Issazadeh S, Fedoseyeva EV, Waaga AM, Yamada A, Sho M, Benichou G, Auchincloss H Jr, Grusby MJ, Khoury SJ, Sayegh MH: The role of CD154-CD40 versus CD28–B7 costimulatory pathways in regulating allogeneic Th1 and Th2 responses in vivo. J Clin Invest 106: 63–72, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Womer KL, Sayegh MH, Auchincloss H Jr: Involvement of the direct and indirect pathways of allorecognition in tolerance induction. Philos Trans R Soc Lond B Biol Sci 356: 639–647, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sho M, Sandner SE, Najafian N, Salama AD, Dong V, Yamada A, Kishimoto K, Harada H, Schmitt I, Sayegh MH: New insights into the interactions between T-cell costimulatory blockade and conventional immunosuppressive drugs. Ann Surg 236: 667–675, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakkis FG, Arakelov A, Konieczny BT, Inoue Y: Immunologic ′ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med 6: 686–688, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Ochando JC, Yopp AC, Yang Y, Garin A, Li Y, Boros P, Llodra J, Ding Y, Lira SA, Krieger NR, Bromberg JS: Lymph node occupancy is required for the peripheral development of alloantigen-specific Foxp3+ regulatory T cells. J Immunol 174: 6993–7005, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Ochando JC, Krieger NR, Bromberg JS: Direct versus indirect allorecognition: Visualization of dendritic cell distribution and interactions during rejection and tolerization. Am J Transplant 6: 2488–2496, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, Gabuzda D: Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med 197: 1701–1707, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu P, Yu YR, Spencer JA, Johnson AE, Vallanat CT, Fong AM, Patterson C, Patel DD: CX3CR1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arterioscler Thromb Vasc Biol 28: 243–250, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW: Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med 201: 1037–1044, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izawa A, Yamaura K, Albin MJ, Jurewicz M, Tanaka K, Clarkson MR, Ueno T, Habicht A, Freeman GJ, Yagita H, Abdi R, Pearson T, Greiner DL, Sayegh MH, Najafian N: A novel alloantigen-specific CD8+PD1+ regulatory T cell induced by ICOS-B7h blockade in vivo. J Immunol 179: 786–796, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Tanaka K, Albin MJ, Yuan X, Yamaura K, Habicht A, Murayama T, Grimm M, Waaga AM, Ueno T, Padera RF, Yagita H, Azuma M, Shin T, Blazar BR, Rothstein DM, Sayegh MH, Najafian N: PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol 179: 5204–5210, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louvet C, Heslan JM, Merieau E, Soulillou JP, Cuturi MC, Chiffoleau E: Induction of Fractalkine and CX3CR1 mediated by host CD8+ T cells in allograft tolerance induced by donor specific blood transfusion. Transplantation 78: 1259–1266, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Lu L, Li W, Fu F, Chambers FG, Qian S, Fung JJ, Thomson AW: Blockade of the CD40-CD40 ligand pathway potentiates the capacity of donor-derived dendritic cell progenitors to induce long-term cardiac allograft survival. Transplantation 64: 1808–1815, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Niimi M, Shirasugi N, Ikeda Y, Kan S, Takami H, Hamano K: Operational tolerance induced by pretreatment with donor dendritic cells under blockade of CD40 pathway. Transplantation 72: 1556–1562, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Markees TG, Pearson T, Cuthbert A, Pearson AL, Shultz LD, Leif J, Phillips NE, Mordes JP, Greiner DL, Rossini AA: Evaluation of donor-specific transfusion sources: Unique failure of bone marrow cells to induce prolonged skin allograft survival with anti-CD154 monoclonal antibody. Transplantation 78: 1601–1608, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Hackstein H, Wang Z, Morelli AE, Kaneko K, Takayama T, Colvin BL, Bein G, Thomson AW: Normal donor bone marrow is superior to Flt3 ligand-mobilized bone marrow in prolonging heart allograft survival when combined with anti-CD40L (CD154). Am J Transplant 2: 609–617, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Game DS, Lechler RI: Pathways of allorecognition: Implications for transplantation tolerance. Transpl Immunol 10: 101–108, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Lechler RI, Batchelor JR: Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med 155: 31–41, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talmage DW, Dart G, Radovich J, Lafferty KJ: Activation of transplant immunity: Effect of donor leukocytes on thyroid allograft rejection. Science 191: 385–388, 1976 [DOI] [PubMed] [Google Scholar]
- 32.Lacy PE, Davie JM, Finke EH: Prolongation of islet allograft survival following in vitro culture (24 degrees C) and a single injection of ALS. Science 204: 312–313, 1979 [DOI] [PubMed] [Google Scholar]
- 33.Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, Nelson PJ: CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int 70: 591–596, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Josien R, Heslan M, Brouard S, Soulillou JP, Cuturi MC: Critical requirement for graft passenger leukocytes in allograft tolerance induced by donor blood transfusion. Blood 92: 4539–4544, 1998 [PubMed] [Google Scholar]
- 35.Gagne K, Brouard S, Guillet M, Cuturi MC, Soulillou JP: TGF-beta1 and donor dendritic cells are common key components in donor-specific blood transfusion and anti-class II heart graft enhancement, whereas tolerance induction also required inflammatory cytokines down-regulation. Eur J Immunol 31: 3111–3120, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Vella JP, Vos L, Carpenter CB, Sayegh MH: Role of indirect allorecognition in experimental late acute rejection. Transplantation 64: 1823–1828, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Lechler R, Ng WF, Steinman RM: Dendritic cells in transplantation: Friend or foe? Immunity 14: 357–368, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Caballero A, Fernandez N, Lavado R, Bravo MJ, Miranda JM, Alonso A: Tolerogenic response: Allorecognition pathways. Transpl Immunol 17: 3–6, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Hornick P: Direct and indirect allorecognition. Methods Mol Biol 333: 145–156, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Jiang S, Herrera O, Lechler RI: New spectrum of allorecognition pathways: Implications for graft rejection and transplantation tolerance. Curr Opin Immunol 16: 550–557, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Rogers NJ, Lechler RI: Allorecognition. Am J Transplant 1: 97–102, 2001 [PubMed] [Google Scholar]
- 42.Baker RJ, Hernandez-Fuentes MP, Brookes PA, Chaudhry AN, Cook HT, Lechler RI: Loss of direct and maintenance of indirect alloresponses in renal allograft recipients: Implications for the pathogenesis of chronic allograft nephropathy. J Immunol 167: 7199–7206, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Hornick PI, Mason PD, Yacoub MH, Rose ML, Batchelor R, Lechler RI: Assessment of the contribution that direct allorecognition makes to the progression of chronic cardiac transplant rejection in humans. Circulation 97: 1257–1263, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Ruedl C, Koebel P, Bachmann M, Hess M, Karjalainen K: Anatomical origin of dendritic cells determines their life span in peripheral lymph nodes. J Immunol 165: 4910–4916, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Corry RJ, Winn HJ, Russell PS: Primarily vascularized allografts of hearts in mice: The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation 16: 343–350, 1973 [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez ER: The pathology of heart transplant biopsy specimens: Revisiting the 1990 ISHLT working formulation. J Heart Lung Transplant 22: 3–15, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Billingham ME, Cary NR, Hammond ME, Kemnitz J, Marboe C, McCallister HA, Snovar DC, Winters GL, Zerbe A: A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant 9: 587–593, 1990 [PubMed] [Google Scholar]
- 48.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA: In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity 17: 211–220, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Probst HC, Tschannen K, Odermatt B, Schwendener R, Zinkernagel RM, Van Den Broek M: Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin Exp Immunol 141: 398–404, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, Noelle RJ, Coyle A, Mellor AL, Khoury SJ, Sayegh MH: A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med 202: 231–237, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, Schuler G: An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 223: 77–92, 1999 [DOI] [PubMed] [Google Scholar]