Abstract
Nocturnal home hemodialysis (NHD) is associated with an increase in hemoglobin level. We hypothesized that NHD enhances the removal of toxins of hematopoietic progenitor cells (HPCs), thereby improving HPC growth and function. Among 16 patients with ESRD, 2 mo of NHD nearly doubled Kt/V per session and significantly lowered both parathyroid hormone levels and serum phosphate concentration. In addition, treatment with NHD improved hemoglobin levels from 113 ± 3 to 125 ± 4 g/L (P = 0.03) without altering erythropoietin requirements or iron status. To assess whether NHD may enhance removal of HPC toxins, we collected paired plasma samples from the same patient during treatment with conventional HD and NHD. In vitro, growth of erythroid (BFU-E) and granulocytic (CFU-GM) colonies was superior when cultured with NHD plasma compared with conventional HD plasma. Differential gene expression profiles obtained from peripheral blood and HPC colonies revealed similar upregulation of genes responsible for HPC mobilization and growth and production of red blood cells. In conclusion, the enhanced clearance by NHD is associated with an improvement in HPC growth and a coordinated increase in expression of genes relevant to production of red blood cells.
Anemia secondary to end-stage renal disease (ESRD) is an important but complex syndrome that directly contributes to significant morbidity and mortality in this patient population.1,2 The main causes of uremia-associated anemia are (1) relative erythropoietin (EPO) deficiency,3 (2) hypoproliferative bone marrow function,4 and (3) reduced survival of red blood cells (RBCs).5 Optimal renal replacement therapy may play a role in correcting the anemia by removing small and possibly middle/large molecules that inhibit erythropoiesis.6 Although the role of dialysis dosage per se on the response to EPO therapy has been proposed,7 it has been largely underexamined in the past. The conversion from conventional hemodialysis (CHD; three times a week, 4 h per session) to nocturnal home hemodialysis (NHD; five to six times a week, 6 to 8 h per session) results in a three- to four-fold increase in uremia clearance.8 This improvement is associated with an increase in hemoglobin level and a reduction of EPO requirement9 without altering RBC survival.5 In addition, our group has documented acute and long-term improvements in anemia associated cardiovascular surrogate outcomes (e.g. superior blood pressure [BP] control, improved flow mediated vasodilation10 and regression of left ventricular hypertrophy11) after conversion to NHD. Most recently, we have reported restoration in the biology of bone marrow derived endothelial progenitor cells in patients undergoing NHD.12 Given that hematopoietic progenitor cells (HPCs) are responsible for the maintenance of RBC, these observations led to the speculation that NHD may improve hemoglobin levels in patients with ESRD without further EPO demand by improved mobilization of bone marrow–derived HPCs into the circulation, enhanced HPC survival and growth, or both. This study was designed to test the uremic effect on HPCs using paired plasma samples obtained from patients while initially on CHD and subsequently on NHD. We hypothesized that NHD enhances the removal of substances that may be toxic or inhibitory to HPC, thereby improving HPC mobilization, growth, and function and resulting in ameliorating anemia management in patients with ESRD.
RESULTS
Clinical Observations
We studied 16 stable patients with ESRD (age 47 ± 2 yr; nine men). Of the 16 patients, 13 were white, two were Asian, and one was Hispanic. Their ESRD vintage was 5.7 ± 1.3 yr. Their ESRD was due to glomerulonephritis (n = 7), polycystic kidney disease (n = 3), diabetes (n = 2), vasculitis (n = 1), thrombotic microangiopathy (n = 1), reflux nephropathy (n = 1), and calcineurin antagonist toxicity (n = 1).
The dialysis dosage received (Kt/V per session) increased significantly after conversion to NHD (from 1.27 ± 0.06 to 2.23 ± 0.09; P < 0.01), and the frequency of dialysis doubled (Table 1). In addition, parathyroid hormone (PTH) levels fell (from 49.0 ± 5.4 to 20.6 ± 6.2 pmol/L; P < 0.05), and plasma phosphate concentration was restored to normal (from 2.1 ± 0.2 to 1.2 ± 0.1 mmol/L; P < 0.01). Concomitantly, there was a fall in mean arterial BP from 123 ± 4 to 106 ± 2 mmHg (P < 0.05) with a decrease in vasoactive medication requirement from 2.5 to 0.5 medications per patient (P < 0.001). Specifically, five of the study cohort were prescribed angiotensin-converting enzyme inhibitors or angiotensin receptor blockers at baseline. After 2 mo of NHD, two patients remained on angiotensin-converting enzyme inhibitors. Plasma albumin concentrations, aspirin use, and iron dosing did not change before and after conversion to NHD.
Table 1.
Hematologic and biochemical parameters before and after conversion to NHDa
| Variables | CHD | NHD
|
|
|---|---|---|---|
| 1 Mo | 2 Mo | ||
| Kt/V per session | 1.27 ± 0.06 | 2.11 ± 0.10b | 2.22 ± 0.10b |
| Weight (kg) | 83 ± 3 | 81 ± 3 | 81 ± 3 |
| Predialysis urea (mmol/L) | 24.8 ± 1.7 | 15.6 ± 1.4b | 16.3 ± 1.7b |
| Hemoglobin (g/L) | 113 ± 3 | 121 ± 3b | 125 ± 4b |
| MCV (fL) | 91 ± 1 | 92 ± 1 | 92 ± 1 |
| RDW (%) | 15.6 ± 0.2 | 16.5 ± 0.6 | 15.6 ± 0.3 |
| MCH (pg) | 30.2 ± 0.5 | 30.9 ± 0.5c | 31.5 ± 0.6b |
| WBC (109/L) | 7.7 ± 0.3 | 7.8 ± 0.4 | 8.1 ± 0.4 |
| Platelet (109/L) | 191 ± 11 | 195 ± 15 | 191 ± 10 |
| Ferritin (μg/L) | 368 ± 94 | 357 ± 76 | 370 ± 70 |
| Iron saturation | 0.24 ± 0.03 | 0.24 ± 0.03 | 0.26 ± 0.02 |
| Intravenous iron dosing (mg/mo) | 129 ± 25 | 114 ± 17 | 114 ± 17 |
| EPO requirement (U/wk) | 9667 ± 2021 | 9917 ± 2001 | 8833 ± 1912 |
| EPO resistance index (U/L per g/wk) | 76 ± 19 | 73 ± 19 | 63 ± 18b |
| Plasma albumin (g/L) | 37.10 ± 1.00 | 36.90 ± 0.70 | 38.20 ± 0.70 |
| Plasma phosphate (mmol/L) | 2.10 ± 0.20 | 1.20 ± 0.10b | 1.20 ± 0.10b |
| Plasma calcium (mmol/L) | 2.35 ± 0.03 | 2.37 ± 0.05 | 2.32 ± 0.04 |
| Plasma PTH (pmol/L) | 49.00 ± 5.40 | 26.40 ± 6.30b | 20.60 ± 6.20b |
MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; RDW, RBC distribution width.
P < 0.05 versus CHD.
P = 0.05 versus CHD.
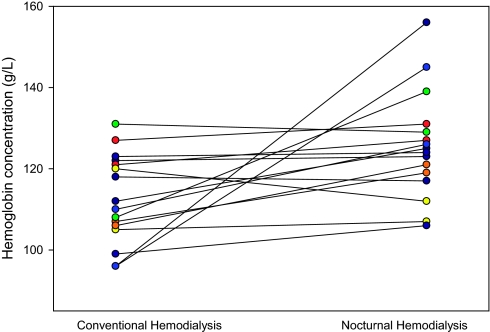
After 2 mo of NHD, hemoglobin levels improved from 113 ± 3 to 125 ± 4 g/L (P = 0.03) without alterations in EPO requirements or iron status (Table 1, Figure 1). Of the 16 study patients, three had a decrease in hemoglobin concentration, whereas the remainder had an increase. The observed reductions in PTH levels were directly correlated with the change in hemoglobin concentrations (P = 0.52, P = 0.04).
Figure 1.
Changes in hemoglobin concentration before and after conversion to nocturnal hemodialysis.
Cell Culture Studies
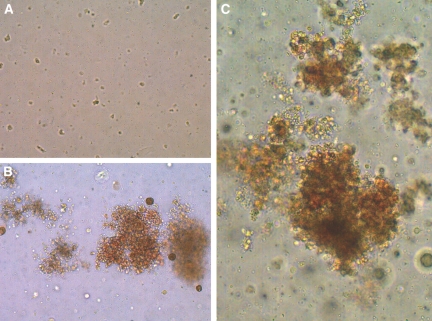
Paired plasma samples obtained from the same patient while on CHD and NHD were examined to determine their ability to support colony formation by BFU-E and CFU-GM obtained from normal donors. The frequency and size of colonies grown in culture from the same target cells was superior with 20% NHD plasma compared with 20% CHD plasma (BFU-E: 190 ± 54 versus 61 ± 30 colonies; P = 0.02 [normal 352 ± 76]; CFU-GM: 94 ± 25 versus 34 ± 16; P = 0.03 [normal 189 ± 11], respectively; Figure 2).
Figure 2.
Representative blast-forming units. (A through C) Erythroid appearance under CHD (A), NHD (B) and normal (C) conditions.
Gene Profiling
We performed gene profiling studies on mononuclear cells obtained from the peripheral blood of patients with ESRD while on CHD and subsequently on NHD. In addition, we conducted the same studies on pooled hematopoietic colonies grown from normal target cells in the presence of plasma derived while on CHD or NHD.
The principal components analysis of the differential gene expression patterns revealed two distinct populations coinciding with their degree of uremic clearance. Peripheral blood obtained from patients on NHD showed differential gene expression profiles that revealed augmentation in genes responsible for HPC mobilization and growth and production of RBCs (Table 2, Figure 1). Of potential candidate genes, significant upregulations in matrix metalloproteinase 9 (MMP-9; 1.65-fold increase), platelet factor 4 (1.69-fold increase), and chemokine receptor 4 (CXCR4; 1.38 fold increase) were noted after 2 mo of NHD. In addition, genes responsible for the biosynthesis of hemoglobin were systematically upregulated (Table 2). Similar expression profiles were obtained from normal target cells cultured in the presence of NHD plasma in comparison with the respective CHD plasma samples. Data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) and are accessible through Gene Expression Omnibus Series accession no. GSE11227.
Table 2.
Selected differential expression of genes under CHD and NHD conditions
| Affymetrix Probe ID | Gene Name | Gene Bank Accession No. | Gene Name | Fold Increase after Conversion to NHD |
|---|---|---|---|---|
| 209116_x_at | HBB | M25079 | Human sickle cell β-globin | 4.50 |
| 214414_x_at | HBA2 | T50399 | Hemoglobin α | 4.29 |
| 211696_x_at | HBB | AF349114 | Hemoglobin β | 4.09 |
| 217414_x_at | HBA2 | V00489 | Human α-globin | 3.64 |
| 211745_x_at | HBA2 | BC005931 | Hemoglobin α 2 | 3.53 |
| 209458_x_at | HBA2 | AF105974 | Hemoglobin α 2 | 3.38 |
| 204018_x_at | HBA2 | NM_000558 | Hemoglobin α 2 | 3.19 |
| 211699_x_at | HBA2 | AF349571 | Hemoglobin α 2 | 2.94 |
| 203434_s_at | MME | AI433463 | Membrane metallo-endopeptidase | 1.77 |
| 206390_x_at | PF4 | NM_002619 | Platelet factor 4 | 1.69 |
| 203936_s_at | MMP9 | NM_004994 | Matrix metalloproteinase 9 | 1.65 |
| 213515_x_at | HBG2 | AI133353 | Hemoglobin gamma | 1.58 |
| 204419_x_at | HBG2 | NM_000184 | Hemoglobin gamma | 1.47 |
| 217489_s_at | IL6R | S72848 | Interleukin 6 receptor | 1.46 |
| 209201_x_at | CXCR4 | L01639 | Chemokine receptor 4 | 1.38 |
| 211919_s_at | CXCR4 | AF348491 | Chemokine receptor 4 | 1.38 |
Plasma PTH levels inversely correlated with CXCR4 expression levels (r = −0.9, P < 0.001). CXCR4 expression was associated with levels of MMP-9 (r = 0.7, P < 0.05) and hemoglobin β (r = 0.8, P < 0.05).
DISCUSSION
Anemia is a common comorbid condition in patients with ESRD. NHD is an emerging mode of intensive hemodialysis that has been shown to offer multiple clinical advantages compared with CHD. Our study is consistent with the concept that augmentation of uremia clearance is associated with an improvement in EPO responsiveness and an increase in hemoglobin concentration in stable patients with ESRD. Furthermore, this study demonstrates that intensification of dialysis dosage by NHD is associated with upregulation of genes responsible for HPC mobilization and growth and production of RBCs. In addition, the frequency and size of colonies grown from HPCs of normal individuals was superior in cultures containing plasma obtained after 2 mo of NHD compared with plasma of the same patient while on CHD. Our observation adds to the existing literature that there is a direct relationship between the dosage of hemodialysis and the responsiveness of the bone marrow in patients with ESRD.
A number of uremic toxins13,14 and metabolites have been implicated as potential EPO toxins, including various amines15 and PTH.4 It is proposed that these substances induce a hypoproliferative bone marrow, thereby suppressing erythropoiesis at an early progenitor cell level. Rao et al.4 examined 18 CHD patients and correlated the degree of bone marrow fibrosis, the severity of secondary parathyroidism, and EPO responsiveness. These investigators found that the dosage of EPO required to achieve an adequate hemoglobin level was directly related to the extent of bone marrow fibrosis postulated to be caused by elevated concentrations of PTH. In addition, Ifudu et al.7 found a direct relationship between hematocrit and urea reduction ratio (URR). After adjustment for confounding variables, it was determined that an 11% increase in URR doubled the likelihood of a patient's achieving a hematocrit level of >30%. Literature also demonstrated the dynamic interaction between activation of osteoblasts by PTH and the expansion, mobilization, and differentiation of HPCs within the microenvironment of the bone marrow niche.16,17 Our data are consistent with the published literature and illustrate a direct relationship between the changes in PTH and hemoglobin after conversion to NHD. Furthermore, the growth of cultured HPCs was facilitated by NHD plasma. Taken together, these observations further strengthen our hypothesis that NHD by removing additional accumulated uremic toxins/metabolites may restore the proliferative function of the bone marrow.
Interaction between HPCs and maintenance of RBCs is a tightly regulated physiologic process. The mobilization, proliferation, and survival of HPCs is controlled by the expression of lineage-specific cytokines.18 EPO, produced in the kidney, is the primary cytokine responsible for expansion and differentiation of the erythroid lineage.3 Under the appropriate stimuli, mobilization and differentiation of CD34+ HPC occurs. Recent advances have characterized the required steps from mobilization of HPCs to differentiation to RBCs. Physiologic stress such as vascular trauma or ischemia induces the release of specific chemokines such as vascular endothelial growth factor (VEGF). These stimulate the upregulation and activation of MMP-9, resulting in the release of stem cell–active cytokines such as stromal-derived factor 1 (SDF-1). Of the various stem cell–active cytokines, SDF-1 and its receptor CXCR4 have been implicated in the mobilization,18 chemotaxis,19 homing,20 survival, and antiapoptosis of HPCs.21 Under the influence of SDF-1, CD34+VEGF1+ HPCs proliferate and are mobilized to the circulation, ultimately leading to the differentiation of RBCs.
Perturbations of normal HPC autocrine/paracrine loops have been documented in CHD patients.22 As shown in our results, the number of HPC-derived colonies was significantly less in cultures containing plasma of CHD patients compared with that of normal control subjects. In addition, other investigators have extracted total RNAs (TRNAs) from 100 colonies per patient and subjected them to complementary DNA expression array studies of 268 growth factors and cytokines and their receptors. A characteristic cluster of genes was identified in the CHD patients compared with normal control subjects; however, given that all CHD patients were receiving similar dosages of HD, the investigators were able to conclude only that HPCs are diminished in quantity and have abnormal gene expression patterns in patients with ESRD.22
Using a genome-wide analytical approach with a before-and-after conversion to NHD design, we were able to identify the set of genes that were differentially expressed as a result of the increase in dialysis dosage. We demonstrated a systematic upregulation of genes responsible for HPC mobilization and growth and production of RBCs both in the peripheral blood samples of our patients and from the cultured HPCs. Importantly, we were able to identify upregulations in CXCR4 and MMP-9, which are critical initiating steps allowing early progenitors to be released from bone marrow niches ultimately into the circulation. Our group also reported that NHD patients have significantly higher levels of circulating CD34+ VE-cadherin+ vascular progenitor cells when compared with age- and gender-matched CHD patients.12 Circulating CD34+ VE-cadherin+ cell number was similar to that of age- and gender-matched healthy control subjects. In vitro migration in response to a VEGF-A stimulus was also higher in NHD patients compared with matched CHD patients, at a level that was comparable to that of healthy control subjects. Furthermore, there was in inverse relationship between vascular progenitor cell number and function, cardiovascular surrogate end points (e.g., left ventricular mass index), and intensity of hemodialysis.12 Our experiment provides a novel observation that PTH levels are inversely associated with CXCR4 expression, which in turn represents an important rate-limiting step for RBC synthesis. Taken together, these complementary results substantiate the toxic effect of uremia on bone marrow. It is tempting to speculate that upregulations of CXCR4 and MMP-9 in conjunction with improvement in PTH levels via nocturnal hemodialysis provide a potential mechanistic pathway to restore bone marrow–derived progenitor responsiveness.
In summary, we have evidence to support the concept that augmented uremia clearance using NHD is accompanied by an increase in hemoglobin without further EPO requirement in stable patients with ESRD. Intensification of dialysis dosage is associated with an upregulation of genes responsible for HPC mobilization and growth and production of RBCs. In addition, NHD plasma facilitated the growth of human HPCs. This study represents the first attempt to study the impact of NHD on HPC biology. Additional experiments using other HPC functional assays and serum-mixing strategies are required to improve our basic understanding of the influence of uremia on bone marrow–derived stem cells. Future work determining the influence of NHD on various inflammatory cytokines and their effects on the bone marrow warrants additional investigations. The true impact of extracorporeal circulation and diabetes status on HPC biology in ESRD also require further studies. Our results are limited by its observational nature and its small sample size. The use of a stable ESRD population and the lack of a randomized, controlled design represented the pilot nature of the results; however, given the important role of HPCs in anemia management in ESRD and that greater EPO responsiveness is associated with better survival,23 we believe our work adds support to the growing benefits of NHD and provides a rationale for further testing the impact of NHD on HPC biology and future potential therapeutic targets on anemia management in patients with ESRD.
CONCISE METHODS
This protocol was approved by the Research Ethics Board of the Toronto General Hospital, University Health Network (Toronto, Ontario, Canada). Medically stable patients who had ESRD (age between 18 and 85 yr) and had received a minimum of 3 mo of CHD and were training for NHD were invited to participate in this study. All patients had stable hemoglobin, dosages of EPO or EPO analog, and iron therapy for a minimum of 3 mo at the time of entry in the study. None of the patients had any acute illness, hospitalization, or symptomatic cardiovascular disease (including congestive heart failure and acute coronary syndrome). Written informed consent was obtained from each patient. Patients were excluded when there was any evidence of blood loss, preexistent bone marrow pathology, previous bone marrow or stem cell transplantation, use of immunosuppressive medications, or presence of autoimmune disorders. Pregnant patients were also excluded.
Clinical Protocol
NHD patients received HD at home for 6 to 8 h, five to six nights per week. Vascular access was achieved through either a long-term internal jugular catheter (Uldall Catheter; Cook Critical Care, Bloomington, IN) or an arteriovenous fistula. Dialysate flow rate of 350 ml/min and blood flow rate of 200 to 300 ml/min was used. F80 polysulfone dialyzers (Fresenius Medical Care, Lexington, MA) or Exceltra 120 dialyzers (Baxter, Chicago, IL) were used. CHD patients received HD for 4 h three times per week via similar vascular access. A blood flow rate of 400 ml/min, a dialysate flow rate of 500 to 750 ml/min, and F80 polysulfone dialyzers (Fresenius Medical Care) were used. Unfractionated heparin was used for anticoagulation on CHD and NHD.
Dialysis dosage per treatment was estimated by equilibrated Kt/V (eKt/V) as described by Daugirdas et al.24: eKt/V = spKt/V − 0.6(spKt/V)/t + 0.03, spKt/V = single-pool Kt/V.25 spKt/V was determined using blood URR.
Patients included consecutive eligible patients who were converted to NHD at the University Health Network. Patient demographic information such as age, gender, ethnicity, cause of ESRD, and comorbid conditions was prospectively collected into a computerized clinical database. Clinical assessment, including weight, height, and BP measurements, were performed at baseline and monthly after conversion to NHD. Biochemical and hematologic parameters (complete blood count, urea, creatinine, albumin, alkaline phosphatase, calcium, phosphate, and PTH) were obtained monthly during the same time intervals. Baseline studies were performed the morning after a CHD day (a minimum of 18 h after dialysis). To minimize circadian variation and replicate steady-state NHD conditions, we performed subsequent experiments at the same time of day (a minimum of 4 h after the regular NHD session).
Differential Gene Expression under CHD and NHD Conditions
Whole blood samples were obtained atraumatically through the clinical protocol already outlined at baseline (under CHD) and after 2 mo of exposure of NHD. The blood samples were processed immediately after withdrawal to maximize the fidelity of the gene expression data.
TRNA was isolated from blood samples using Trizol Reagent (Life Technologies/BRL, Grand Island, NY) following the manufacturer's protocol. The quality of TRNA was assessed using the Agilent 2100 Bioanalyzer (version A.02.01S1232; Agilent Technologies, Santa Clara, CA). Only RNA with the OD ratio of 1.99 to 2.00 at 260/280 was used for microarray analysis. Hybridizations were performed on the Human HG-U133_PLUS2 GeneChip (Affymetrix, Santa Clara, CA). Samples were prepared for hybridization according to standard Affymetrix instructions and performed at the Genomic Core Facility at the Hospital for Sick Children. Microarrays were performed in compliance with accepted Microarray Experimental Guidelines.26
To monitor and compare differential gene expression in CHD and NHD samples, data obtained from GCOS (GeneChip Operating software) absolute analysis of all of the arrays were analyzed and clustered using GENESPRING 7.0 (Agilent Technologies). After filtering, one-way ANOVA (nonequal variance) was performed and differentially expressed genes were identified by hierarchical clustering. The same method was used to establish gene expression profiles from pooled hemopoietic colonies grown from normal peripheral blood stem cell donors in the presence of either CHD or NHD plasma obtained from the same patient.
Effect of Uremic Plasma on Normal HPCs
As described previously,27 HPC-derived colonies from normal individuals were grown in semisolid cultures supported by normal heparinized human plasma and sources of growth factors. The latter included human recombinant growth factors IL-3 (50 ng), GM-CSF (50 ng), SCF (50 ng), and EPO (2 U) and 10% phytohemaglutinin-conditioned medium. The need for human plasma at a concentration of 20% provided the opportunity to compare the growth supporting effect of normal plasma with plasma derived from patients with ESRD while initially on CHD and subsequently on NHD. Thus, plasma of each patient on study was evaluated under both dialysis conditions. Normal peripheral HPCs served as targets. They were obtained from consenting healthy donors of G-CSF–mobilized hemopoietic allografts. The cultures were scored for the presence of BFU-E–derived erythroid burst and CFU-GM–derived granulocyte-macrophage colonies. Individual colonies were removed, and TRNA was prepared. Differential gene expression analysis was performed using methods outlined already.
Data Analysis
Descriptive data are presented as means ± SE. The primary outcome measure was the difference in HPC colonies among normal control subjects and CHD and NHD patients. Differentially expressed genes were identified by hierarchical clustering before and after conversion to NHD. Mann-Whitney U test was used for comparison of continuous variables between two groups. ANOVA was used for multiple comparisons of a continuous variable among three groups of patients. Spearman correlation was used to investigate potential associations between variables of interest. All statistical tests were two tailed with a P < 0.05 taken to indicate significance. SPSS 10 (SPSS Inc., Chicago, IL) was used for all statistical analyses.
DISCLOSURES
None.
Acknowledgments
This study was supported by Physicians’ Services Inc. Foundation Grant 06-30. C.T.C. holds the R Fraser Elliott Chair in Home Dialysis. P.P.L. is the Heart & Stroke/Polo Chair Professor of Medicine and Physiology at the University of Toronto. Part of the gene expression array analysis is supported through grants from the Heart & Stroke Foundation and the Canadian Institutes of Health Research.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Ma JZ, Ebben J, Xia H, Collins AJ: Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 10: 610–619, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Xia H, Ebben J, Ma JZ, Collins AJ: Hematocrit levels and hospitalization risks in hemodialysis patients. J Am Soc Nephrol 10: 1309–1316, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Nissenson AR, Nimer SD, Wolcott DL: Recombinant human erythropoietin and renal anemia: Molecular biology, clinical efficacy, and nervous system effects. Ann Intern Med 114: 402–416, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Rao DS, Shih MS, Mohini R: Effect of serum parathyroid hormone and bone marrow fibrosis on the response to erythropoietin in uremia. N Engl J Med 328: 171–175, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Ly J, Marticorena R, Donnelly S: Red blood cell survival in chronic renal failure. Am J Kidney Dis 44: 715–719, 2004 [PubMed] [Google Scholar]
- 6.Locatelli F, Del Vecchio L: Dialysis adequacy and response to erythropoietic agents: What is the evidence base? Nephrol Dial Transplant 18[Suppl 8]: viii29–viii35, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Ifudu O, Feldman J, Friedman EA: The intensity of hemodialysis and the response to erythropoietin in patients with end-stage renal disease. N Engl J Med 334: 420–425, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Pierratos A: New approaches to hemodialysis. Annu Rev Med 55: 179–189, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Schwartz DI, Pierratos A, Richardson RM, Fenton SS, Chan CT: Impact of nocturnal home hemodialysis on anemia management in patients with end-stage renal disease. Clin Nephrol 63: 202–208, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Chan CT, Harvey PJ, Picton P, Pierratos A, Miller JA, Floras JS: Short-term blood pressure, noradrenergic, and vascular effects of nocturnal home hemodialysis. Hypertension 42: 925–931, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Chan CT, Floras JS, Miller JA, Richardson RM, Pierratos A: Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney Int 61: 2235–2239, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Chan CT, Li SH, Verma S: Nocturnal hemodialysis is associated with restoration of impaired endothelial progenitor cell biology in end-stage renal disease. Am J Physiol Renal Physiol 289: F679–F684, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Yamada S, Kataoka H, Kobayashi H, Ono T, Minakuchi J, Kawano Y: Identification of an erythropoietic inhibitor from the dialysate collected in the hemodialysis with PMMA membrane (BK-F). Contrib Nephrol 125: 159–172, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Vanholder R, De Smet R: Pathophysiologic effects of uremic retention solutes. J Am Soc Nephrol 10: 1815–1823, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Radtke HW, Rege AB, LaMarche MB, Bartos D, Bartos F, Campbell RA, Fisher JW: Identification of spermine as an inhibitor of erythropoiesis in patients with chronic renal failure. J Clin Invest 67: 1623–1629, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvi LM: Osteoblastic activation in the hematopoietic stem cell niche. Ann N Y Acad Sci 1068: 477–488, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT: Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425: 841–846, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Rabbany SY, Heissig B, Hattori K, Rafii S: Molecular pathways regulating mobilization of marrow-derived stem cells for tissue revascularization. Trends Mol Med 9: 109–117, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC: The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med 185: 111–120, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T: Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 283: 845–848, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Broxmeyer HE, Kohli L, Kim CH, Lee Y, Mantel C, Cooper S, Hangoc G, Shaheen M, Li X, Clapp DW: Stromal cell-derived factor-1/CXCL12 directly enhances survival/antiapoptosis of myeloid progenitor cells through CXCR4 and G(alpha)i proteins and enhances engraftment of competitive, repopulating stem cells. J Leukoc Biol 73: 630–638, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Nakao K, Wada J, Ota K, Ichikawa H, Akagi S, Okamoto A, Hida K, Nagake Y, Makino H: Perturbation of autocrine/paracrine loops of burst-forming units of erythroid-derived cells in rHuEPO-hyporesponsive hemodialysis patients. Am J Kidney Dis 41: 624–636, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Kilpatrick RD, Critchlow CW, Fishbane S, Besarab A, Stehman-Breen C, Krishnan M, Bradbury BD: Greater epoetin alfa responsiveness is associated with improved survival in hemodialysis patients. Clin J Am Soc Nephrol 3: 1077–1083, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jindal KK, Manuel A, Goldstein MB: Percent reduction in blood urea concentration during hemodialysis (PRU): A simple and accurate method to estimate Kt/V urea. ASAIO Trans 33: 286–288, 1987 [PubMed] [Google Scholar]
- 25.Daugirdas JT, Greene T, Depner TA, Gotch FA, Star RA: Relationship between apparent (single-pool) and true (double-pool) urea distribution volume. Kidney Int 56: 1928–1933, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC, Gaasterland T, Glenisson P, Holstege FC, Kim IF, Markowitz V, Matese JC, Parkinson H, Robinson A, Sarkans U, Schulze-Kremer S, Stewart J, Taylor R, Vilo J, Vingron M: Minimum information about a microarray experiment (MIAME): Toward standards for microarray data. Nat Genet 29: 365–371, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Messner HA, Fauser AA, Buick R, Chang LJ, Lepine J, Curtis JC, Senn J, McCulloch EA: Assessment of human pluripotent hemopoietic progenitors and leukemic blast-forming cells in culture. Haematol Blood Transfus 26: 246–250, 1981 [DOI] [PubMed] [Google Scholar]