Abstract
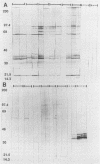
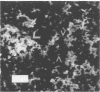
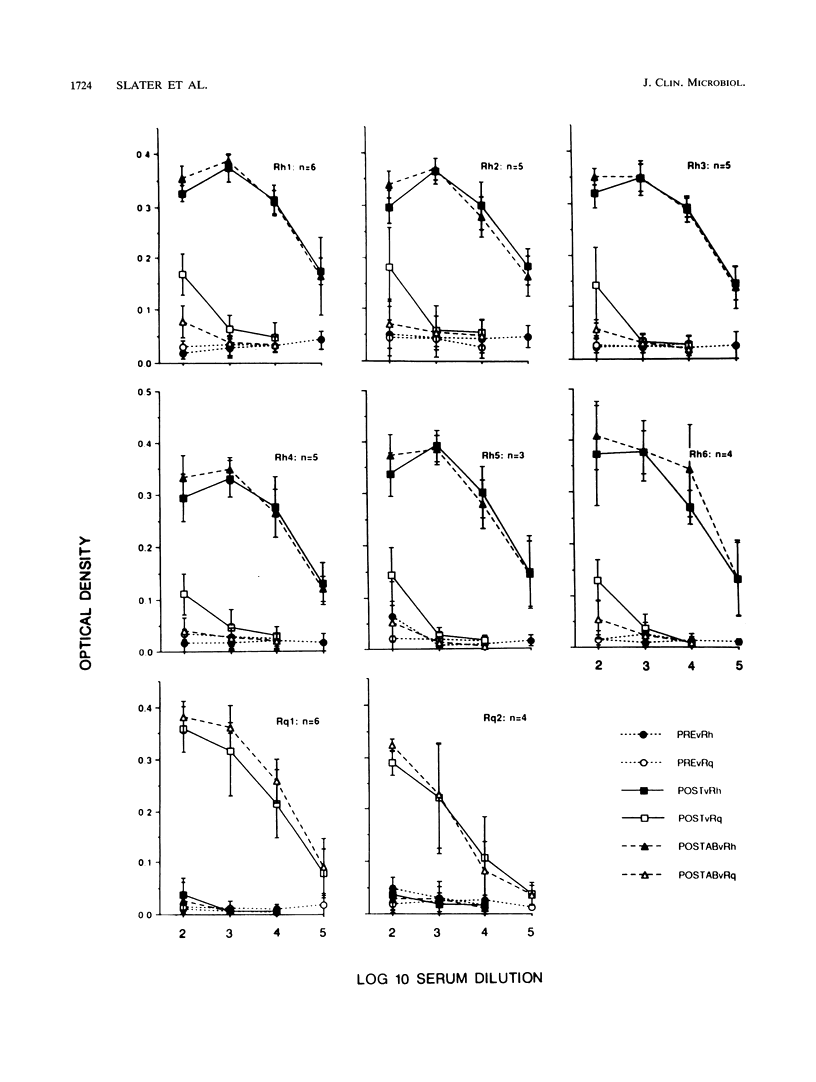
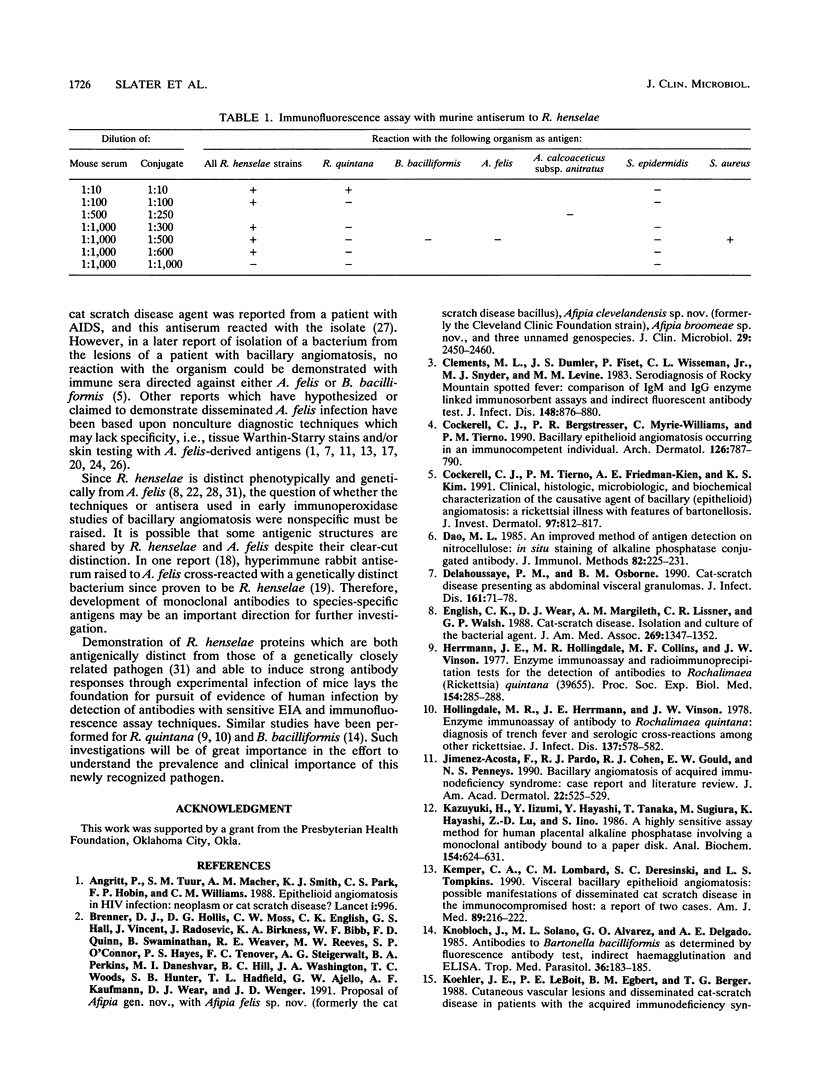
Rochalimaea henselae causes persistent bacteremia, bacillary angiomatosis, and parenchymal bacillary peliosis. Detection of a specific antibody response to R. henselae infection may represent an alternative to cultivation as a means of diagnosis. We assessed the specificity of induced murine antibodies for antigens from R. henselae and the closely related species R. quintana. Groups of CD-1 mice were inoculated with whole organisms of six strains of R. henselae and two of R. quintana. Pre- and postinoculation blood specimens were collected. Enzyme immunosorbent assays were performed by using as antigens preparations of immunogenic proteins from one isolate of R. henselae or from the R. quintana type strain. These demonstrated high specificity of R. henselae-induced antibodies for proteins of R. henselae and of R. quintana-induced antibodies for proteins of R. quintana. Protein preparations extracted from all of the strains were separated electrophoretically. After their transfer to membranes, immunoblots were performed by using 1:1,000 dilutions of all of the pre- and postinoculation sera in combination with proteins from all of the strains. Preinoculation sera had minimal reactivity. All of the R. henselae-induced immune sera reacted with numerous proteins of all of the R. henselae strains but cross-reacted minimally with proteins of R. quintana. Immune sera from R. quintana-inoculated mice had similar species specificity. An immunofluorescence assay was developed by using antiserum to one strain of R. henselae. A 1:1,000 dilution yielded fluorescence with all strains of R. henselae but with none of R. quintana, Bartonella bacilliformis, or Afipia felis. Acinetobacter calcoaceticus subsp. anitratus was also unreactive with a dilution of 1:500. A 1:10 dilution yielded weak fluorescence with R. quintana but none with Staphylococcus epidermidis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angritt P., Tuur S. M., Macher A. M., Smith K. J., Park C. S., Hobin F. P., Myrie-Williams C. Epithelioid angiomatosis in HIV infection: neoplasm or cat-scratch disease? Lancet. 1988 Apr 30;1(8592):996–996. doi: 10.1016/s0140-6736(88)91813-2. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Hollis D. G., Moss C. W., English C. K., Hall G. S., Vincent J., Radosevic J., Birkness K. A., Bibb W. F., Quinn F. D. Proposal of Afipia gen. nov., with Afipia felis sp. nov. (formerly the cat scratch disease bacillus), Afipia clevelandensis sp. nov. (formerly the Cleveland Clinic Foundation strain), Afipia broomeae sp. nov., and three unnamed genospecies. J Clin Microbiol. 1991 Nov;29(11):2450–2460. doi: 10.1128/jcm.29.11.2450-2460.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements M. L., Dumler J. S., Fiset P., Wisseman C. L., Jr, Snyder M. J., Levine M. M. Serodiagnosis of Rocky Mountain spotted fever: comparison of IgM and IgG enzyme-linked immunosorbent assays and indirect fluorescent antibody test. J Infect Dis. 1983 Nov;148(5):876–880. doi: 10.1093/infdis/148.5.876. [DOI] [PubMed] [Google Scholar]
- Cockerell C. J., Bergstresser P. R., Myrie-Williams C., Tierno P. M. Bacillary epithelioid angiomatosis occurring in an immunocompetent individual. Arch Dermatol. 1990 Jun;126(6):787–790. [PubMed] [Google Scholar]
- Cockerell C. J., Tierno P. M., Friedman-Kien A. E., Kim K. S. Clinical, histologic, microbiologic, and biochemical characterization of the causative agent of bacillary (epithelioid) angiomatosis: a rickettsial illness with features of bartonellosis. J Invest Dermatol. 1991 Nov;97(5):812–817. doi: 10.1111/1523-1747.ep12487507. [DOI] [PubMed] [Google Scholar]
- Dao M. L. An improved method of antigen detection on nitrocellulose: in situ staining of alkaline phosphatase conjugated antibody. J Immunol Methods. 1985 Oct 10;82(2):225–231. doi: 10.1016/0022-1759(85)90354-0. [DOI] [PubMed] [Google Scholar]
- Delahoussaye P. M., Osborne B. M. Cat-scratch disease presenting as abdominal visceral granulomas. J Infect Dis. 1990 Jan;161(1):71–78. doi: 10.1093/infdis/161.1.71. [DOI] [PubMed] [Google Scholar]
- English C. K., Wear D. J., Margileth A. M., Lissner C. R., Walsh G. P. Cat-scratch disease. Isolation and culture of the bacterial agent. JAMA. 1988 Mar 4;259(9):1347–1352. doi: 10.1001/jama.259.9.1347. [DOI] [PubMed] [Google Scholar]
- Herrmann J. E., Hollingdale M. R., Collins M. F., Vinson J. W. Enzyme immunoassay and radioimmunoprecipitation tests for the detection of antibodies to Rochalimaea (Rickettsia) quintana. Proc Soc Exp Biol Med. 1977 Feb;154(2):285–288. doi: 10.3181/00379727-154-39655. [DOI] [PubMed] [Google Scholar]
- Hirano K., Iiizumi Y., Hayashi Y., Tanaka T., Sugiura M., Hayashi K., Lu Z. D., Iino S. A highly sensitive assay method for human placental alkaline phosphatase involving a monoclonal antibody bound to a paper disk. Anal Biochem. 1986 May 1;154(2):624–631. doi: 10.1016/0003-2697(86)90038-2. [DOI] [PubMed] [Google Scholar]
- Hollingdale M. R., Herrmann J. E., Vinson J. W. Enzyme immunoassay of antibody to Rochalimaea quintana: diagnosis of trench fever and serologic cross-reactions among other rickettsiae. J Infect Dis. 1978 May;137(5):578–582. doi: 10.1093/infdis/137.5.578. [DOI] [PubMed] [Google Scholar]
- Jimenez-Acosta F., Pardo R. J., Cohen R. J., Gould E. W., Penneys N. S. Bacillary angiomatosis of acquired immunodeficiency syndrome: case report and literature review. J Am Acad Dermatol. 1990 Mar;22(3):525–529. doi: 10.1016/s0190-9622(08)80400-8. [DOI] [PubMed] [Google Scholar]
- Kemper C. A., Lombard C. M., Deresinski S. C., Tompkins L. S. Visceral bacillary epithelioid angiomatosis: possible manifestations of disseminated cat scratch disease in the immunocompromised host: a report of two cases. Am J Med. 1990 Aug;89(2):216–222. doi: 10.1016/0002-9343(90)90301-s. [DOI] [PubMed] [Google Scholar]
- Knobloch J., Solano L., Alvarez O., Delgado E. Antibodies to Bartonella bacilliformis as determined by fluorescence antibody test, indirect haemagglutination and ELISA. Trop Med Parasitol. 1985 Dec;36(4):183–185. [PubMed] [Google Scholar]
- Koehler J. E., LeBoit P. E., Egbert B. M., Berger T. G. Cutaneous vascular lesions and disseminated cat-scratch disease in patients with the acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. Ann Intern Med. 1988 Sep 15;109(6):449–455. doi: 10.7326/0003-4819-109-6-449. [DOI] [PubMed] [Google Scholar]
- LeBoit P. E., Berger T. G., Egbert B. M., Yen T. S., Stoler M. H., Bonfiglio T. A., Strauchen J. A., English C. K., Wear D. J. Epithelioid haemangioma-like vascular proliferation in AIDS: manifestation of cat scratch disease bacillus infection? Lancet. 1988 Apr 30;1(8592):960–963. doi: 10.1016/s0140-6736(88)91779-5. [DOI] [PubMed] [Google Scholar]
- Lenoir A. A., Storch G. A., DeSchryver-Kecskemeti K., Shackelford G. D., Rothbaum R. J., Wear D. J., Rosenblum J. L. Granulomatous hepatitis associated with cat scratch disease. Lancet. 1988 May 21;1(8595):1132–1136. doi: 10.1016/s0140-6736(88)91952-6. [DOI] [PubMed] [Google Scholar]
- Lucey D., Dolan M. J., Moss C. W., Garcia M., Hollis D. G., Wegner S., Morgan G., Almeida R., Leong D., Greisen K. S. Relapsing illness due to Rochalimaea henselae in immunocompetent hosts: implication for therapy and new epidemiological associations. Clin Infect Dis. 1992 Mar;14(3):683–688. doi: 10.1093/clinids/14.3.683. [DOI] [PubMed] [Google Scholar]
- Margileth A. M., Wear D. J., English C. K. Systemic cat scratch disease: report of 23 patients with prolonged or recurrent severe bacterial infection. J Infect Dis. 1987 Mar;155(3):390–402. doi: 10.1093/infdis/155.3.390. [DOI] [PubMed] [Google Scholar]
- Milam M. W., Balerdi M. J., Toney J. F., Foulis P. R., Milam C. P., Behnke R. H. Epithelioid angiomatosis secondary to disseminated cat scratch disease involving the bone marrow and skin in a patient with acquired immune deficiency syndrome: a case report. Am J Med. 1990 Feb;88(2):180–183. doi: 10.1016/0002-9343(90)90471-o. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Holzer G., Wallace P. L., Hollis D. G. Cellular fatty acid compositions of an unidentified organism and a bacterium associated with cat scratch disease. J Clin Microbiol. 1990 May;28(5):1071–1074. doi: 10.1128/jcm.28.5.1071-1074.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Dudas K. C., Mylotte J. M., Apicella M. A. A subtyping system for nontypable Haemophilus influenzae based on outer-membrane proteins. J Infect Dis. 1983 May;147(5):838–846. doi: 10.1093/infdis/147.5.838. [DOI] [PubMed] [Google Scholar]
- Pilon V. A., Echols R. M. Cat-scratch disease in a patient with AIDS. Am J Clin Pathol. 1989 Aug;92(2):236–240. doi: 10.1093/ajcp/92.2.236. [DOI] [PubMed] [Google Scholar]
- Relman D. A., Falkow S., LeBoit P. E., Perkocha L. A., Min K. W., Welch D. F., Slater L. N. The organism causing bacillary angiomatosis, peliosis hepatis, and fever and bacteremia in immunocompromised patients. N Engl J Med. 1991 May 23;324(21):1514–1514. doi: 10.1056/NEJM199105233242117. [DOI] [PubMed] [Google Scholar]
- Rocco V. K., Roman R. J., Eigenbrodt E. H. Cat scratch disease. Report of a case with hepatic lesions and a brief review of the literature. Gastroenterology. 1985 Dec;89(6):1400–1406. [PubMed] [Google Scholar]
- Schlossberg D., Morad Y., Krouse T. B., Wear D. J., English C. K., Littman M. Culture-proved disseminated cat-scratch disease in acquired immunodeficiency syndrome. Arch Intern Med. 1989 Jun;149(6):1437–1439. [PubMed] [Google Scholar]
- Slater L. N., Welch D. F., Hensel D., Coody D. W. A newly recognized fastidious gram-negative pathogen as a cause of fever and bacteremia. N Engl J Med. 1990 Dec 6;323(23):1587–1593. doi: 10.1056/NEJM199012063232303. [DOI] [PubMed] [Google Scholar]
- Slater L. N., Welch D. F., Min K. W. Rochalimaea henselae causes bacillary angiomatosis and peliosis hepatis. Arch Intern Med. 1992 Mar;152(3):602–606. [PubMed] [Google Scholar]
- Vinson J. W. In vitro cultivation of the rickettsial agent of trench fever. Bull World Health Organ. 1966;35(2):155–164. [PMC free article] [PubMed] [Google Scholar]
- Welch D. F., Pickett D. A., Slater L. N., Steigerwalt A. G., Brenner D. J. Rochalimaea henselae sp. nov., a cause of septicemia, bacillary angiomatosis, and parenchymal bacillary peliosis. J Clin Microbiol. 1992 Feb;30(2):275–280. doi: 10.1128/jcm.30.2.275-280.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]