Abstract
Absence of connexin 40 (Cx40) leads to ectopic juxtaglomerular renin expression and abrogates recruitment of renin-expressing cells in the adult kidney but does not disturb renin expression during kidney development. To find an explanation for these observations, we aimed to analyze the expression pattern of major vascular Cxs in normal juxtaglomerular epithelioid cells, in recruited renin-expressing cells, and in fetal renin-expressing cells. We found that during kidney development, the appearance of renin-producing cells paralleled the expression of Cx40 and, to a lesser extent, Cx45 but not other Cxs. In the adult kidney, juxtaglomerular epithelioid cells expressed Cx40 and lesser amounts of Cx37 and Cx43 but not Cx45, which localized to arteriolar smooth muscle cells. Recruitment of renin-producing cells in adult kidneys in response to long-term salt deprivation of mice correlated with the reappearance of only Cx40. Cx40-null renin-producing cells did not express Cx37, Cx43, or Cx45. These findings suggest that Cx40 expression is a characteristic of renin-producing cells in the kidney, and it seems to be essential in the recruitment of renin-producing cells in the adult but not the fetal kidney.
It has long been known that the juxtaglomerular epithelioid (JGE) renin-producing cells of the kidney form gap junctions among each other and with their neighbors.1–3 The functional meaning of this gap junctional coupling remained obscure until recently. Gap junctions are formed by connexins (Cxs),4 and information available suggests that JGE cells express Cx40 and Cx37.5–8 Indirect evidence for an important function of Cxs for JGE cells was first derived from the observation that replacement of Cx43 by the smaller Cx32 blunts the activation of renin secretion in response to a drop of renal perfusion pressure.9 This finding, however, is difficult to understand if JGE cells do not express Cx43. More clear evidence for the role of gap junctions in JGE cells was recently derived from mice lacking Cx40, which is considered as the main Cx of JGE cells. Deletion of Cx40, in fact, abrogates all apparent gap junctions in JGE cells.10 Cx40-deficient mice have severe defects in the feedback control of renin secretion, rendering them massively hyperreninemic and hypertensive.7,11 Moreover, lack of Cx40 leads to abnormalities in the location of renin-expressing cells in the adult kidney. During kidney development, when renin expression switches on and off in the walls of developing preglomerular arteries and arterioles,12–14 there is no obvious difference in the spatiotemporal renin expression pattern between mice with and without Cx40. At the end of this developmental process, usually characteristic JGE cells appear, which form the media layer of the terminal part of the afferent arterioles by replacing typical vascular smooth muscle cells (VSMCs). Instead of actin-myosin filaments, the cells are characterized by numerous renin storage granules, which leads to an epithelium-like appearance, coining the term JGE cells.15 In the absence of Cx40, however, these JGE cells do not develop. Instead, ectopic renin-producing cells appear, which infiltrate the periglomerular interstitium.10 In addition, the recruitment of renin producing cells along the walls of preglomerular vessels,16,17 which can be observed in normal kidneys under chronic stimulations of the renin system, does not occur in the absence of Cx40.10 In normal kidneys, this characteristic retrograde recruitment is probably due to reversible metaplastic transformation of VSMCs into renin-producing cells.18 The reasons for this particular distribution pattern of renin-expressing cells in Cx40-deficient mice, namely normal development, ectopic localization in the juxtaglomerular area and lack of retrograde recruitment, are unknown.
One may speculate about the existence of two states of renin-producing cells: A mature form that depends on Cx40 and a more immature form that is independent of Cx40. Because the impact of Cxs goes in parallel with the differentiation state of cells, we hypothesized that the different forms of renin-expressing cells in the kidney might differ in their Cx expression pattern; therefore, we aimed to analyze the Cx expression pattern in normal JGE cells, in recruited renin-expressing cells, and in fetal renin-expressing cells.
We found that expression of Cx40 was a common characteristic of renin-expressing cells at any stage. Fetal but not adult renin-producing cells also expressed Cx45. Finally, typical JGE cells showed co-regulation with Cx37 and Cx43.
RESULTS
Normal Adult Kidneys
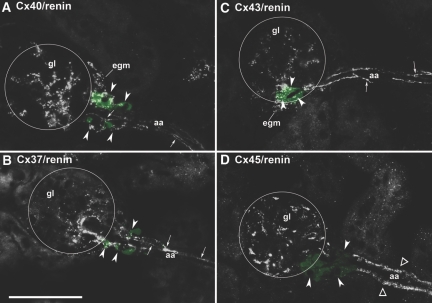
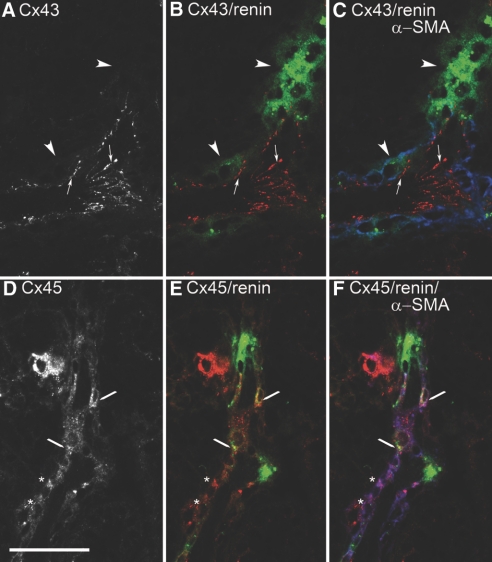
In normal adult mouse kidneys, we found renin immunoreactivity at the typical juxtaglomerular position. Renin-producing cells with the characteristic cubic appearance of JGE cells continued the layer of smooth muscle cells in the walls of afferent arterioles at the entrance into the glomerular capillary network (Figure 1). Cx40 immunoreactivity was found in association with JGE cells, with endothelial cells of preglomerular vessels, and with extra- and intraglomerular structures, whereas preglomerular VSMCs displayed no Cx40 immunoreactivity (Figure 1A). Cx40 immunoreactivity in renin-expressing cells as well as in intraglomerular cells appeared in a punctuated pattern, whereas that in endothelial cells produced a more continuous pattern (Figure 1A). Cx37 immunoreactivity was found in association with endothelial cells of afferent arterioles, with cells at the glomerular vascular poles, and less intensive also with intraglomerular cells. Co-immunostaining for renin revealed a co-regulation of Cx37 with renin (Figure 1B). Similar to Cx40, Cx37 immunoreactivity in renin-expressing cells as well as in intraglomerular cells produced a dotted pattern, whereas that in endothelial cells appeared more as continuous lines. Cx43 immunoreactivity was constantly found with endothelial cells of afferent and efferent arterioles as well as endothelial cells of concomitant veins, partially with JGE cells and constantly with intraglomerular cells (Figure 1C). VSMCs of afferent arterioles were devoid of Cx43 immunoreactivity. Like Cx40 and Cx37 immunoreactivity, Cx43 immunoreactivity in renin-expressing cells as well as in intraglomerular cells produced a punctuated pattern, whereas that in endothelial cells was more continuous. Cx45 immunoreactivity was found in intraglomerular cells and in VSMCs of preglomerular arteries/arterioles. Notably, it was absent from renin-expressing cells (Figure 1D).
Figure 1.
(A through D) Histologic sections of kidney cortex from a mouse kept on normal-salt diet stained for Cx40 (A), Cx37 (B), Cx43 (C), Cx45 (D) together with renin (green color). Bar = 50 μm. Circles highlight glomerulus (gl); egm, extraglomerular mesangium; aa, afferent arteriole; arrowheads highlight renin-expressing cells; arrows indicate endothelial Cx staining; ▵, Cx45 staining in smooth muscle cells. Cx37, Cx40, and Cx43 are found in association with renin-expressing cells and with endothelial cells of the aa. Whereas Cx40 and Cx43 immunoreactivity extends more evenly over the whole glomeruli, Cx37 immunoreactivity is more focused to the entrance of the aa into the capillary network. Cx45 immunoreactivity is not associated with renin-expressing cells but clearly is seen in VSMCs of aa and in intraglomerular cells.
Adult Kidneys with Modulated Renin System
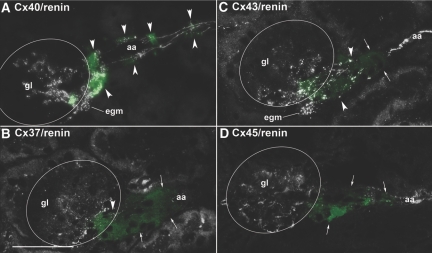
In kidneys of adult mice on treatment with an angiotensin-converting enzyme inhibitor (ACEI) plus low-salt diet, renin immunoreactivity not only was markedly increased in the juxtaglomerular area but also appeared in the walls of afferent arterioles more distal from the glomerular vascular poles, indicating recruitment of renin-expressing cells (Figure 2). Also in kidneys with stimulated renin system, Cx40 was associated with renin-expressing cells, not only in JGE cells but also in upstream recruited cells of the afferent arterioles (Figure 2A, Supplemental Figure 1). In contrast, no Cx37 immunoreactivity was found in recruited renin-expressing cells during treatment with low-salt and ACEI. It seemed even to decrease not only in JGE cells but also in the afferent endothelium (Figure 2B). Cx43 immunoreactivity seemed to disappear from renin-expressing cells with increasing distance from the glomerular vascular pole but was present in the afferent, efferent, and venous endothelium and in intraglomerular cells (Figure 2C). Cx45 immunoreactivity was absent from all renin-expressing cells of mice treated with low-salt diet in combination with an ACEI but remained visible in VSMCs and intraglomerular cells (Figure 2D).
Figure 2.
(A through D) Histologic sections of kidney cortex from a mouse kept on low-salt diet in combination with the ACEI enalapril (10 mg/kg per d), stained for Cx40 (A), Cx37 (B), Cx43 (C), and Cx45 (D) together with renin (green color). Bar = 50 μm. Circles highlight gl; arrowheads highlight renin-expressing cells; arrows indicate renin immunoreactivity without immunoreactivity of the respective Cx. Treatment of the mice led to an upstream recruitment of renin-expressing cells along the aa. All renin positive cells also stained for Cx40, whereas Cx37 immunoreactivity was absent from renin-expressing cells but still present in the endothelium of aa. Cx43 immunoreactivity was associated with renin-expressing cells close to the glomerular vascular poles but faded in renin-expressing cells with increasing distance to the vascular pole. Cx45 immunoreactivity did not co-localize with renin.
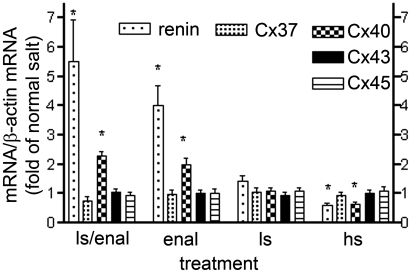
Co-recruitment of renin and Cx40 in these kidneys was paralleled by increases of the respective mRNAs (Figure 3), whereas that of Cx43 and Cx45 did not change and that of Cx37 even tended to decrease. Such parallel changes of renin and Cx40 mRNA but not of other Cx mRNAs turned out as a more general phenomenon occurring during a variety of maneuvers stimulatory or inhibitory for the renin system (Figure 3).
Figure 3.
Renin, Cx37, Cx40, Cx43, and Cx45 mRNA abundance in kidney cortex of mice treated with a combination of low-salt diet (0.02% wt/wt) with the ACEI enalapril (10 mg/kg per d; ls/enal), with enalapril (10 mg/kg per d) alone (enal), with low-salt diet (0.02% wt/wt; ls), with normal-salt diet (0.6% wt/wt), or with high-salt diet (4% wt/wt; hs). Data are means ± SEM of five mice in each group. *P < 0.05 versus vehicle-treated mice on normal-salt diet.
Fetal Kidneys
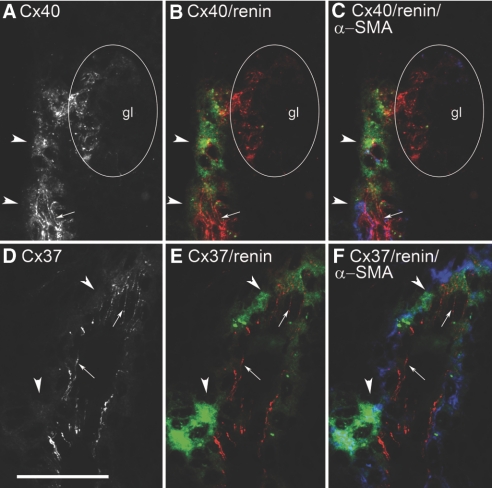
During development of the kidneys, renin expression starts in the walls of larger arterial vessels, shifts to smaller vessels, and remains restricted to the terminal portions of afferent arterioles in adult kidneys. To determine the Cx expression pattern of fetal renin-producing cells located in the walls of lager vessels, we analyzed kidney sections obtained 17 d after onset of gestation. As shown in Figure 4, distinct smooth muscle–like cells containing α-smooth muscle actin (α-SMA) in the walls of larger arteries expressed renin. Already in these cells, we found a prominent punctated pattern of Cx40 immunoreactivity (Figure 4, A through C), in addition to a strong staining of the endothelium and also of maturing glomeruli. Cx37 immunoreactivity was also found in the endothelium but not in renin-expressing cells or in other α-SMA–containing cells (Figure 4, D through F). Cx43 immunoreactivity was found in the endothelium of developing arteries and veins (Figure 5, A through C) and in S-shaped bodies (Supplemental Figure 2) but not in α-SMA–containing cells or in renin-expressing cells. In the developing kidney, Cx45 immunoreactivity was seen already in maturing glomeruli and faintly in the walls of arteries/arterioles including renin-expressing smooth muscle cells (Figure 5, D through F).
Figure 4.
Histologic sections of kidney cortex from a normal 17-d-old mouse fetus. (A) Immunostaining for Cx40 alone. (B) Co-immunostaining for renin (green) and Cx40 (red). (C) Co-immunostaining for renin (green), α-SMA (blue), and Cx40 (red). (D) Immunostaining for Cx37 alone. (E) Co-immunostaining for renin (green) and Cx37(red). (F) Co-immunostaining for renin (green), α-SMA (blue), and Cx37 (red). Bar = 50 μm. Arrowheads indicate regions of renin expression; arrows indicate endothelial Cx staining; circle indicates gl.
Figure 5.
Histologic sections of kidney cortex from a normal 17-d-old mouse fetus. (A) Immunostaining for Cx43 alone. (B) Co-immunostaining for renin (green) and Cx43 (red). (C) Co-immunostaining for renin (green), α-SMA (blue), and Cx43 (red). (D) Immunostaining for Cx45 alone. (E) Co-immunostaining for renin (green) and Cx45 (red). (F) Co-immunostaining for renin (green), α-SMA (blue), and Cx45 (red). Bar = 50 μm. Arrowheads indicate regions of renin expression without Cx staining; arrows indicate endothelial Cx staining; lines, Cx45 co-localizing with renin; *, Cx45 in smooth muscle cells with low Cx45 staining.
Adult Kidneys Lacking Cx40
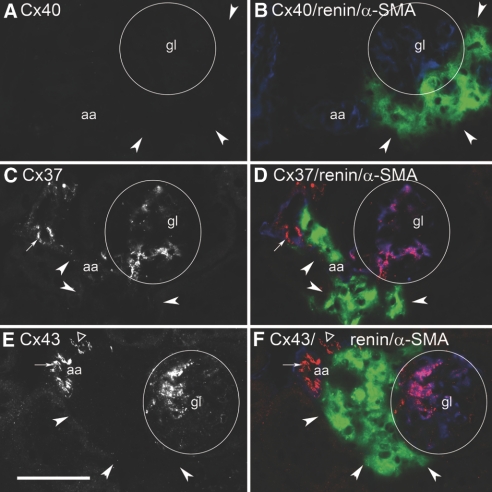
Deficiency of Cx40 causes a displacement of renin-expressing cells from the walls of afferent arterioles to the periglomerular interstitium (Figure 6, B, D, and F). To obtain information about the Cx expression pattern of these altered renin-producing cells, we determined Cx37, Cx43, and Cx45 immunoreactivity in Cx40-deficient kidneys. Interruption of the Cx40 gene was confirmed by the complete lack of Cx40 immunoreactivity in these kidneys (Figure 6, A and B). Cx37 immunoreactivity was not found in the renin-expressing cells (Figure 6, C and D). Immunoreactivity for Cx37, less than in wild-type kidneys, was found in the afferent endothelium and in intraglomerular structures (Figure 6, C and D). Cx43 immunoreactivity was not found in renin-expressing cells, but it was clearly seen in the afferent and efferent endothelium and in intraglomerular structures (Figure 6, E and F). The ectopic renin-expressing cells also lacked Cx45, which was preserved in VSMCs and in intraglomerular cells (data not shown).
Figure 6.
Histologic sections of kidney cortex from a Cx40-deficient mouse kept on a normal-salt diet. (A) Immunostaining for Cx40 alone. (B) Co-immunostaining for renin (green), Cx40 (red), and α-SMA (blue). (C) Immunostaining for Cx37 alone. (D) Co-immunostaining for renin (green), Cx37 (red), and α-SMA (blue). (E) Immunostaining for Cx43 alone. (F) Co-immunostaining for renin (green), Cx43 (red), and α-SMA (blue). Bar = 50 μm. Circles highlight gl; arrowheads indicate renin-expressing cells; arrows indicate endothelial Cx staining; ▵, staining for Cx43 in venous endothelium.
DISCUSSION
Our data indicate that Cx40 is a general characteristic of renin-producing cells in the kidney, because it is coexpressed with renin not only in JGE cells but also in smooth muscle cells of larger vessels of fetal kidneys and also during modulations of the renin system in adult kidneys. Apparently, co-regulation of Cx40 with renin is essential for the correct formation of JGE cells and also for the reversible metaplastic transformation of vascular smooth muscle into renin-producing cells in the adult kidney but not for the development of renin-producing cells in the fetal kidney.10 This may indicate the existence of two different forms of renin-producing cells, namely a mature form including JGE cells being subject to controlled metaplastic transformation and a more immature form existing during fetal development. The existence of two different forms would also be supported by the expression pattern of Cx45. In mature kidneys, Cx45 and renin expression seem to be mutually exclusive, whereas co-regulation of Cx45 with renin occurs in fetal renin-producing cells. Similar to findings in other organs,19–21 Cx45 was mainly seen in arteriolar VSMCs both in fetal and in adult kidney. In addition, Cx45 was found in glomerular cells, thereby supporting a previous notion.22
Co-regulation of renin and of Cx40 but not of other Cxs was also confirmed on the mRNA level. First evidence that Cx40 but not Cx37 or Cx43 expression might change with the recruitment of renin-producing cells can already be derived from a recent study7 showing that in mice with unilateral renal artery stenosis only Cx40 but not Cx37 or Cx43 mRNA changed in parallel with renin mRNA.
In accordance with the results of previous studies,5–7 our data confirm the expression of Cx37 in JGE cells and in endothelial cells, whereas smooth muscle cells of afferent arterioles are negative for Cx37.5–9 In contrast to previous reports, our data also suggest that mature JGE cells but not fetal renin-producing cells also express Cx43. This observation could help in the understanding of why replacement of Cx43 by Cx32 affects the function of JGE cells.9 We could not confirm weak Cx43 staining in VSMCs of afferent arterioles, which, however, seems to be variable (cf. 23).
Conclusions about Cx43 localization are generally limited by the fact that Cx43 antibodies might occasionally cross-react with Cx40.24 From three lines of evidence, we infer that the staining seen for Cx43 in this study is in fact reflecting Cx43. First, Cx43 antibodies from different providers produced the same results. Second, Cx43 immunoreactivity did not constantly overlap with Cx40 immunoreactivity. Finally, Western blotting of renal tissue with the Cx43 antibody produced the same results in wild-type and in Cx40-deficient mice.
Conversely, co-regulation of Cx37 and also of Cx43 with Cx40 seems to be specific for typical JGE cells. In analogy with other tissues, one may therefore speculate about the existence of heterotypic hemi- or holochannels in JGE cells.25–27 The partially overlapping defects in the pressure control of the renin system seen by replacement of Cx43 or by deletion of Cx407,9,11 could support a functional role of heterotypic Cx40/43 channels. The expression of Cx37 and Cx43 expression in JGE cells, however, seems to be more variable than the expression of Cx40. Thus, stimulation of the renin system by an ACEI in combination with low-salt diet even decreased Cx37 expression in JGE cells, whereas diabetes modulated Cx43 expression in JGE cells.6
The central role of Cx40 in mature renin-producing cells is also emphasized by the Cx expression pattern in Cx40-deficient, renin-producing cells. In accordance with a previous report,7 our data now demonstrate that these ectopic periglomerular renin-expressing cells lacking Cx40 are also free from Cx37, Cx43, and Cx45, which provides an explanation for the lack of gap junctions between these cells.10 The complete lack of these vascular Cxs raises the question of whether the absence of Cx37 and Cx43 is secondary to the lack of Cx40, which might be essential for the formation of heterotypic gap junctions and/or might form a scaffold for the other Cxs.28 Supportive of such an explanation could be the recent observations that Cx37 expression decreased also in Cx40-deficient endothelial cells.7,29 Another explanation could be that an intermediate, more immature form of juxtaglomerular renin-producing cells that does not express the Cx37, Cx43, and Cx45 exists, similar to the vessel-associated renin-producing cells during kidney development, which are also devoid of Cx37 and Cx43. In this view, the co-regulation of Cx37, Cx40, and Cx43 might constitute a differentiation signal to form typical JGE cells. At the present state of knowledge, we cannot exclude a role of also other vascular Cxs for the differentiation of JGE cells.
CONCISE METHODS
Animal Experiments
All animal experiments were performed according to the guidelines for the care and use of laboratory animals published by the US National Institutes of Health and were approved by the local ethics committee. The experiments were conducted on 14- to 16-wk-old male C57 Bl/6 mice. In addition, three C57 Bl/6 day 17 fetuses, four Cx45–green fluorescence protein, and four adult (3 mo) homozygous Cx40−/− mice30 were examined.
Male mice were assigned to the following groups:
Enalapril treatment: Four mice were given the ACEI enalapril (10 mg kg/d) for 5 d in the drinking water.
Salt diets: Mice were maintained for 10 d on food balanced in all respects except for a low (0.02% NaCl, Ssniff) or a high (4% NaCl, Ssniff) sodium content (n = 4 for each treatment and genotype).
Combination of low-salt diet with enalapril treatment: Four mice were fed a low-sodium diet for 10 d. For the last 5 d of the low-sodium diet, the mice received enalapril (10 mg kg/d) in the drinking water
Mice were deeply anesthetized with sevoflurane and killed by cervical dislocation. The kidneys were removed quickly and frozen in liquid nitrogen.
Determination of β-Actin, Renin, Cx37, Cx40, Cx43, and Cx45 mRNA by Real-Time PCR
Total RNA was isolated from the frozen kidneys as described by Chomczynski and Sacchi.31 The cDNA was synthesized by Maloney murine leukemia virus reverse transcriptase (Superscript; Invitrogen, Karlsruhe, Germany) and amplified with primers as specified in Table 1. For mRNA quantification, real-time reverse transcriptase–PCR was performed using a Light Cycler Instrument (Roche Diagnostics Corp., Grenzach, Germany) and the QuantiTect SYBR Green PCR kit (Qiagen, Hilden, Germany) and β-actin as a control. For verification of the accuracy of the amplicon, a melting curve analysis was done after amplification and PCR products were analyzed on an ethidium bromide–stained agarose gel.
Table 1.
Primer sequences used for cDNA amplification
| Primer | Sequence |
|---|---|
| β-Actin | |
| sense | 5′-cgggatccccgccctaggcaccagggtg-3′ |
| antisense | 5′-ggaattaggctggggtgttgaaggtctcaaa-3′ |
| Renin | |
| sense | 5′-atgaagggggtgtctgtgggg-3′ |
| antisense | 5′-atgcggggagggtgggcacct-3′ |
| Cx37 | |
| sense | 5′-gggagataaaggcacgaagg-3′ |
| antisense | 5′-atgagagcccgttgtaggtg-3′ |
| Cx40 | |
| sense | 5′-tttggcaagtcacggcaggg-3′ |
| antisense | 5′-tgtcactatggtagccctgag-3′ |
| Cx43 | |
| sense | 5′-tcctgggtacaagctggtcactgg-3′ |
| antisense | 5′-gctgctggctctgctggaagg-3′ |
| Cx45 | |
| sense | 5′-gaacttgatgatccgggtgcttat-3′ |
| antisense | 5′-atgggggttgttttggtgatggta-3′ |
Immunohistochemistry for Renin, Cx37, Cx40, Cx43, Cx45, Green Fluorescence Protein, and α-SMA
Renin antibody (chicken anti-mouse) was generated by Davids Biotechnologie (Regensburg, Germany). Antibodies used for immunostaining of Cxs were purchased from commercial providers: Anti-Cx40 (goat anti-mouse; Santa Cruz Biotechnology, Santa Cruz, CA), anti-Cx37 (rabbit anti-mouse; Alpha Diagnostic Int., San Antonio, TX), anti-Cx43 (rabbit anti-mouse; Sigma, St. Louis, MO, and Zymed, Basel, Switzerland), anti-Cx45 (rabbit anti-mouse32), and anti–α-SMA (mouse anti-mouse; Beckman Coulter Immunotech, Marseille, France).
Kidneys were frozen unfixed in TissueTek OCT embedding medium (Sakura Finetek, Heppenheim, Germany) and sectioned at 5 μm with a cryostat. Without further storing, sections were fixed in methanol at −20°C for 20 min; washed three times in PBS; and blocked in a buffer containing PBS, 1% BSA, and 10% horse serum for 30 min. Primary antibodies were diluted in the same blocking solution using anti-Cx40 (1:100), anti-Cx37 (0.5 mg/ml; 1:200), anti-Cx43 (1:800 [Sigma] and 1:200 [Zymed]), anti-Cx45 (1:800), anti-renin (1:200), and anti–α-SMA (1:10) in respective combinations, incubating sections at 4°C overnight. On the next day, sections were washed three times in PBS containing 1% BSA and incubated with combinations of Cy2, TRITC, or Cy5 secondary antibodies (Dianova, Hamburg, Germany) for 90 min at room temperature diluted 1:400, 1:300, and 1:400, respectively. After washing in PBS, sections were mounted with Dakocytomation Glycergel mounting medium (Dako, Glostrup, Denmark) and viewed with an Axiovert Microscope (Zeiss, Jena, Germany).
Statistical Analysis
Values are given as means ± SEM. Differences between groups were analyzed by ANOVA and Bonferroni adjustment for multiple comparisons. P < 0.05 was considered statistically significant.
NOTE ADDED IN PROOF
While this manuscript was under revision, an article reporting the expression of Cx45 in the juxtaglomerular apparatus was published (Hanner F, et al. Am J Physiol Regul 295: R371–R380, 2008).
DISCLOSURES
None.
Acknowledgments
This work was financially supported by the Deutsche Forschungsgemeinschaft (SFB 699).
We thank Julia von Maltzahn and Klaus Willecke (University of Bonn, Bonn, Germany) for providing us with mice expressing green fluorescence protein–tagged Cx45 under the control of the Cx45 promoter.
The skillful technical help provided by Anna M’Bangui and Susanne Lukas is gratefully acknowledged.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Taugner R, Schiller A, Kaissling B, Kriz W: Gap junctional coupling between the JGA and the glomerular tuft. Cell Tissue Res 186: 279–285, 1978 [DOI] [PubMed] [Google Scholar]
- 2.Taugner R, Kirchheim H, Forssmann WG: Myoendothelial contacts in glomerular arterioles and in renal interlobular arteries of rat, mouse and Tupaia belangeri. Cell Tissue Res 235: 319–325, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Mink D, Schiller A, Kriz W, Taugner R: Interendothelial junctions in kidney vessels. Cell Tissue Res 236: 567–576, 1984 [DOI] [PubMed] [Google Scholar]
- 4.Sohl G, Willecke K: Gap junctions and the connexin protein family. Cardiovasc Res 62: 228–232, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Hwan Seul K, Beyer EC: Heterogeneous localization of connexin 40 in the renal vasculature. Microvasc Res 59: 140–148, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Hill CE: Differential connexin expression in preglomerular and postglomerular vasculature: accentuation during diabetes. Kidney Int 68: 1171–1185, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, Waeber G, Meda P, Haefliger JA: Loss of connexin 40 alters renin production causing hypertension. Kidney Int 72: 814–822, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Haefliger JA, Demotz S, Braissant O, Suter E, Waeber B, Nicod P, Meda P: Connexins 40 and 43 are differentially regulated within the kidneys of rats with renovascular hypertension. Kidney Int 60: 190–201, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Haefliger JA, Krattinger N, Martin D, Pedrazzini T, Capponi A, Döring B, Plum A, Charollais A, Willecke K, Meda P: Connexin43-dependent mechanism modulates renin secretion and hypertension. J Clin Invest 116: 405–413, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtz L, Schweda F, de Wit C, Kriz W, Witzgall R, Warth R, Sauter A, Kurtz A, Wagner C: Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J Am Soc Nephrol 18: 1103–1111, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Wagner C, de Wit C, Kurtz L, Grünberger C, Kurtz A, Schweda F: Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res 100: 556–563, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Kon Y, Hashimoto Y, Kitagawa H, Kudo N: An immunohistochemical study on the embryonic development of renin-containing cells in the mouse and pig. Anat Histol Embryol 18: 14–26, 1989 [DOI] [PubMed] [Google Scholar]
- 13.Gomez RA, Lynch KR, Sturgill BC, Elwood JP, Chevalier RL, Carey RM, Peach MJ: Distribution of renin mRNA and its protein in the developing kidney. Am J Physiol 257: F850–F858, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Sauter A, Machura K, Neubauer B, Kurtz A, Wagner C: Spatiotemporal development of renin expression in the mouse kidney. Kidney Int 73: 43–51, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Hackenthal E, Paul M, Ganten D, Taugner R: Morphology, physiology, and molecular biology of renin secretion. Physiol Rev 70: 1067–1116, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Taugner R, Marin-Grez M, Keilbach R, Hackenthal E, Nobiling R: Immunoreactive renin and angiotensin II in the afferent glomerular arterioles of rats with hypertension due to unilateral renal artery constriction. Histochemistry 76: 61–69, 1982 [DOI] [PubMed] [Google Scholar]
- 17.Gomez RA, Chevalier RL, Everett AD, Elwood JP, Peach MJ, Lynch KR, Carey RM: Recruitment of renin gene-expressing cells in adult rat kidneys. Am J Physiol 259: F660–F665, 1990 [DOI] [PubMed] [Google Scholar]
- 18.Cantin M, Araujo-Nascimento MD, Benchimol S, Desormeaux Y: Metaplasia of smooth muscle cells into juxtaglomerular cells in the juxtaglomerular apparatus, arteries, and arterioles of the ischemic (endocrine) kidney: An ultrastructural-cytochemical and autoradiographic study. Am J Pathol 87: 581–602, 1977 [PMC free article] [PubMed] [Google Scholar]
- 19.Rummery NM, Hill CE: Vascular gap junctions and implications for hypertension. Clin Exp Pharmacol Physiol 31: 659–667, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Haefliger JA, Nicod P, Meda P: Contribution of connexins to the function of the vascular wall. Cardiovasc Res 62: 345–356, 2004 [DOI] [PubMed] [Google Scholar]
- 21.de Wit C, Wölfle SE, Höpfl B: Connexin-dependent communication within the vascular wall: Contribution to the control of arteriolar diameter. Adv Cardiol 42: 268–283, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Butterweck A, Gergs U, Elfgang C, Willecke K, Traub O: Immunochemical characterization of the gap junction protein connexin45 in mouse kidney and transfected human HeLa cells. J Membr Biol 141: 247–256, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Wagner C: Functions of connexins in the renal circulation. Kidney Int 73: 547–555, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Zimmerli SC, Masson F, Cancela J, Meda P, Hauser C: Cutting edge: Lack of evidence for connexin-43 expression in human epidermal Langerhans cells. J Immunol 179: 4318–4321, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Moreno AP: Biophysical properties of homomeric and heteromultimeric channels formed by cardiac connexins. Cardiovasc Res 62: 276–286, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Cottrell GT, Wu Y, Burt JM: Functional characteristics of heteromeric Cx40-Cx43 gap junction channel formation. Cell Commun Adhes 8: 193–197, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Valiunas V, Gemel J, Brink PR, Beyer EC: Gap junction channels formed by coexpressed connexin40 and connexin43. Am J Physiol Heart Circ Physiol 281: H1675–H1689, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Herve JC, Bourmeyster N, Sarrouilhe D, Duffy HS: Gap junctional complexes: From partners to functions. Prog Biophys Mol Biol 94: 29–65, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Simon AM, McWhorter AR: Decreased intercellular dye-transfer and downregulation of non-ablated connexins in aortic endothelium deficient in connexin37 or connexin40. J Cell Sci 116: 2223–2236, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Kirchhoff S, Nelles E, Hagendorff A, Krüger O, Traub O, Willecke K: Reduced cardiac conduction velocity and predisposition to arrhythmias in connexin40-deficient mice. Curr Biol 8: 299–302, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 32.Dedek K, Schultz K, Pieper M, Dirks P, Maxeiner S, Willecke K, Weiler R, Janssen-Bienhold U: Localization of heterotypic gap junctions composed of connexin45 and connexin36 in the rod pathway of the mouse retina. Eur J Neurosci 24: 1675–1686, 2006 [DOI] [PubMed] [Google Scholar]