Abstract
In May 2005, preliminary trial results pronouncing the effectiveness of Herceptin (trastuzumab) for treatment of early-stage breast cancer were disseminated at a high-profile scientific meeting. Herceptin was subsequently approved for use in the public healthcare systems of Canada and the United Kingdom, although the differences between the two decision timelines were stark. The authors compared UK and Canadian newspaper coverage of the Herceptin story to assess how it may have been “hyped” in each country. They analyzed a diverse sample of newspapers and coded clippings for reporters' framing of the drug's efficacy, costs and funding approval process. Canadian news coverage preceded formal publication of the trial results, while UK coverage mirrored major national events. Reporters in both countries used predominantly individualistic perspectives and framed Herceptin's efficacy in salutary terms. Framing of costs was more neutral in Canadian than in UK newspapers. Funding approval framing focused on inequitable access in the UK and timeliness in Canada. News coverage of drug access stories varies across jurisdictions in terms of intensity and some aspects of framing. Such variations likely reflect different journalistic practices and dominant political rhetoric. Greater attention should be given to the role that news coverage of drug access plays in shaping public opinion and policy action, especially when this coverage precedes scientific debate.
Abstract
En mai 2005, les résultats préliminaires d'une étude faisant valoir l'efficacité de l'Herceptin (trastuzumab) pour le traitement du stade précoce du cancer du sein étaient diffusés au cours d'une rencontre scientifique de haut calibre. Par la suite, l'usage de l'Herceptin était autorisé dans les systèmes de santé au Canada et au Royaume-Uni, bien qu'il y ait une grande différence entre les calendriers de décision respectifs des deux pays. Les auteurs ont comparé la couverture de la presse écrite au Canada et au Royaume-Uni afin d'évaluer à quel point le cas a fait l'objet de battage dans chacun des pays. Ils ont analysé un échantillon de quotidiens et de coupures de presse afin de cerner comment les journalistes ont fait état de l'efficacité du médicament, de ses coûts et du processus d'autorisation. Au Canada, la couverture médiatique a précédé la publication officielle des résultats de l'étude, tandis qu'au Royaume-Uni la couverture suivait les principales étapes nationales. Dans les deux pays, les journalistes ont adopté un point de vue principalement personnel et ont abordé l'efficacité de l'Herceptin en termes favorables. La question des coûts a été abordée de façon plus neutre au Canada qu'au Royaume-Uni. L'approche au sujet de l'autorisation de financement a porté, au Royaume-Uni, sur un accès équitable et, au Canada, sur un accès en temps opportun. Entre les deux pays, l'intensité de la couverture médiatique des cas d'accès aux médicaments, ainsi que certains aspects de l'approche, présentent des différences. Une telle variation reflète probablement des différences dans les pratiques journalistiques et dans le discours politique dominant. Il faut porter plus d'attention au rôle que joue la couverture médiatique sur l'accès aux médicaments dans l'opinion publique et dans les initiatives politiques, particulièrement si la couverture précède le débat scientifique.
New and emerging healthcare technologies present some of the greatest challenges to policy makers responsible for making public coverage decisions. New therapies hold out great promise to potential beneficiaries. As such, they challenge public policy makers to find the “right” balance between ensuring safety and efficacy, and timely and equitable access to new treatments. Their tendency to be introduced at higher prices than existing therapies raises additional concerns about affordability (CIHI 2008). Drug review bodies such as the National Institute for Health and Clinical Excellence (NICE) in the United Kingdom and the Common Drug Review in Canada have been charged with the task of assessing the clinical and cost effectiveness of new therapies against existing therapies to ensure “value for money” in reimbursement decisions (McMahon et al. 2006). Alongside these rigorous assessment efforts, new, potentially life-saving technologies continue to be the source of popular human-interest stories covered by the health news media (Geller et al. 2002). “New therapy” stories can offer important sources of information to patients; they also shape public expectations and act as instruments for policy advocacy (Benelli 2003; Braun 2003). The reporting of new and emerging cancer therapies, in particular, attracts sustained media interest (Bartlett et al. 2002) given cancer's prominence as a leading cause of death in developed countries (Statistics Canada 2004; Niederlaender 2006). Recently, the reimbursement decisions associated with these therapies have intensified media interest in these stories, prompting renewed scrutiny of media roles in the policy process.
For close to a decade, the health research community has critiqued the accuracy and framing of health news reporting, often under the banner of the media hyping research (Scheule 1999; Moynihan et al. 2000; Cooper and Yukimura 2002; Schwitzer 2003; Schwartz and Woloshin 2004). Compressed news cycles and an increasingly concentrated and competitive media industry encourage the reporting of early results for promising new therapies. High-profile international scientific meetings provide venues for rapid and widespread dissemination of these results, facilitated by sophisticated press releases (Woloshin and Schwartz 2002). This type of media coverage can be problematic when press releases and news stories overlook important study information or downplay treatment risks (Woloshin and Schwartz 2002, 2006a; Smith et al. 2005; Cassels and Lexchin 2008), or if they understate the preliminary nature of the findings (Schwartz et al. 2002). The clinical and policy risks associated with this type of reporting have been articulated (Woloshin and Schwartz 2006b) along with calls for empirical scrutiny of the effects of “media hyping” on public opinion and coverage policy decisions for new technologies (Chen and Siu 2001; Cassels et al. 2003; Driedger and Eyles 2003; Bubela and Caulfield 2004; Smith 2005).
We used the cancer therapy drug, trastuzumab (Herceptin), as a case study to empirically examine the attributes of hyping in the health news media through a cross-national comparative study of Canadian and UK print news coverage. While the phenomenon of “media hyping” has been previously explored in the literature (Bubela and Caulfield 2004), comparative studies that produce an enriched understanding of what shapes or constrains it are much less common. In May 2005, clinical trial results of trastumuzab use for treating HER2-positive patients with early-stage breast cancer were presented in a late-breaking session at the American Society for Clinical Oncology (ASCO) meeting in Orlando, Florida (Tuma 2005). The results demonstrated an absolute benefit in terms of disease-free survival at two years of 8.4% for patients receiving trastumuzab in conjunction with standard chemotherapy, compared to patients receiving standard chemotherapy only (Piccart-Gebhart et al. 2005). Results presented at this scientific meeting received front-page coverage in the Canadian news media. In hailing trastumuzab's benefits and decrying the absence of public funding for this treatment, the media transformed a medical breakthrough story into an access story (Abraham 2005; Priest 2005a,b). Several Canadian provinces, including British Columbia, Saskatchewan and Ontario, moved quickly to announce public funding for trastumuzab, at a cost of Cdn$35,000–$45,000 per year (Hume 2005; Priest 2005e), in advance of the publication of study results in October 2005 (Piccart-Gebhart et al. 2005; Romond et al. 2005). This policy response drew accolades and a national newspaper award for story coverage that spurred Canadian provincial governments to action (Moore 2006).
In the United Kingdom, trastumuzab was recommended for use in the National Health Service (NHS), at an annual cost equivalent to the cost in Canada, after the National Institute of Health and Clinical Excellence (NICE) completed its formal review, almost a year after the first Canadian provincial government funding decision had been made (Mayor 2006). Despite different decision trajectories, concerns arose in both countries about the influence of news media coverage on the public's understanding of the therapy, including its associated costs, benefits and risks, and the media's role in precipitating policy action (Kondro and Sibbald 2005; Smith 2005; Collier 2006; Thomas 2006; Keidan 2007). We examine whether and how this access story was “hyped” by the media in each of these jurisdictions and consider the implications of these findings for future public and policy debates about the funding of novel and emerging medical therapies.
Methods
Design
Media studies typically focus on the production, representation or reception of media messages (Seale 2003). We employed a media content analysis design to examine how the “Herceptin access story” was represented by Canadian and UK newspaper media. A quantitative analytic approach, common to media content analysis studies (Brodie et al. 1998; Arvai and Mascarenhas 2001; Collins et al. 2006), was used to facilitate statistical comparisons between the two countries.
A comparative approach to the study allowed us to determine whether coverage of an internationally relevant health news story differs between countries operating in different policy contexts. In the United Kingdom, drug funding decisions are made by the NHS using recommendations issued by NICE. Since 2002, primary care trusts (PCTs) operating within the NHS have been “obliged to provide funding for drugs within 3 months of a NICE recommendation” (McMahon et al. 2006: 345). The lack of any additional funding to act on these recommendations places enormous funding pressures on the PCTs. Canada's Common Drug Review (CDR) is a similarly centralized review body, but its listing recommendations are less binding than those issued by NICE. CDR has a clearly mandated advisory role that allows Canada's provincial and territorial governments to determine locally whether or not they will follow its listing recommendations. This role is similar to the status of NICE's guidance prior to 2002 (McMahon et al. 2006).
We reviewed Canadian and UK newspapers during a time in which public debate surrounding the use of Herceptin was prominently featured in each jurisdiction's newspapers. A 21-month study period (January 2005 to September 2006) was chosen to capture key events in the Herceptin access story chronology (i.e., the months leading up to the presentation of early trial results until a few months after the NICE policy recommendation for England and Wales).
Analytic framework
Hyping has been defined as “excessive publicity and the ensuing commotion” (Friend et al. 1997: 668), facilitated by extravagant claims or exaggerations (Thompson 1995). In the context of this study, publicity refers to the news media coverage (including its quantity and timing), claims refers to the messages conveyed through the story coverage and commotion refers to the opinions and behaviours that such coverage may have elicited.
We assessed the first two dimensions of hyping (publicity and claims) in the Canadian and UK newspaper coverage of the Herceptin access story by constructing two variables – intensity and framing. The intensity variable allows us to portray aspects of publicity, although it is unable to determine its excessiveness objectively. Commotion, the third dimension of media hyping, requires a media reception study and is beyond the scope of this study. The intensity variable refers to the timing and quantity of news coverage over the life of the story. Framing refers to the way in which issues are presented in the news: “framing is the subtle selection of certain aspects of an issue by the media to make them more important” (Dearing and Rogers 1996: 64). This variable was assessed using one general story framing variable and three theme-specific framing variables. Based on our analytic variables, coverage that hyped the Herceptin access story would have preceded publication of the full trial results, framed the story in patient-centric ways, exaggerated the benefits of the drug and/or downplayed patient risks and societal costs associated with funding the drug.
Sample
We chose a purposive sample of seven newspapers (three UK and four Canadian) to reflect a cross-section of national-level print news media with different readerships (e.g., socio-economic status, gender, age), political orientations (left, centrist, right) and multiple targets of influence (e.g., policy elites and general public). The UK newspaper sample included the Guardian, the Daily Mail and the Financial Times. The Canadian sample, incorporating French- and English-language newspapers, included the Globe and Mail, the Toronto Star, La Presse and Le Devoir. Using the electronic databases Lexis-Nexis and Factiva, we searched all newspapers for the keyword “Herceptin” (“Herceptine” for French-language newspapers) between January 1, 2005 and September 30, 2006. Only articles that discussed issues pertaining to the drug's efficacy, costs or funding approval process were included in the sample. Excluded articles typically focused on business perspectives (e.g., manufacturer share prices) or personal aspects of the story where only indirect references to Herceptin were made (e.g., life with breast cancer).
Data collection and analysis
Selected articles were coded using a quantitative codebook consisting primarily of nominal variables. The codebook was developed inductively through a trial coding process. This process involved two coders applying an initial set of codes to a small sample of clippings; codes were compared, revised and applied to another set of trial clippings to ensure coding consistency. All subsequent article coding was performed by a single coder. The codebook included the following basic variables of analytic interest: country of origin, headline, newspaper, author and date. Articles were also coded for four framing variables ( Table 1): one general frame, based on the perspective from which the story was written (i.e., individualistic/patient-focused vs. broader society), and three drug-specific thematic frames (efficacy, costs and the funding approval process). We hypothesized that stories that adopted an individualistic general frame would be more likely to employ thematic frames that exaggerated the effectiveness of Herceptin and downplayed the costs of approving the drug (i.e., salutary framing). Consequently, news clippings that employed these frames would be more likely to have hyped the story. All statistical analyses employed the Pearson chi-square test at the 95% level of confidence.
TABLE 1.
Framing codes
| Frame | Codes | Code descriptions | |
|---|---|---|---|
| General framing | Story perspective |
|
|
| Specific framing | Efficacy |
|
|
| Costs |
|
|
|
| Funding approval |
|
|
Results
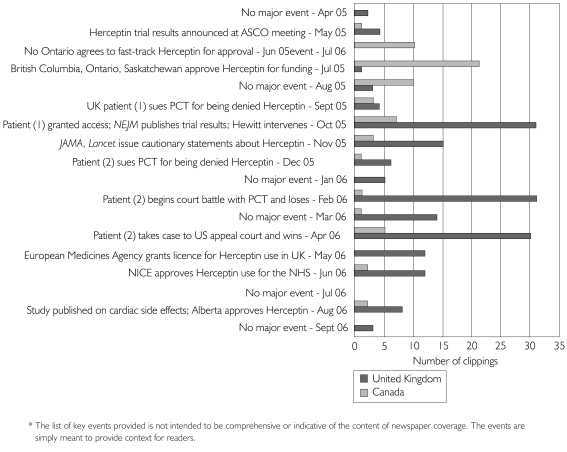
The word Herceptin or Herceptine was identified over the study period in 403 clippings. Of these, 248 (181 UK, 67 Canadian) met the study inclusion criteria (Table 2). The number of clippings retrieved for each country is reported for each of the months in which they appeared throughout the data collection period (Figure 1). Clipping numbers are reported alongside key events in the Herceptin story chronology, although the coverage in any given month does not exclusively correspond to these events. Key reported events were scientific, e.g., debate over clinical practice changes and safety concerns (Anonymous 2005; Grove 2005; Hortobagyi 2005; Wilson 2005); political, e.g., intervention from NHS Health Secretary Patricia Hewitt (Meikle 2005); legal, e.g., UK patient suing her PCT (Cook 2005); and policy related, e.g., Ontario government announcing funding (Priest 2005e). Figure 1 reveals that over two-thirds (67%) of the Canadian coverage preceded publication of the trial results in scientific journals (Piccart-Gebhart et al. 2005; Romond et al. 2005). Much of the Canadian coverage occurred over the summer months immediately following the May ASCO scientific meeting and reported concerns about patients experiencing difficulties accessing the drug (Priest 2005a,b,d). These stories were swiftly followed by successive provincial government funding announcements (Hume 2005; Priest 2005e). In contrast, only 8% of the UK coverage preceded the publication of the trial results; a large proportion of the news coverage coincided with the publication of the results in October 2005, including editorials calling for “cooler heads to prevail” regarding Herceptin's benefits (Cookson 2005), as well as high-profile legal (Tait 2006; Wheldon 2006) and political events (Boseley 2006; Jackson 2006).
TABLE 2.
Framing differences between Canadian and UK newspapers
| Canadian newspapers (Total N=67) | UK newspapers (Total N=181) | Chi-squared analysis of Canada-UK totals | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frame | Variable | The Globe and Mail (N=40) | The Toronto Star (N=10) | La Presse (N=14) | Le Devoir (N=3) | Total (%) | The Guardian (N=71) | The Financial Times (N=28) | The Daily Mail (N=82) | Total (%) | p-value |
| Story perspective (N=248) | Individualistic | 29 | 7 | 8 | 3 | 47 (70.1) | 48 | 14 | 64 | 126 (69.6) | 0.935 |
| Societal | 11 | 3 | 6 | 0 | 20 (29.9) | 23 | 14 | 18 | 55 (30.4) | ||
| Efficacy (N=197) | Salutary | 22 | 5 | 7 | 1 | 35 (62.5) | 34 | 11 | 52 | 97 (68.8) | 0.397 |
| Cautionary | 13 | 3 | 4 | 1 | 21 (37.5) | 20 | 8 | 16 | 44 (31.2) | ||
| Costs (N=175) | Salutary | 9 | 0 | 1 | 0 | 10 (25.0) | 17 | 3 | 36 | 56 (41.5) | <0.001 |
| Cautionary | 6 | 4 | 1 | 0 | 11 (27.5) | 30 | 13 | 13 | 56 (41.5) | ||
| Neutral | 12 | 3 | 3 | 1 | 19 (47.5) | 9 | 1 | 13 | 23 (17.0) | ||
| Funding Approval (N=200) | Timeliness | 13 | 4 | 2 | 0 | 19 (45.2) | 15 | 7 | 15 | 37 (23.4) | 0.017 |
| Equity | 13 | 3 | 1 | 0 | 17 (40.5) | 36 | 11 | 50 | 97 (61.4) | ||
| Undemocratic | 5 | 1 | 0 | 0 | 6 (14.3) | 13 | 8 | 3 | 24 (15.2) | ||
FIGURE 1.
Monthly coverage in Canada and the UK (tracked against key events*)
Our framing analysis revealed similarities and differences in the reporting between the two countries (Table 2). Close to 70% of stories in both countries adopted an individualistic story frame. Similarly, close to two-thirds of stories in both countries framed drug efficacy in salutary terms. In contrast, between-country comparisons of the framing of costs revealed statistically significant differences. UK stories were polarized and tended to adopt either a salutary (42%) or a cautionary frame (42%), while Canadian stories were more likely to present the drug costs neutrally (48%). Funding approval process framing also differed significantly between the two countries. Timeliness of access was more often featured in Canadian stories (45% vs. 23%), while the UK coverage focused on equity of access (61% vs. 41%).
Individual, newspaper-specific findings point to within-country differences. In Canada, the Globe and Mail generated the greatest proportion of articles with an individualistic general story frame. Compared to the other Canadian newspapers, the Globe and Mail's coverage was also more likely to frame Herceptin's effectiveness and costs in salutary terms. Among the UK papers, the Daily Mail tended to frame its coverage in a similar manner to Canada's Globe and Mail, while the Financial Times offered the most moderated or cautionary coverage of the “Herceptin access story” among the UK sample.
Our hypothesis that stories adopting individualistic story frames would be associated with salutary, theme-specific frames was confirmed by our findings (Table 3). When the UK and Canadian data were combined, stories that were generally framed from a societal rather than an individualistic perspective were more likely to cautiously frame the drug's efficacy (52% vs. 27%) and costs (78% vs. 18%). In addition, stories with general societal frames were less likely to frame the funding approval process as excessively long (13% vs. 35%).
TABLE 3.
Relationships between general framing (story perspective) and specific framing (efficacy, costs, funding approval)
| Frame | Variable | Story perspective | p-value | |
|---|---|---|---|---|
| Individualistic (%) | Societal (%) | |||
| Efficacy (N=197) | Salutary | 108 (73.5) | 24 (48.0) | 0.001 |
| Cautionary | 39 (26.5) | 26 (52.0) | ||
| Costs (N=175) | Salutary | 57 (49.1) | 9 (15.3) | <0.001 |
| Cautionary | 21 (18.1) | 46 (78.0) | ||
| Neutral | 38 (32.8) | 4 (6.8) | ||
| Funding approval (N=200) | Timeliness of access | 48 (34.5) | 8 (13.1) | <0.001 |
| Equity of access | 84 (60.4) | 30 (49.2) | ||
| Undemocratic | 7 (5.0) | 23 (37.7) | ||
Discussion
Our findings highlight several themes that warrant discussion and further examination. First, what should we make of the differences in the intensity of the news coverage between the two countries depicted in Figure 1? The Canadian story reached a crescendo in the summer of 2005 following the presentation of the trial results at the May ASCO scientific meeting. It then lost considerable momentum before the trial results were published and critically reviewed by the scientific media in October and November 2005. In contrast, why didn't the UK newspapers feature the same international medical breakthrough story until the publication of the trial results in peer-reviewed journals later that Fall? One explanation is that the location of the medical meeting in North America may have inhibited the flow of information to other parts of the world, although the globalization of media communications and the international profile of this particular scientific meeting challenge this explanation. Alternatively, perhaps there are systematic differences between journalistic practices in Canada and the UK when it comes to the reporting of medical news stories. Or perhaps other UK news stories were privileged over this one. The media studies literature offers limited guidance in interpreting these results. More robust explanatory analyses of significant timing discrepancies for the same major medical breakthrough news story are needed, particularly when the policy decisions that follow closely on the heels of these stories pre-empt the processes of peer review and scientific deliberation.
Our framing results offer some preliminary insights into the role of political and institutional context in shaping news reporting. Both countries adopted primarily individualistic general story frames and framed the drug's efficacy in salutary terms, suggesting that the framing of these types of stories is independent of jurisdictional context. These results support prior media studies, which have documented similar approaches towards individualistic news reporting and the hyping of medical breakthrough stories (Moynihan et al. 2000; Ransohoff and Ransohoff 2001; Bubela and Caulfield 2004). The differences between the two countries in their framing of costs and the funding approval processes suggest that policy context plays a role in shaping news reporting. The tendency of UK journalists to weigh in more definitively on the cost issues associated with Herceptin suggests greater willingness within the UK mainstream news media to publicly scrutinize issues related to cost containment, opportunity costs and cost-effectiveness of new cancer therapies. Moreover, differences in the framing of the news coverage of the funding approval process between the two countries reflect their different political and institutional contexts for decision-making. In the United Kingdom, news reporting concentrated on longstanding “post-code lottery” concerns raised about the NHS (Chapman 2006; Hall 2006), while the focus on timeliness in Canadian coverage reflected the dominance of this theme in Canadian health policy rhetoric (Lloyd 2005; Priest 2005c,d).
Based on our conceptualization of hyping, our intensity and framing data lead us to conclude that the Canadian version of the Herceptin access story was indeed hyped relative to that of the United Kingdom. Most of the coverage (67%) preceded the publication of the trial results in a major medical journal, understated the preliminary nature of the findings and provided decision-makers, patients and the general public with inadequate information upon which to base informed opinions on the issue. In addition, most of the Canadian coverage framed the efficacy of Herceptin in salutary terms (i.e., exaggerated the benefits to patients) (63%), and framed the costs of approving Herceptin in either salutary or neutral terms (i.e., downplayed the costs to society) (73%). The funding approval framing contributed equally to hyping in both countries, by playing up politically charged issues that resonate with their respective publics. That the theme-specific salutary frames were significantly associated with individualistic framing underscores our conclusion that this story had been hyped in Canadian coverage. While much of the UK coverage framed the story in a similar manner, the timing of the coverage (following the publication of scientific studies and editorials) provided less of a vehicle for story hyping than that observed in the Canadian media.
Limitations
There are several limitations to our study. First, we were unable to draw any causal inferences about the relationship between the media coverage and the policy events. The bestowing of a national newspaper award for reporting that exerts a major influence over public policy suggests that at least some of the Herceptin story coverage in Canada likely influenced funding decisions (Moore 2006). But without a detailed documentation of the precise chain of events, we can only speculate about these relationships. Moreover, we have nothing more than anecdotal accounts about how the story played to a broad range of readers, the extent to which various publics (either mass or attentive) were aware of the story, the degree of enlightenment, anxiety or confusion it may have prompted among patients and the types of behaviour that ultimately resulted. If Canadian public policy was influenced by the coverage of this story in the news, surely such decisions, which result in significant investments and disinvestments of public resources, deserve to be informed by something more than the presumed impact of media stories on audiences. We encourage future media–policy studies to examine the “commotion” arising from these stories, which was beyond the scope of this study.
Second, the small and uneven samples for each country presented challenges to the statistical analysis. We could not make any inferences about what would constitute an appropriate quantity of coverage for this story, and thus could not adequately measure the “excessiveness” dimension of hyping. Our sample size prohibited us from conducting cross-newspaper or within-country statistical analyses. Information on clipping type (e.g., news, column, editorial) and author type (e.g., reporter, columnist, editor) was often ambiguous or absent from the electronic databases, preventing us from conducting deeper analyses of these variables. While the Globe and Mail is a national newspaper, the remaining three Canadian newspapers in our sample are provincially based, in Ontario and Quebec. Our findings would have been more robust had we been able to compare the framing of this particular story with previous stories to determine whether these stories are being covered more neutrally now than in the past. Finally, we did not assess the accuracy of the news coverage with respect to the reporting of the clinical trial findings; we chose to focus on the analysis of “news hyping,” which has received less attention from health policy researchers despite its potential role in shaping key policy decisions.
Conclusions
Our findings demonstrate that reporting of a major medical news access story, drawing from the same evidence base and leading to comparable policy outcomes, can vary between jurisdictions. These differences can be revealed in both the timing and quantity of the coverage. Some patterns of framing transcend jurisdictional boundaries (e.g., general story frame, drug efficacy), while others reflect different journalistic practices and emphases on dominant political rhetoric within respective jurisdictions (e.g., costs and drug approval processes). As health policy access stories continue to proliferate in the health news media, media–policy research studies need to move beyond documenting what is in the news to examine more rigorously why and how these health policy news stories are being reported, and the consequent effects on public attitudes towards, understanding of and engagement in these health policy debates.
Contributor Information
Julia Abelson, McMaster University, Hamilton, ON.
Patricia A. Collins, Simon Fraser University, Vancouver, BC.
References
- Abraham C. Cancer Clinic Opens the Door for Private Care. The Globe and Mail. 2005. Aug 22, p. A1.
- Anonymous Herceptin and Early Breast Cancer: A Moment for Caution. Lancet. 2005;366:1673. doi: 10.1016/S0140-6736(05)67670-2. [DOI] [PubMed] [Google Scholar]
- Arvai J.L., Mascarenhas M.J. Print Media Framing of the Environmental Movement in a Canadian Forestry Debate. Environmental Management. 2001;27(5):705–14. doi: 10.1007/s002670010181. [DOI] [PubMed] [Google Scholar]
- Bartlett C., Sterne J., Egger M. What Is Newsworthy? Longitudinal Study of the Reporting of Medical Research in Two British Newspapers. British Medical Journal. 2002;325(7355):81–84. doi: 10.1136/bmj.325.7355.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benelli E. The Role of Media in Steering Public Opinion on Healthcare Issues. Health Policy. 2003;63(2):179–86. doi: 10.1016/s0168-8510(02)00064-7. [DOI] [PubMed] [Google Scholar]
- Boseley S. Charities Welcome Draft Approval for Breast Cancer Drug. The Guardian. 2006. Jun 9, p. 6.
- Braun S. The History of Breast Cancer Advocacy. Breast Journal. 2003;9:S101–3. doi: 10.1046/j.1524-4741.9.s2.13.x. [DOI] [PubMed] [Google Scholar]
- Brodie M., Brady L., Altman D.E. Media Coverage of Managed Care: Is There a Negative Bias? Health Affairs. 1998;17(1):9–25. doi: 10.1377/hlthaff.17.1.9. [DOI] [PubMed] [Google Scholar]
- Bubela T.M., Caulfield T.A. Do the Print Media ‘Hype’ Genetic Research? A Comparison of Newspaper Stories and Peer-Reviewed Research Papers. Canadian Medical Association Journal. 2004;170:1399–407. doi: 10.1503/cmaj.1030762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Institute for Health Information (CIHI) Drug Expenditure in Canada, 1985 to 2007. Ottawa: Author; 2008. [Google Scholar]
- Cassels A., Hughes M.A., Cole C., Mintzes B., Lexchin J., McCormack J.P. Drugs in the News: An Analysis of Canadian Newspaper Coverage of New Prescription Drugs. Canadian Medical Association Journal. 2003;168:1133–37. [PMC free article] [PubMed] [Google Scholar]
- Cassels A., Lexchin J. How Well Do Canadian Media Outlets Convey Medical Treatment Information? Initial Findings from a Year and a Half of Media Monitoring by Media Doctor Canada. Open Medicine. 2008;2(1):20–23. [PMC free article] [PubMed] [Google Scholar]
- Chapman J. Postcode Lottery ‘Denies Cancer Victims Best Care’. The Daily Mail. 2006. Jan 26, p. 27.
- Chen X., Siu L.L. Impact of the Media and the Internet on Oncology: Survey of Cancer Patients and Oncologists in Canada. Journal of Clinical Oncology. 2001;19:4291–97. doi: 10.1200/JCO.2001.19.23.4291. [DOI] [PubMed] [Google Scholar]
- Collier J. Panorama: Herceptin: Wanting the Wonder Drug. British Medical Journal. 2006;332(7537):368. [Google Scholar]
- Collins P., Abelson J., Pyman H., Lavis J. Are We Expecting Too Much from Print Media: An Analysis of Newspaper Coverage of the 2002 Canadian Healthcare Reform Debate. Social Science and Medicine. 2006;63:89–112. doi: 10.1016/j.socscimed.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Cook E. Cancer Victim Sues to Get Wonder Drug on the NHS. The Daily Mail. 2005. Sep 19, p. 5.
- Cookson C. Medical Opinion Split on Use of Herceptin for Early Breast Cancer. The Financial Times. 2005. Nov 11, p. 6.
- Cooper C.P., Yukimura D. Science Writers' Reactions to a Medical ‘Breakthrough’ Story. Social Science and Medicine. 2002;54:1887–96. doi: 10.1016/s0277-9536(01)00160-5. [DOI] [PubMed] [Google Scholar]
- Dearing J.W., Rogers E.M. Thousand Oaks, CA: Sage Publications; 1996. Agenda-Setting. [Google Scholar]
- Driedger S.M., Eyles J.D. Different Frames, Different Fears: Communicating about Chlorinated Drinking Water and Cancer in the Canadian Media. Social Science and Medicine. 2003;56:1279–93. doi: 10.1016/s0277-9536(02)00128-4. [DOI] [PubMed] [Google Scholar]
- Friend D., Keelet J., Liebman D., Sutherland F., editors. Canadian Dictionary of the English Language. Scarborough, ON: ITP Nelson; 1997. [Google Scholar]
- Geller G., Bernhardt B.A., Holtzman N.A. The Media and Public Reaction to Genetic Research. Journal of the American Medical Association. 2002;287:773. [PubMed] [Google Scholar]
- Grove M.L. Adjuvant Trastuzumab for Breast Cancer: An Increasingly Common Ethical and Economic Conundrum. British Medical Journal. 2005;331(7526):1202. doi: 10.1136/bmj.331.7526.1202-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S. English Women Lose Out in Herceptin's ‘Postcode Lottery’. The Guardian. 2006. Apr 10, p. 5.
- Hortobagyi G.N. Trastuzumab in the Treatment of Breast Cancer. New England Journal of Medicine. 2005;353(16):1734–36. doi: 10.1056/NEJMe058196. [DOI] [PubMed] [Google Scholar]
- Hume M. BC Agrees to Pay for Vaunted Cancer Drug. The Globe and Mail. 2005. Jul 12, p. A6.
- Jackson A. Decision Awaited on Breast Cancer Drug. The Financial Times. 2006. May 25, p. 4.
- Keidan J. Sucked into the Herceptin Maelstrom. British Medical Journal. 2007;334(7583):18. doi: 10.1136/bmj.39080.481551.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondro W., Sibbald B. Patient Demand and Politics Push Herceptin Forward. Canadian Medical Association Journal. 2005;173(4):347–48. doi: 10.1503/cmaj.050883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd D. Why the Drug Delay? The Globe and Mail. 2005. Jul 14, p. A16.
- Mayor S. NICE Approves Trastuzumab for Early Stage Breast Cancer. British Medical Journal. 2006;332:1409. doi: 10.1136/bmj.332.7555.1409-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon M., Morgan S., Mitton C. The Common Drug Review: A Nice Start for Canada? Health Policy. 2006;77(3):339–51. doi: 10.1016/j.healthpol.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Meikle J. Hewitt Clears Way for Use of Costly Breast Cancer Drug. The Guardian. 2005. Oct 26, p. 4.
- Moore O. The Globe Wins Michener Award. The Globe and Mail. 2006. Apr 12, p. A8.
- Moynihan R., Bero L., Ross-Degnan D., Henry D., Lee K., Watkins J., Mah C., Soumerai S.B. Coverage by the News Media of the Benefits and Risks of Medications. New England Journal of Medicine. 2000;342:1645–50. doi: 10.1056/NEJM200006013422206. [DOI] [PubMed] [Google Scholar]
- Niederlaender E. Luxembourg: Eurostat; 2006. Causes of Death in the EU. [Google Scholar]
- Piccart-Gebhart M.J., Procter M., Leyland-Jones B., Goldhirsch A., Untch M., Smith I., Gianni L., Baselga J., Bell R., Jackisch C., Cameron D., Dowsett M., Barrios C.H., Steger G., Huang C.S., Andersson M., Inbar M., Lichinitser M., Lang I., Nitz U., Iwata H., Thomssen C., Lohrisch C., Suter T.M., Ruschoff J., Suto T., Greatorex V., Ward C., Straehle C., McFadden E., Dolci M.S., Gelber R.D. Trastuzumab after Adjuvant Chemotherapy in Her2-Positive Breast Cancer. New England Journal of Medicine. 2005;353(16):1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- Priest L. New Cancer Drug Limited to Few. The Globe and Mail. 2005a. Jun 7, p. A1.
- Priest L. A Case of Growing Impatience. The Globe and Mail. 2005b. Jun 8, p. A1.
- Priest L. Ontario Will Fast-Track Breast Cancer Drug. The Globe and Mail. 2005c. Jun 24, p. A1.
- Priest L. Cancer-Drug Delay Could Kill Women, Study Says. The Globe and Mail. 2005d. Jun 27, p. A3.
- Priest L. Ontario Will Make Herceptin Widely Available. The Globe and Mail. 2005e. Jul 22, p. A1.
- Ransohoff D.F., Ransohoff R.M. Sensationalism in the Media: When Scientists and Journalists May Be Complicit Collaborators. Effective Clinical Practice. 2001;4:185–88. [PubMed] [Google Scholar]
- Romond E. Advances in Monoclonal Antibody Therapy for Breast Cancer: Joint Analysis of Nsabp-B-31 and Ncctg-N9831.. Paper presented at the 2005 Association of Clinical Oncologists Annual Meeting (Virtual Meeting); Orlando, Florida. 2005. [Google Scholar]
- Romond E.H., Perez E.A., Bryant J., Suman V.J., Geyer C.E., Jr., Davidson N.E., Tan-Chiu E., Martino S., Paik S., Kaufman P.A., Swain S.M., Pisansky T.M., Fehrenbacher L., Kutteh L.A., Vogel V.G., Visscher D.W., Yothers G., Jenkins R.B., Brown A.M., Dakhil S.R., Mamounas E.P., Lingle W.L., Klein P.M., Ingle J.N., Wolmark N. Trastuzumab Plus Adjuvant Chemotherapy for Operable Her2-Positive Breast Cancer. New England Journal of Medicine. 2005;353(16):1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- Scheule D. Framing as a Theory of Media Effects. Journal of Communication. 1999;49:103–22. [Google Scholar]
- Schwartz L.M., Woloshin S. The Media Matter: A Call for Straightforward Medical Reporting. Annals of Internal Medicine. 2004;140:226–28. doi: 10.7326/0003-4819-140-3-200402030-00015. [DOI] [PubMed] [Google Scholar]
- Schwartz L.M., Woloshin S., Baczek L. Media Coverage of Scientific Meetings: Too Much, Too Soon? Journal of the American Medical Association. 2002;287(21):2859–63. doi: 10.1001/jama.287.21.2859. [DOI] [PubMed] [Google Scholar]
- Schwitzer G. How the Media Left the Evidence Out in the Cold. British Medical Journal. 2003;326:1403–4. [Google Scholar]
- Seale C. Health and Media: An Overview. Sociology of Health and Illness. 2003;25:513–31. doi: 10.1111/1467-9566.t01-1-00356. [DOI] [PubMed] [Google Scholar]
- Smith D.E., Wilson A.J., Henry D.A. Monitoring the Quality of Medical News Reporting: Early Experience with Media Doctor. Medical Journal of Australia. 2005;183(4):190–93. doi: 10.5694/j.1326-5377.2005.tb06992.x. [DOI] [PubMed] [Google Scholar]
- Smith M. Oh, Canada: Public Outcry Pushed Demand for Trastuzumab for Early-Stage Breast Cancer. Journal of the National Cancer Institute. 2005;97(18):1327. doi: 10.1093/jnci/dji327. [DOI] [PubMed] [Google Scholar]
- Statistics Canada. Age-Standardized Mortality Rates by Selected Causes, by Sex, 2007. 2004 Retrieved January 5, 2009. < http://www40.statcan.ca/l01/cst01/health30a.htm>.
- Tait N. Cancer Sufferer Loses Drug Fight. The Financial Times. 2006. Feb 16, p. 5.
- Thomas P. Herceptin: Clinical Trial by Media – It Doesn't Cure Cancer, It Has Worrying Adverse Effects, and Is Very Expensive. So How Did Herceptin Become the Latest Breast Cancer Miracle Drug? Ecologist. 2006;36(7):14–21. [Google Scholar]
- Thompson D., editor. The Concise Oxford Dictionary. 9th ed. New York: Oxford University Press; 1995. [Google Scholar]
- Tuma R.S. Trastuzumab Trials Steal Show at ASCO Meeting. Journal of the National Cancer Institute. 2005;97(12):870–71. doi: 10.1093/jnci/97.12.870. [DOI] [PubMed] [Google Scholar]
- Wheldon J. Mother Who Was Refused New Cancer Drug Begins Court Battle. The Daily Mail. 2006. Feb 6, p. 20.
- Wilson E.C.F. Trastuzumab for Early Breast Cancer Raises Important Issues. British Medical Journal. 2005;331(7523):1023. doi: 10.1136/bmj.331.7523.1023-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloshin S., Schwartz L.M. Press Releases: Translating Research into News. Journal of the American Medical Association. 2002;287(21):2856–58. doi: 10.1001/jama.287.21.2856. [DOI] [PubMed] [Google Scholar]
- Woloshin S., Schwartz L.M. Media Reporting on Research Presented at Scientific Meetings: More Caution Needed. Medical Journal of Australia. 2006a;184(11):576–80. doi: 10.5694/j.1326-5377.2006.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Woloshin S., Schwartz L.M. What's the Rush? The Dissemination and Adoption of Preliminary Research Results. Journal of the National Cancer Institute. 2006b;98(6):372–73. doi: 10.1093/jnci/djj115. [DOI] [PubMed] [Google Scholar]