Abstract
The medial temporal cortex (MTC) has been implicated in the pathogenesis of pediatric major depressive disorder (MDD). Eleven MDD-case control pairs underwent proton magnetic resonance spectroscopic imaging. N-acetyl-aspartate was lower in left MTC (27%) in MDD patients versus controls. Lower N-acetyl-aspartate concentrations in MDD patients may reflect reduced neuronal viability.
Keywords: Depression, Hippocampus, N-Acetyl-Aspartate
1. INTRODUCTION
Current models of major depressive disorder (MDD) propose that neuronal dysfunction, loss, or damage in the hippocampus is a hallmark of the disease (Sapolsky, 2000). Consistent with reports in adults with MDD (Sheline et al., 1999; MacQueen et al., 2003), smaller hippocampal volumes have been noted in pediatric MDD (MacMaster and Kusumakar 2004; MacMaster et al., 2007) although conflicting reports do exist (Rosso et al., 2005). The aim of this study is to determine if hippocampal neuronal viability, as represented by medial temporal cortex (MTC) N-Acetyl-Aspartate (NAA) concentrations, is impaired in pediatric treatment-naïve MDD as compared to healthy controls. Studies of pediatric MDD patients minimize potential confounds such as treatment intervention and illness duration; and may clarify potential neurodevelopmental abnormalities related to the pathogenesis of the disease.
2. METHODS
2.1. Sample
Eleven treatment-naïve pediatric outpatients with MDD (10−16 years of age) and 11 healthy subjects were case-control matched for age, gender, weight, height, parental socioeconomic status and handedness. The Kiddie Schedule for Affective Disorders and Schizophrenia (Kaufman et al., 1997) was administered to all children and their parents (diagnostic criteria confirmed by DR, YM). Exclusion criteria for all patients and controls included any lifetime history of bipolar disorder, obsessive-compulsive disorder, tic disorder, psychosis, attention deficit hyperactivity disorder, conduct disorder, eating disorder, post-traumatic stress disorder, significant medical or neurological disease, autism, mental retardation or learning disability. Controls also had no axis I DSM-IV diagnosis or any first degree relative with DSM-IV axis I disorder. All studies adhered to the regulations of the Wayne State University Human Investigation Committee. Legal guardians gave written informed consent, and all participants provided written assent prior to initiating this investigation. Clinical measures are listed in table 1.
Table 1.
Demographic, Clinical and Imagine Information (Mean ± Standard Deviation)
| Item | Healthy Controls | MDD Patients | U | P-value |
|---|---|---|---|---|
| Age | 14.48 ± 1.82 | 14.13 ± 1.99 | 53.00 | 0.622 |
| Sex | 6 male, 5 female | 6 male, 5 female | - | - |
| Height | 65.64 ± 4.97 | 63.55 ± 5.54 | 41.00 | 0.199 |
| Weight | 141.64 ± 36.06 | 124.36 ± 27.03 | 39.00 | 0.182 |
| Socioeconomic Status | 2.91 ± 0.54 | 2.64 ± 0.67 | 45.50 | 0.259 |
| CDRS | - | 59.00 ± 9.52 | - | - |
| Hamilton Depression | - | 22.36 ± 8.36 | - | - |
| Hamilton Anxiety | - | 9.55 ± 5.41 | - | - |
| Duration of Illness (Years) | - | 0.69 ± 0.57 | - | - |
| Right N-Acetyl-Aspartate | 11.05 ± 3.17 | 10.45 ± 1.80 | 51.00 | 0.533 |
| Right Choline | 2.23 ± 0.98 | 1.93 ± 0.58 | 53.00 | 0.622 |
| Right Creatine | 8.57 ± 3.12 | 7.32 ± 1.91 | 56.00 | 0.768 |
| Left N-Acetyl-Aspartate | 12.30 ± 1.44 | 8.99 ± 2.63 | 16.00 | 0.003 |
| Left Choline | 2.50 ± 1.17 | 2.17 ± 0.32 | 53.00 | 0.622 |
| Left Creatine | 8.25 ± 2.18 | 7.54 ± 2.41 | 45.00 | 0.332 |
2.2. Imaging
Magnetic resonance (MR) studies were performed at the Children's Hospital of Michigan on a 1.5T Signa scanning system (General Electric, Horizon 5.7 software, Milwaukee, WI). All MR scans were reviewed to rule out any clinically significant abnormalities. The volumetric MRI and proton MR spectroscopic imaging (1H MRSI; TE = 272 msec, TR = 2300 msec, four 15mm thick slices, 2.5mm gaps; 24 × 24cm FOV, 32 × 32 phase encoding) acquisition and analysis procedures have been described previously (Farchione et al., 2002; Smith et al., 2003). A nominal voxel size of 0.8 mL was obtained (7.5mm-right/left × 7.5mm- anterior/posterior × 15mm-superior/inferior). 1H MRSI data was collected with a validated phantom replacement methodology using a robust multi-slice spin-echo spectroscopic imaging sequence with outer volume suppression originally developed at the National Institute of Health (Duyn and Moonen, 1993; Soher et al., 1996). The CSX and IMAX software packages (Dr. Peter Barker, Johns Hopkins University) were used for quantitative spectral processing as described in our earlier reports (Farchione et al., 2002; Smith et al., 2003). Regions of interest in left and right MTC were designated manually on the anatomic images (slice landmarks include: crus cerebri – typically medial to the hippocampus voxel, inferior colliculus, occipital horn of the lateral ventricle – more prominent some patients). The spectrum was automatically displayed with metabolites undergoing an automated peak non-linear least squares fitting procedure in the frequency domain. Processing parameters included: a magnitude calculation, a 3Hz exponential filter to remove residual water, and zero filling in the time domain by a factor of 4 (256 to 1024 points). Data was collected on absolute NAA, choline compounds (Cho) and creatine/phosphocreatine (Cr). Data is reported in institutional units. A single trained rater (A.R.; inter-class reliability 0.96−0.97), blind to any identifying information, performed all analysis.
2.3. Analysis
Due to the small sample size, non-parametric Mann-Whitney tests were used to examine differences between groups. Given the uniqueness of the sample, we believe that presentation of these pilot findings is warranted. To correct for multiple comparisons, alpha was set at p = 0.008. In an exploratory analysis, Spearman correlations between significantly different metabolites and clinical/demographic variables were also examined.
3. RESULTS
No differences were noted between the groups with regard to age, height, weight and socioeconomic status. Left MTC NAA was lower (27% lower) in treatment-naïve patients with MDD compared to healthy controls (U = 16.00, P = 0.003). No significant differences were noted for right MTC NAA. No differences were observed for Cho and Cr concentrations in either the right or left MTC (See table 1). No significant correlations were noted between left MTC concentrations and clinical scores or duration of illness. Left MTC NAA was demonstrated a trend with age in the controls (ρ = 0.58, P = 0.06) but not in the MDD patients (ρ = −0.19, P = 0.57). Using a Fisher r to z transformation, a trend for difference between the two group's correlations between MTC and age was noted (z = 1.71, P = 0.09). Interestingly, right MTC NAA was not correlated with age in the controls (ρ = −0.33, P = 0.33) but was in the MDD patients (ρ = 0.64, P = 0.04). These correlations differed significantly (z = −2.2, P = 0.03).
4. DISCUSSION
We found lower left MTC NAA in treatment-naïve patients with MDD compared to healthy controls. Left MTC NAA did not correlate with measures of depression or anxiety. These findings are consonant with reports in adults with MDD (Sheline et al., 1999; MacQueen et al., 2003) and pediatric MDD (MacMaster and Kusumakar 2004; MacMaster et al., 2007) noting smaller hippocampal volumes in MDD. One hypothesized mechanism that may be responsible for lower NAA in MDD may be a cortisol induced neuronal toxicity (Sapolsky, 2000). Nocturnal cortisol is elevated in pediatric MDD (Dahl et al., 1991; Kutcher et al., 1991) and cortisol can cause neuronal death and injury, especially in the hippocampus (Sapolsky, 2000). Further studies using concurrent imaging and endocrine markers are warranted. Our findings are consistent with reports noting left sided abnormalities in MDD (Soares and Mann 1007). The lack of a correlation between age and MTC NAA in the MDD patients (while present in controls) is interesting, albeit preliminary given the cross-sectional study design. However, smaller MTC NAA concentrations may not be a risk factor for MDD. Hippocampal NAA changes may also be a result of the illness, with repeated episodes causing a reduction in neuronal viability (Sheline et al., 1999; MacQueen et al., 2003). It should be noted that duration of illness did not correlate with left MTC NAA. Additionally, these findings may not be specific to MDD, as many other conditions demonstrate changes to hippocampal structure and chemistry (i.e. Blasi et al., 2004). Other limitations of this study include the small sample size, possibility of type II error, and the limited ability to compose a voxel solely of hippocampal tissue. Furthermore, the Mann-Whitney does not have utility to show a group by side interaction, limiting this study further. This report provides fresh evidence of left hippocampal dysfunction in treatment naïve pediatric MDD.
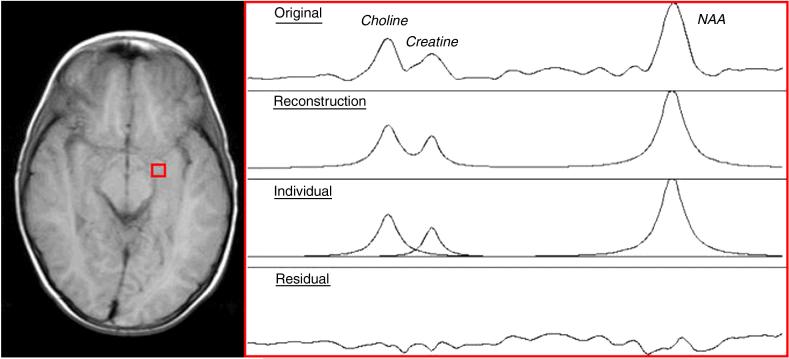
Figure 1.
Voxel placement (left) and sample spectra (right)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Blasi G, Bertolino A, Brudaglio F, Sciota D, Altamura M, Antonucci N, Scarabino T, Weinberger DR, Nardini M. Hippocampal neurochemical pathology in patients at first episode of affective psychosis: a proton magnetic resonance spectroscopic imaging study. Psychiatry Research. 2004;131(2):95–105. doi: 10.1016/j.pscychresns.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Ryan ND, Puig-Antich J, Nguyen NA, al-Shabbout M, Meyer VA, Perel J. 24-hour cortisol measures in adolescents with major depression: a controlled study. Biological Psychiatry. 1991;30:25–36. doi: 10.1016/0006-3223(91)90067-v. [DOI] [PubMed] [Google Scholar]
- Duyn JH, Moonen CT. Fast proton spectroscopic imaging of human brain using multiple spin-echoes. Magnetic Resonance in Medicine. 1993;30:409–414. doi: 10.1002/mrm.1910300403. [DOI] [PubMed] [Google Scholar]
- Farchione TR, Moore GJ, Rosenberg DR. Proton magnetic resonance spectroscopic imaging in pediatric major depression. Biological Psychiatry. 2002;52:86–92. doi: 10.1016/s0006-3223(02)01340-9. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kutcher S, Malkin D, Silverberg J, Marton P, Williamson P, Malkin A, Szalai J, Katic M. Nocturnal cortisol, thyroid stimulating hormone, and growth hormone secretory profiles in depressed adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:407–414. doi: 10.1097/00004583-199105000-00009. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Kusumakar V. Hippocampal volume in early onset depression. BMC Medicine. 2004;2:2. doi: 10.1186/1741-7015-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMaster FP, Mirza Y, Szeszko PR, Kmiecik LE, Easter PC, Taormina SP, Lynch M, Rose M, Moore GJ, Rosenberg DR. Amygdala and hippocampal volumes in familial pediatric major depressive disorder. Biological Psychiatry. doi: 10.1016/j.biopsych.2007.05.005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, Yurgelun-Todd DA. Amygdala and hippocampus volumes in pediatric major depression. Biological Psychiatry. 2005;57:21–26. doi: 10.1016/j.biopsych.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Soares JC, Mann JJ. The anatomy of mood disorders--review of structural neuroimaging studies. Biological Psychiatry. 1997;41:86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biological Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. Journal of Neuroscience. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EA, Russell A, Lorch E, Banerjee SP, Rose M, Ivey J, Bhandari R, Moore GJ, Rosenberg DR. Increased medial thalamic choline found in pediatric patients with obsessive-compulsive disorder versus major depression or healthy control subjects: a magnetic resonance spectroscopy study. Biological Psychiatry. 2003;54:1399–405. doi: 10.1016/s0006-3223(03)00474-8. [DOI] [PubMed] [Google Scholar]
- Soher BJ, Hurd RE, Sailasuta N, Barker PB. Quantitation of automated single-voxel proton MRS using cerebral water as an internal reference. Magnetic Resonance in Medicine. 1996;36:335–339. doi: 10.1002/mrm.1910360302. [DOI] [PubMed] [Google Scholar]