Abstract
Understanding the pathogenesis of albuminuria in diabetic nephropathy is important to improve methods for early diagnosis and treatment. In this study, we addressed whether albuminuria in diabetes results from altered glomerular filtration and/or altered processing of filtered albumin by the proximal tubule. Type 1 diabetic Munich Wistar rats developed albuminuria after 12 wk of diabetes. Intravital two-photon microscopy revealed similar glomerular permeability in the diabetic and control animals, assessed using both albumin-Alexa568 and 69-kD FITC-dextran; however, diabetic animals demonstrated significantly less filtered fluorescent albumin in renal proximal tubule (PT) cells compared with control animals. We also observed increased albumin-derived urinary peptide excretion in diabetic animals, and hyperglycemia modulated this peptideuria. In conclusion, in the early stages of diabetic nephropathy, the PT plays a major role in the development of albuminuria, which may be preceded by peptideuria.
There has been much focus on the kidney filter as the primary factor for the manifestation of albuminuria in kidney disease, including diabetic nephropathy.1,2 Many studies have emphasized the importance of various structural elements of the glomerular capillary wall (GCW), including podocytes, in governing albuminuria in diabetes3,4; however, previous studies were unable to make direct measurements of GCW albumin permeability. There is now a debate as to whether the albuminuria that develops in diabetes and other renal diseases is of glomerular or PT origin.5 Here, we use intravital two-photon microscopy of infused albumin-Alexa568 and 69-kD FITC-dextran to examine directly and for the first time the in vivo processes of albumin filtration and proximal reabsorption in an animal model of diabetes.
We induced type 1 diabetes in rats using streptozotocin (STZ), a drug that targets and destroys β cells of the pancreas, producing a widely used and recognized model of insulin-dependent diabetes. Analysis of physiologic parameters after 12 wk of diabetes demonstrated significantly decreased body weight and increases in blood glucose, kidney weight, and osmotic diuresis accompanied by increased water intake, versus age-matched control rats (Supplemental Table 1). Analysis of urinary albumin excretion demonstrated significantly increased intact albumin excretion in the diabetic versus control groups (P < 0.05; Figure 1), consistent with the induction of diabetic nephropathy.
Figure 1.
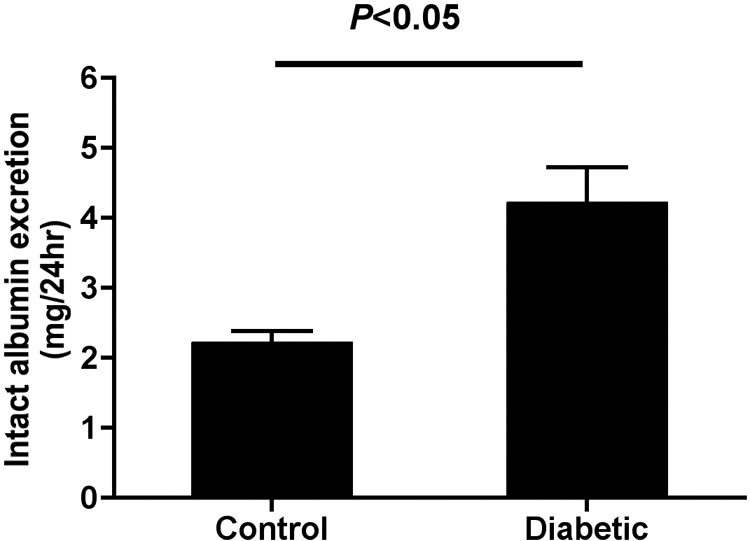
Intact urinary albumin excretion is increased in 12-wk diabetic rats. Urinary intact albumin excretion was measured by ELISA for rat serum albumin in 24-h urine samples of 12-wk diabetic rats and age-matched controls. Diabetic rats had significantly increased urinary intact albumin excretion (P < 0.05 control versus diabetic; control n = 4, diabetic n = 4).
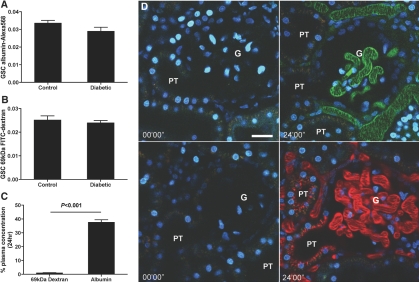
Intravital two-photon microscopy was used to determine the origin of the albuminuria. Anesthetized rats were administered an intravenous injection of rat serum albumin conjugated to Alexa568 (albumin-Alexa568; red).6 After the injection, fluorescent albumin was immediately filtered across glomerular capillaries, entered the urinary space, and avidly bound to the base of the PT cell brush border in both control and diabetic animals (Supplemental Movies 1 and 2, respectively). Superficial glomeruli and associated PTs were imaged for the first 3 min after albumin-Alexa568 injection, and short movies were also recorded at 12 and 24 min after injection for use in determining the albumin glomerular-sieving coefficient (GSC). We calculated GSC as the ratio of albumin-Alexa568 (or FITC-dextran in some experiments) in the glomerular capillary blood space versus that in the Bowman's space at the interface with the S1 segment, after correction for background fluorescence (see the Concise Methods section). Results shown in Figure 2A reveal that the GSC of albumin in control rats was 0.0340 ± 0.0032 (n = 4), which is in agreement with the GSC that we previously reported for albumin using this method.6 Importantly, the albumin GSC in the diabetic rats was not significantly different from that in the control rats, with a value of 0.0290 ± 0.0047 (n = 4), despite the presence of albuminuria in the diabetic animals. To corroborate these data, we took a low polydisperse 69-kD FITC-dextran (which has a radius of 5.3 to 6.5 nm)7 and determined its sieving coefficient also over 24 min after injection using the same technique. In control rats (Figure 2B), FITC-dextran had a GSC of 0.0250 ± 0.0041 (n = 4); the diabetic rats had a similar GSC of 0.0240 ± 0.0021 (n = 5). This result is in agreement with the GSC measurement obtained for similar dextrans in an independent study in nondiabetic rats using a glomerular uptake technique, which reported a GSC of 0.022.8 This agreement between two different methods further substantiates the use of two-photon technology to quantify albumin GSC. All GSCs were time-independent throughout the 24-min period of measurement. These results reveal, therefore, that despite a doubling of intact albumin levels in the urine (Figure 1), there is no detectable change in glomerular permeability for albumin or for a 69-kD uncharged FITC-dextran at 12 wk after diabetes induction in this animal model, suggesting that the observed albuminuria has a nonglomerular origin.
Figure 2.
The GSC of albumin and 69-kD FITC-dextran is unchanged in 12-wk diabetic versus control rats. (A) GSC of albumin-Alexa568 in 12-wk diabetic and aged-matched control rats demonstrates no significant change in the GSC for albumin (control n = 4, diabetic n = 4; 13 data points for control animals and 13 data points for diabetic animals over 24 min). (B) GSC of 69-kD FITC-dextran in 12-wk diabetic and aged-matched control rats demonstrates no significant change in the GSC for dextran, confirming the results obtained for albumin GSC (control n = 4, diabetic n = 5; 16 data points for control animals and 12 data points for diabetic animals taken over 24 min). (C) The plasma clearance of albumin-Alexa and 69-kD FITC-dextran over 24 h demonstrates that dextran is quickly cleared from the plasma over 24 h but a large portion of albumin is retained in the plasma despite the two molecules’ having similar GSC (albumin n = 4, dextran n = 4). (D) Representative pictures showing before and 24 min after injection of green 69-kD FITC-dextran (top) and red albumin-Alexa568 (bottom) in 12-wk control rats. Albumin (red) can be seen distributed in PT cells, whereas dextran (green) uptake is negligible. Bar = 30 μm. G, glomerulus; PT, proximal tubule.
Analysis of the proportion of albumin and dextran remaining in the plasma during 24 h in control rats (Figure 2C) revealed that only 0.92 ± 0.64% (n = 4) of the injected 69-kD FITC-dextran remained in the circulation after 24 h, whereas a much larger percentage of injected albumin-Alexa568 (37.6 ± 3.8%; n = 4) remained. Note that the serum concentration was also affected by redistribution into the extracellular volume space and other vascular compartments such as the lymph, in addition to elimination by the kidney. Given that these molecules have essentially the same GSCs, this strongly supports the theory that filtered albumin is rescued and returned to the circulation by the PT epithelium, as we proposed previously.6,9 This is further supported by the distribution of the albumin-Alexa and FITC-dextran in the S1 segment of the PT 24 min after injection, where albumin is seen distributed across PT cells of the S1 segment immediately leading from the Bowman's capsule, whereas negligible dextran uptake is observed (Figure 2D). Previous evidence for the retrieval pathway is supported by (1) the use of toxins to inhibit tubular uptake, resulting in high levels of albumin in the urine10; (2) the recovery of filtered albumin in the peritubular capillary9; and (3) the distribution of immunogold-labeled endogenous albumin in PT cells by electron microscopy6 and the distribution of exogenous fluorescence albumin in PT cells over time.6 It should be noted, however, that the exact nature of the cellular processes involved in the retrieval of glomerular filtered albumin still remains to be fully elucidated.
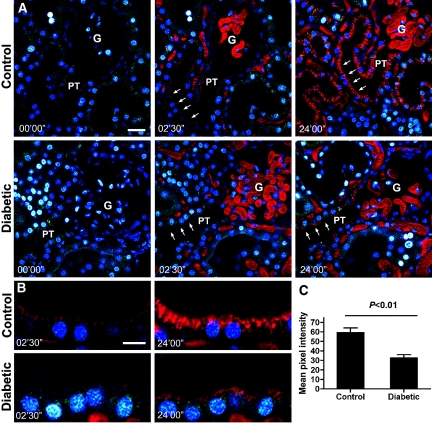
Analysis of albumin endocytosis and the distribution of albumin in the PT was carried out in 12-wk diabetic versus control rats by intravital two-photon microscopy. Over time, a strikingly different distribution of endocytosed albumin in PT cells could be observed in the diabetic versus control rat kidneys at 2.5 and 24 min after albumin injection (Figure 3A). Albumin was localized mainly toward the apical pole in the diabetic animals compared with age-matched control animals (Figure 3B). The analysis of mean fluorescence intensity for fluorescently labeled albumin in S1 PT segments immediately adjoining the glomerulus revealed an approximate 45% reduction in the amount of albumin-associated fluorescence in the cells of diabetic versus control rat kidneys at 24 min after albumin-Alexa568 injection (Figure 3C; P < 0.01; n = 4 for each group). This suggests a significant change in PT handling of albumin in diabetes.
Figure 3.
Albumin distribution in PT cells is reduced in diabetes. (A) Representative pictures of the distribution of albumin in the kidney of 12-wk diabetic rats and age-matched control rats. Images shown are before albumin-Alexa568 (red) injection and 2.5 min and 24 min after albumin-Alexa injection. Albumin uptake (red) can be seen in the S1 segment of the PT (arrows). This uptake is dramatically reduced in the diabetic group. Bar = 30 μm. (B) Enlarged picture of the region highlighted by arrows in A. The enlarged picture demonstrates the dramatic change in the distribution of albumin in PT cells. Bar = 15 μm. (C) Quantification of the amount of albumin distributed along the S1 segment throughout the cell (P < 0.01 control versus diabetic; control n = 4, diabetic n = 4). G, glomerulus; PT, proximal tubule.
The amount of albumin handled by the renal PT given a GSC of 0.03 is in the vicinity of approximately 1 g/d per kidney in rats. This suggests that changes in the renal handling of albumin such as that demonstrated in Figure 3 should induce significant albuminuria; however, analysis of intact albumin in the urine revealed a relatively small two-fold increase to approximately 4 mg/d intact albumin in the diabetic group (Figure 1). Thus, we endeavored to determine whether there were any changes in total albumin excretion in the urine. Albumin in urine was previously shown to be present in two forms.11 The majority (approximately 150 mg/d in rats) is present as albumin-derived fragments and is “immuno-unreactive ” in immunobased assays that are used for the quantification of albuminuria. The second form of urinary albumin is intact albumin (approximately 1 mg/d in rats), which is “immuno-reactive.” The immuno-unreactive albumin-derived fragments from humans were characterized previously as having molecular weights in the range of 300 to 500 Da using radiolabeled albumin and the Biuret protein assay.12 Furthermore, peptideuria in diabetes has previously been noted and has been confirmed to be albumin derived using radiolabeled albumin and the Biuret assay.13
In this study, the analysis of 24-h urine samples from our 12-wk diabetic and control rats revealed a 2.5-fold increase in total protein excretion from 118.2 ± 16.1 to 302 ± 12.7 mg/d (P < 0.01; Figure 4A). This total protein analysis is not specific for albumin and may include other proteins of both filtered and cellular origin; however, the increase in total protein excretion in diabetes is thought to be mainly albumin derived, because analysis of the fractional clearance (Fc) of [14C]albumin also revealed an approximately 2.2-fold increase in total albumin clearance (P < 0.001; Figure 4B). Consistent with previous studies, the GFR was not significantly different between the groups (control 3.70 ± 0.24 versus diabetic 3.63 ± 0.37). To determine whether this apparent peptideuria induced in diabetes was related to potential STZ toxicity on PT cells, we conducted a short-term diabetes study in which STZ diabetes was induced in the presence and absence of strict insulin treatment. The results revealed that peptideuria could be induced within 4 d of prolonged hyperglycemia and could be ameliorated by strict glycemic control (Figure 4C). Furthermore, we demonstrated that significant changes in peptideuria preceded changes in intact albuminuria, suggesting that peptideuria may be an earlier and more sensitive marker of renal dysfunction in diabetes and may also play a role in diabetic nephropathy progression (Figure 4C).
Figure 4.
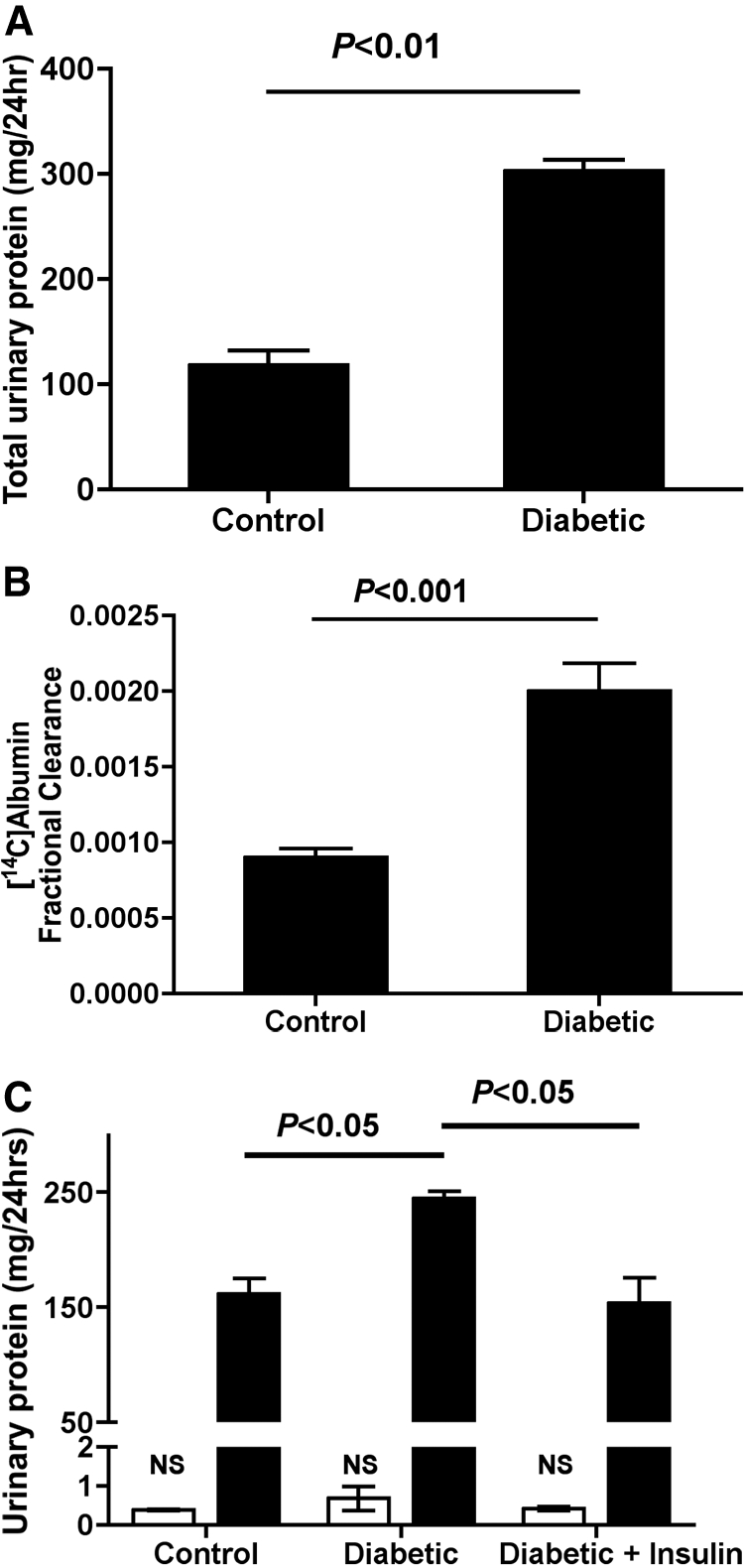
Total urinary protein excretion. (A) Total protein excretion in 12-wk diabetic and age-matched control rats demonstrated significantly increased total urinary protein excretion in the diabetic versus control groups (P < 0.01 control versus diabetic; control n = 4, diabetic n = 4). (B) Fractional clearance of [14C]albumin in 12-wk diabetic rats demonstrates an approximately 2.2-fold increase in total albumin excretion (P < 0.001 control versus diabetic; control n = 7, diabetic n = 9). (C) Total protein excretion (▪) at 4 d after STZ-induced diabetes induction resulted in significantly increased total protein excretion that could be prevented through strict glycemic control (P < 0.05 control versus diabetic; P < 0.05 diabetic versus diabetic + insulin). Analysis of intact albumin excretion by ELISA (□) reveals no significant increase in intact albumin excretion 4 d after STZ-induced diabetes induction despite significant and relatively considerable increases in total protein excretion (control n = 3, diabetic n = 3, diabetic + insulin n = 3). NS, not significant.
The results presented here demonstrate two major findings: (1) That the onset of albuminuria is not associated with changes in GCW permeability and (2) that intact albuminuria is preceded by albumin-derived peptideuria whose excretion is in the nephrotic range and is again not associated with changes in glomerular permeability but rather is due to changes in the PT handling of albumin in the hyperglycemic state. With the current trend toward insulin misuse for the purpose of weight loss among patients with diabetes termed, “diabulimia,”14,15 the importance of diagnostics for the detection of early changes in renal function is highlighted. Peptideuria may indeed be an important marker for the early diagnosis and monitoring of diabetic nephropathy onset and progression, particularly in patients who have just received a diagnosis of diabetes or those with poorly controlled glycemia levels. Furthermore, the potential renal toxicity of these high levels of peptideuria and their role in the progression of diabetic nephropathy are an important new area of investigation.
In conclusion, these findings support an important paradigm shift in our understanding of the renal processing of albumin in diabetes and highlights the PT as an important element for albuminuria manifestation. The finding that considerable changes in the excretion of urinary albumin occurs during the very early stages of diabetic nephropathy, independent of glomerular permeability changes, offers important new insights into the development of new early diagnostics and treatments that may target the renal PT and glucose control.
CONCISE METHODS
Animals
All animal experiments were approved by the Institutional Committee on Research Animal Care, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals Guidelines and protocols established by the Institutional Animal Care and Use Committee were strictly followed. Male Munich-Wistar rats 200 to 250 g (Simonsen Laboratories, Gilroy, CA) or male Sprague-Dawley rats 200 to 250 g were randomly assigned to one of two groups: Control or diabetes. Before the induction of diabetes, animals were weighed, blood glucose was analyzed, and a blood sample and 24-h urine sample were collected (via metabolic cage). For STZ (Sigma-Aldrich, St. Louis, MO) diabetes induction, animals were fasted overnight and diabetes was induced with a single intravenous injection of STZ 50 mg/kg body wt dissolved in citrate buffer (pH 4.5) as described previously.16 Diabetic rats were treated with 2 IU of Lantus insulin glargine (Sanofi Aventis, Bridgewater, NJ) every second day to prevent ketoacidosis and were given free access to standard rat food and water. Measurements of water consumption, body weight, urine volume, and kidney weight were recorded at the end of the 12-wk time course. For the short-term diabetes study, animals were divided into three groups: Control, diabetic, and diabetic + insulin. Diabetes was induced as outlined already, and the diabetic alone group received no insulin treatment. Conversely, the diabetic + insulin group received up to 8 IU of Lantus insulin glargine twice daily to prevent hyperglycemia.
Physiologic Parameters
Intact albumin excretion was assayed using Nephrat competitive ELISA assay for rat serum albumin (Exocell, Philadelphia, PA). Total urinary protein was measured by total protein assay (micro-Lowry; Sigma-Aldrich). GFR was measured by creatinine assay as described previously.17 Blood glucose was measured by glucometer. BP was directly monitored via a femoral arterial line connected to a Kent Scientific Corp. (Torrington, CT) pressure transducer and monitored using DASYLab software 6.0. In all subjects a broad pulsatile tracing was observed showing both systolic and diastolic pressures indicative of correct placement of the line within the arterial vasculature.
Albumin Fractional Clearance
Albumin fractional clearance was calculated as [urinary albumin/plasma albumin] × [urine flow rate/GFR]. Rat serum albumin (Sigma Chemical Co.) was carbon-14–labeled with 125 μCi [14C]formaldehyde (NEN Life Science Products, Boston, MA) using a modified reductive methylation technique.18 ALZET osmotic pumps, Model 2001 (Durect Corp., Cupertino, CA) were used to deliver [14C]albumin. For osmotic pump implantation, rats were anesthetized by Forthane (Abbott Australasia Pty Ltd., Kurnell, Australia) inhalation, and an osmotic pump was implanted subcutaneously between the scapulae using sterile technique 1 wk before the end of the 12-wk study. At day 7 after osmotic pump implantation, rats were placed in metabolic cages for the collection of a 24-h urine sample. A corresponding blood sample was taken for the analysis of [14C]albumin fractional clearance.
Two-Photon Microscopy
All imaging was conducted using a Bio-Rad MRC-1024MP two-photon laser scanning microscope (Hercules, CA) mounted on a Nikon Diaphot inverted stage platform (Fryer, Huntley, IL) using a Ti:Sapphire laser (Spectra-Physics, Franklin, MA) as described previously.19 The microscope stage was warmed using a ReptiTherm heating pad (Zoo Med Laboratories, San Luis Obispo, CA) and animals were maintained at 37°C. Fluorescence probes were dissolved in isotonic saline and injected into the femoral vein of rats in a steady bolus of 0.1 to 0.4 ml over 5 s. In this study, nonsaturating levels of fluorescence were used. The magnification of the objective used was a ×60 water immersion lens with a 1.2 numerical aperture or a ×20 multi-immersion lens with a 0.75 numerical aperture. Images taken for quantification were obtained with the ×60 water immersion lens. Images were taken at a depth between 5 and 20 μm into the tissue. When free Alexa dye was injected into rats, it was rapidly filtered and concentrated in distal tubules. Importantly, it was not taken up by PTs (Supplemental Movie 3). This indicates that the presence of free dye and its association with other plasma proteins are not responsible for our observations. Furthermore, Alexa fluorescence in the plasma was associated only with a single band at the molecular weight of albumin, indicating that it does not associate with other proteins (Supplemental Figure 1).
Image Processing
Raw data collected were analyzed, quantified, and displayed using Metamorph 6.1 (Universal Imaging/Molecular Devices, Downingtown, PA). Areas of interest were outlined, and average fluorescence intensities were generated and reported after background subtraction. Glomeruli were chosen by screening a low-magnification image of the kidney and then selecting a glomerulus in which we could visualize its associated S1 segment. We then focused on that particular glomerulus and S1 segment during the introduction of the bolus (into the femoral vein) of fluorescently labeled material and during the course of the experiment. In all cases, we observed rapid and continuous perfusion of the glomerulus during the experiment. We previously demonstrated that values obtained for filtered material at saturating and nonsaturating plasma levels are in agreement. In this study, nonsaturating levels of fluorophores were used, and all values recorded for fluorescence detection were above background. Average capillary fluorescence, excluding the erythrocytes that were seen in all capillaries, was not significantly different between the groups (control 162.6 ± 19.4 versus diabetic 161.7 ± 15.4).
Analysis of Albumin Uptake
Still frames from two-photon movies at 24 min taken with a ×60 water immersion lens with a 1.2 numerical aperture were captured and exported into IPLab spectrum. Four control kidneys and four diabetic kidneys were compared. An area of interest was drawn around the S1 PT and analyzed for mean pixel intensity. The mean area analyzed in both groups was not significantly different (control 26,718 ± 13,859 and diabetic 30,595 ± 21,309 pixels).
Proteins and Probes
Rat serum albumin (Fraction V; Sigma-Aldrich) was conjugated to Alexa568 (Molecular Probes, Carlsbad, CA) according to the manufacturer's instructions for amine-reactive probes as previously reported.6 All samples were dialyzed overnight to remove cacodylate and free dye before injection into animals. FITC-dextran had a number average Mn of 45,500 Da, weight average molecular weight of 69,700, and average radius of 5.3 to 6.5 nm (gift from Pharmacia, Uppsala, Sweden).7
Statistical Analysis
Statistics were measured by a two-tailed t test. Data are means ± SEM unless noted otherwise.
DISCLOSURES
None.
Acknowledgments
This work was supported by grants from the Juvenile Diabetes Research Foundation International (L.M.R. and W.D.C.). National Institutes of Health grant DK 061594 was awarded to B.A.M. to establish a George M. O'Brien Center for advanced renal microscopy analysis at the Indiana Centre for Biologic Microscopy. The Microscopy Core Facility of the MGH Program in Membrane Biology receives support from National Institutes of Health grant DK38452 (D.B.), the Boston Area Diabetes and Endocrinology Research Center (DK57521), and the Center for the Study of Inflammatory Bowel Disease (DK43341). We thank Dr. Tanya Osicka and Steve Sastra for performing the fractional clearance studies.
This work was presented in part at the 68th scientific session of the American Diabetes Association; June 6 through 10, 2008; San Francisco, CA; and the 41st annual meeting of the American Society of Nephrology; November 4 through 9, 2008; Philadelphia, PA.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
See related editorial, “Albuminuria, Wherefore Art Thou?” on pages 455–457.
REFERENCES
- 1.Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J: Modification of kidney barrier function by the urokinase receptor. Nat Med 14: 55–63, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Coward RJ, Welsh GI, Koziell A, Hussain S, Lennon R, Ni L, Tavaré JM, Mathieson PW, Saleem MA: Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes 56: 1127–1135, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Wolf G, Chen S, Ziyadeh FN: From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 54: 1626–1634, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Comper WD, Haraldsson B, Deen WM: Resolved: Normal glomeruli filter nephrotic levels of albumin. J Am Soc Nephrol 19: 427–432, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD: The normal kidney filters nephrotic levels of albumin that is retrieved by the proximal tubule cell: Retrieval is disrupted in nephrotic states. Kidney Int 71: 504–513, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Hemmelder MH, de Jong PE, de Zeeuw D: A comparison of analytical procedures for measurement of fractional dextran clearances. J Lab Clin Med 132: 390–403, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Asgeirsson D, Venturoli D, Fries E, Rippe B, Rippe C: Glomerular sieving of three neutral polysaccharides, polyethylene oxide and bikunin in rat: Effects of molecular size and conformation. Acta Physiol 191: 237–246, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Eppel GA, Osicka TM, Pratt LM, Jablonski P, Howden B, Glasgow EF, Comper WD: The return of glomerular filtered albumin to the rat renal vein. Kidney Int 55: 1861–1870, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Osicka TM, Pratt LM, Comper WD: Glomerular capillary wall permeability to albumin and horseradish peroxidase. Nephrology 2: 199–212, 1996 [Google Scholar]
- 11.Osicka TM, Comper WD: Protein degradation during renal passage in normal kidneys is inhibited in experimental albuminuria. Clin Sci 93: 65–72, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Strong KJ, Osicka TM, Comper WD: Urinary-peptide excretion by patients with and volunteers without diabetes. J Lab Clin Med 145: 239–246, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Greive KA, Osicka TM, Russo LM, Comper WD: Nephrotic-like proteinuria in experimental diabetes. Am J Nephrol 23: 38–46, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Yan L: ′Diabulimia’ a growing problem among diabetic girls. Nephrol News Issues 21: 36–38, 2007 [PubMed] [Google Scholar]
- 15.Goebel-Fabbri AE, Fikkan J, Franko DL, Pearson K, Anderson BJ, Weinger K: Insulin restriction and associated morbidity and mortality in women with type 1 diabetes. Diabetes Care 31: 415–419, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Russo LM, Osicka TM, Brammar GC, Candido R, Jerums G, Comper WD: Renal processing of albumin in diabetes and hypertension in rats. Am J Nephrol 23: 61–70, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Di Giorgio J: Creatinine and creatine. In: Clinical Chemistry: Principles and Techniques, edited by Henry RJ, Cannon DC, Winkelman JW, Hagerstown, MD, Harper and Row, 1974, pp 541–548
- 18.Eng LA: Production and characterization of [14C]protein A, a long lived immunological reagent. J Immunol Methods 81: 239–243, 1985 [DOI] [PubMed] [Google Scholar]
- 19.Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA: Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol 283: C905–C916, 2002 [DOI] [PubMed] [Google Scholar]