Abstract
More women are living with and surviving breast cancer, because of improvements in breast cancer care. Trastuzumab (Herceptin®▾) has significantly improved outcomes for women with HER2-positive tumours. Concerns about the cardiac effects of trastuzumab (which fundamentally differ from the permanent myocyte loss associated with anthracyclines) led to the development of cardiac guidelines for adjuvant trials, which are used to monitor patient safety in clinical practice. Clinical experience has shown that the trial protocols are not truly applicable to the breast cancer population as a whole, and exclude some women from receiving trastuzumab, even though they might benefit from treatment without long-term adverse cardiac sequelae. Consequently, five oncologists who recruited patients to trastuzumab trials, some cardiologists with whom they work, and a cardiovascular lead general practitioner reviewed the current cardiac guidelines in the light of recent safety data and their experience with adjuvant trastuzumab. The group devised recommendations that promote proactive pharmacological management of cardiac function in trastuzumab-treated patients, and that apply to all patients who are likely to receive standard cytotoxic chemotherapy. Key recommendations include: a monitoring schedule that assesses baseline and on-treatment cardiac function and potentially reduces the overall number of assessments required; intervention strategies with cardiovascular medication to improve cardiac status before, during, and after treatment; simplified rules for starting, interrupting and discontinuing trastuzumab; and a multidisciplinary approach to breast cancer care.
Keywords: trastuzumab, cardiac, monitoring, UK, recommendations
It has been known since the 1960s that some cancer treatments can affect heart function (Tan et al, 1967). Optimal breast cancer chemotherapy regimens include multiple agents, some with known cardiac side effects. Risk factors for the development of chemotherapy-related congestive heart failure (CHF) include cumulative anthracycline dose (Von Hoff et al, 1979) and increasing patient age (Doyle et al, 2005; Pinder et al, 2007). As the age of the population diagnosed with cancer in the Western world increases and survival improves, the development of treatment-related CHF becomes more important.
The addition of trastuzumab to conventional adjuvant (post-surgical) cytotoxic chemotherapy in individuals with HER2-positive disease has been one of the most important advances in the management of early breast cancer. Five trials recruiting more than 12 000 patients with breast cancer have shown a consistent 50% reduction in the risk of recurrence (Piccart-Gebhart et al, 2005; Romond et al, 2005; Joensuu et al, 2006; Slamon et al, 2006; Smith et al, 2007). However, in the pivotal registration trial of trastuzumab in metastatic breast cancer, there was an unexpectedly high incidence of CHF. This was most evident when trastuzumab was co-administered with doxorubicin (Seidman et al, 2002). Concern about cardiac safety influenced the design of trials evaluating trastuzumab in early breast cancer. In four of the five studies, trastuzumab followed anthracycline administration, and prospective cardiac function monitoring was included in all of the trials. A reduced incidence of heart failure was seen in these adjuvant (post-surgery) trials, compared with experience in the metastatic setting (Romond et al, 2005; Tan-Chiu et al, 2005; Smith et al, 2007; Suter et al, 2007).
The present UK guidance on the use of adjuvant trastuzumab (NICE, 2006a) is based on the protocol for the European registration trial (HERceptin Adjuvant; HERA) (Piccart-Gebhart et al, 2005; Smith et al, 2007). The trial excluded women with a left ventricular ejection fraction (LVEF) of <55% – a level above the lower limit of normal (LLN) in many UK institutions. As a result, current guidance excludes many women with normal cardiac function from receiving trastuzumab. Moreover, monitoring recommendations used serial assessment of LVEF, with interruption or cessation of trastuzumab in response to changes in LVEF or symptoms of CHF. There were no recommendations for the prevention or management of cardiac dysfunction. The limitations of the strict application of a trial protocol to the whole population are recognised in the NCRI guidelines for the use of adjuvant trastuzumab which ‘…are therefore precautionary and may well be simplified with further clinical experience’ (NCRI, 2005).
Aims and methodology
Five breast cancer oncologists who contributed to HERA from regional centres across the United Kingdom, some cardiologists from the same centres, and a cardiovascular lead general practitioner, met to consider the available data on the impact of trastuzumab on cardiac function. They reviewed the present trastuzumab cardiac guidelines, and drafted new recommendations based on the latest published data and their combined experience. These recommendations have been endorsed by the NCRI.
Chemotherapy-related cardiotoxicity in clinical practice
During chemotherapy delivery, the clinical focus is appropriately on acute, often symptomatic, but short-term adverse effects of drug treatment (Partridge et al, 2001). Acute cardiac effects include myocardial dysfunction, ischaemia, hypotension, hypertension, oedema, QT-interval prolongation, bradyarrhythmia, tachyarrhythmia, and thromboembolism. These toxicities are associated with specific drugs, doses, and schedules. In contrast, late-onset treatment-related cardiac toxicity can significantly affect long-term quality of life or even increase mortality. Late effects are under-reported, and can be missed completely if they occur after specialist follow-up is complete. The importance of the long-term needs of cancer survivors, particularly the elderly, is increasingly recognised (Erban and Lau, 2006; Department of Health, 2007).
Anthracycline-related cardiac toxicity is characterised by dose-related and irreversible loss of cardiac myocytes. There is reduced contractility in a proportion of the remaining cells (Table 1). Clinical cardiac failure has been associated with anthracyclines since 1973 (Lefrak et al, 1973). Characteristic permanent ultrastructural abnormalities of the myocardium are seen in endocardial biopsies taken from anthracycline-treated patients with vacuoles, myofibrillar disarray, and dropout on electron microscopy. The changes are present from the earliest administration of the drug, and have recently been referred to as Type I chemotherapy-related cardiac dysfunction (CRCD) (Ewer and Lippman, 2005). The left ventricle is mechanically disadvantaged and can be in a ‘stressed’ state, but function is optimised by compensatory mechanisms and there may be no clinically apparent manifestations for many years.
Table 1. Chemotherapy-related cardiac dysfunction (Ewer and Lippman, 2005).
| Type I (myocardial damage) | Type II (myocardial dysfunction) | |
|---|---|---|
| Characteristic agent | Doxorubicin | Trastuzumab |
| Clinical course, response to CRCD therapy | May stabilise, but underlying damage appears to be permanent and irreversible; recurrence in months or years may be related to sequential cardiac stress | High likelihood of recovery (to or near baseline cardiac status) in 2–4 months (reversible) |
| Dose effects | Cumulative, dose-related | Not dose-related |
| Mechanism | Free-radical formation, oxidative stress/damage | Blocked ErbB2 signalling |
| Ultrastructure | Vacuoles; myofibrillar disarray and dropout; necrosis (changes resolve over time) | No apparent ultrastructural abnormalities |
| Non-invasive cardiac testing | Decreased ejection fraction by ultrasound or nuclear determination: global decrease in wall motion | Decreased ejection fraction by ultrasound or nuclear determination: global decrease in wall motion |
| Effect of rechallenge | High probability of recurrent dysfunction that is progressive, may result in intractable heart failure and death | Increasing evidence for the relative safety of rechallenge; additional data needed |
| Effect of late sequential stress | High likelihood of sequential stress-related cardiac dysfunction | Low likelihood of sequential stress-related cardiac dysfunction |
Abbreviation: CRCD=chemotherapy-related cardiac dysfunction.
Reproduced with permission from the American Society of Clinical Oncology, from Ewer and Lippman, 2005.
The incidence and severity of clinically apparent cardiotoxicity are related to pre-existing medical conditions, previous cardiac exposure to radiation, and patient age (Tan et al, 1967; Doyle et al, 2005; Ng et al, 2006). There is also a clear relationship between anthracycline dose, and the incidence of cardiac dysfunction and clinical heart failure, which has led to restriction of the cumulative dose of anthracyclines administered (typically <450 mg m−2 for doxorubicin, <720 mg m−2 for epirubicin) (Von Hoff et al, 1979).
Cardiac effects of trastuzumab
The cardiac (myocyte) effects of trastuzumab differ fundamentally from those of anthracyclines; in particular, trastuzumab does not cause myocyte loss. In patients with trastuzumab-related cardiac dysfunction, myocytes appear histologically normal; changes may be seen only by using electron microscopy, in keeping with a reversible cardiomyopathy (Guarneri et al, 2006), and this has led to the classification of trastuzumab-related CRCDs as Type II (Table 1) (Ewer and Lippman, 2005; Guarneri et al, 2006), as opposed to the irreversible changes associated with anthracyclines (Type I CRCD).
There is evidence that trastuzumab-related left ventricular systolic dysfunction (LVSD) is mediated through the binding of trastuzumab to the extracellular domain of the HER2 protein present on cardiac myocytes, blocking ErbB2-ErbB4 signalling. This results in the disabling of an important cell-protective, growth-promoting pathway within the myocardium. The pathway is required for cell survival and continuing function, and seems to be stimulated when the myocardium encounters adverse haemodynamic or other stress, such as that associated with anthracyline therapy. Withdrawal of trastuzumab allows return of function of the pathway and reversal of LVEF decline, in contrast to the permanent myocyte dysfunction and loss caused by anthracyclines. This proposed mechanism is consistent with the increase in cardiac effects when trastuzumab is used concomitantly with anthracyclines.
The incidence of serious adverse events during trastuzumab monotherapy is low and the most frequently reported acute adverse effect of trastuzumab therapy is a hypersensitivity-like infusion reaction (Adamo et al, 2007; Herceptin PI, 2007). The antiproliferative side effects commonly associated with cytotoxic chemotherapy have not been reported. However, when trastuzumab was added to chemotherapy in the pivotal registration trial, CHF was reported in an unexpectedly high number of patients, prompting a detailed retrospective cardiac evaluation (Slamon et al, 2001). Anthracycline-naive patients received doxorubicin, and anthracycline-pretreated patients received paclitaxel, to avoid an excessive cumulative anthracycline dose. Paclitaxel monotherapy was associated with a 1% incidence of CHF; concurrent use of trastuzumab increased this to 13%. Patients receiving concurrent anthracycline and trastuzumab had a 27% incidence of symptomatic heart failure (16% NYHA Grade III/IV). Trastuzumab-associated LVSD was not dose-related, and appeared to improve with standard medical management including angiotensin-converting enzyme (ACE) inhibitors.
In adjuvant trials, there were stringent cardiac eligibility criteria, and trastuzumab was interrupted or discontinued in response to the development of CHF or following protocol-defined changes in LVEF. In a large North American study (NSABP B-31) (Rastogi et al, 2007), patients with HER2-positive, node-positive early breast cancer, and an LVEF >50% received 4 cycles of anthracycline-containing chemotherapy followed by 4 cycles of paclitaxel. Patients were randomised to receive trastuzumab for a total of 1 year, starting concurrently with paclitaxel. In the control arm (no trastuzumab), 10 of 872 (1.3%) patients with a normal post-anthracycline LVEF had confirmed cardiac events (9 CHF, 1 death), compared with 35 of 932 (3.9%) patients in the trastuzumab-treated arm (35 CHF, no deaths). Trastuzumab was discontinued because of cardiac dysfunction in 15.6% of patients. Age >50 years, prescription of antihypertensive medication, and post-anthracycline LVEF values of 50–54% (0.50–0.54) were all associated with an increased risk of CHF.
The European HERA trial evaluated the effects of using trastuzumab after completion of all chemotherapy; 94% of patients received anthracyclines. A post-chemotherapy LVEF of 55% was required in HERA, compared with 50% in the US study, and there was an in-built delay of several months between the completion of (cytotoxic) chemotherapy and initiation of trastuzumab (for either 1 or 2 years). No cardiac deaths occurred, the rate of severe heart failure was <1% (Smith et al, 2007; Suter et al, 2007), and only 4.3% of patients discontinued trastuzumab because of cardiac dysfunction (Suter et al, 2007). The reduction in cardiac event rate has been attributed to the higher baseline LVEF requirement and the protocol-driven delay between the end of cytotoxic therapy and start of trastuzumab (Smith et al, 2007). Experience from one US cancer centre showed a median time to decline in LVEF during trastuzumab therapy of 4.5 months (Ewer et al, 2005). After cessation of trastuzumab (37 of 38 patients), and commencement of standard treatment for LVSD (31 patients), the mean time to recovery of LVEF was 1.5 months.
Revised treatment and monitoring algorithm for trastuzumab
The current algorithm for monitoring LVEF and interrupting trastuzumab treatment was derived from the HERA clinical trial protocol (Figure 2). The high incidence of cardiac dysfunction seen in the preceding metastatic breast cancer trials led to stringent inclusion criteria, and many women with normal left ventricular function were excluded from the trial. There was no attempt to ameliorate cardiac risk factors or actively manage asymptomatic LVEF decline during the study, and the only permissible response was interruption or discontinuation of drug. There is greater understanding of the pathophysiology of trastuzumab-related LVSD, and in particular its potential reversibility. The clinical trial population does not map onto the whole population of women with HER2-positive breast cancer, some of whom, on the basis of arbitrarily set cardiac criteria, are excluded from receiving trastuzumab, even though they might benefit from treatment without long-term adverse cardiac sequelae. The algorithm has been re-evaluated to allow proactive intervention that facilitates the initiation and continuation of trastuzumab therapy, where it may previously have been omitted or discontinued.
Key recommendations
Baseline cardiac assessments before cytotoxic chemotherapy
Patients receiving chemotherapy may be considered to be at increased risk of developing cardiac dysfunction – Stage A heart failure in ACC/AHA guidelines (Bonow et al, 2005). The following assessments are recommended before initiating chemotherapy, for all patients with HER2-positive breast cancer. Although these recommendations relate only to HER2-positive tumours, similar pre-treatment assessments would be desirable in any patient for whom cytotoxic chemotherapy is being considered.
- Medical history
- ○ To determine previous cardiac events and risk factors.
- Physical examination
- ○ Blood pressure (hypertension is a potent and modifiable risk factor for the development of cardiac dysfunction, and should be assessed before each cycle of treatment – a blood pressure of >140/85 mmHg should be treated with an ACE inhibitor).
- ○ Auscultation of the heart to identify murmurs (significant valvular heart disease is a risk factor for cardiac dysfunction).
- ○ Signs of heart failure (raised venous pressure, crepitations over the lung fields or pedal oedema).
- 12-lead electrocardiogram (ECG) – looking for possible markers of structural heart disease including left ventricular damage/dysfunction
- ○ For arrhythmias (atrial fibrillation, atrial flutter, heart block).
- ○ For evidence of previous myocardial infarction (Q-waves, left bundle branch block).
- ○ For evidence of left ventricular hypertrophy.
- Left ventricular ejection fraction measurement using Echo or radionucleotide multiple-gated acquisition (MUGA) scan.
- ○ This baseline test is not recommended in present NCRI guidelines. The associated resource use is offset by a reduction in the requirement for testing during trastuzumab administration.
In routine clinical practice, LVEF can be measured with Echo or radionucleotide MUGA scan. Where the ECG is significantly abnormal at baseline, Echo is recommended whenever satisfactory images can be obtained, because it provides additional information on heart valve function.
Serial LVEF assessments are required to guide treatment, and a high degree of reproducibility is essential; MUGA has some advantages in this respect, but requires the use of significant ionising radiation (Healthcare Commission, 2000). The same monitoring modality should be used throughout the course of treatment and, where possible, this should also include the same operator, machine, and calculation algorithm. Each institution should establish a normal range for the methods used. Left ventricular ejection fraction is a dynamic physiological function varying from day to day depending on heart rate and loading conditions, but every effort must be made to provide an accurate and precise assessment of LVEF to guide clinical decisions about trastuzumab therapy, in the same way as this information is essential for implantable cardioverter defibrillator (NICE, 2006b) and cardiac resynchronisation therapies (NICE, 2006c). Clinicians interpreting LVEF values should consider confounding factors if they receive unexpected results.
Interventions at baseline
Referral to a cardiologist
This is recommended for patients who have evidence of LVSD, cardiac dysrhythmias, or structural heart disease, including evidence of previous infarction or significant modifiable cardiovascular risk factors, such as poorly controlled hypertension.
Modification of planned chemotherapy regimen
Assessment of baseline LVEF before chemotherapy in all patients informs the choice of cytotoxic regimen. Patients with low or borderline LVEF may benefit from a non-anthracycline-containing regimen. The combination of docetaxel and cyclophosphamide has been compared with doxorubicin and cyclophosphamide, and has shown at least equivalent efficacy (Jones et al, 2006). Prophylactic ACE inhibitor therapy may also be considered for such patients.
The use of ACE inhibitors to control hypertension
Hypertension is a potent modifiable risk factor for the development of heart failure during breast cancer treatment, with a statistically significant hazard ratio of 1.45 (95% CI, 1.39–1.52) (Pinder et al, 2007). Blood pressure should be measured in all breast cancer patients at routine oncology clinic visits, and where readings are above the second Joint British Societies’ guidelines (British Cardiac Society et al, 2005), target of <140/85 mmHg, they should be treated with an ACE inhibitor licensed for the treatment of heart failure (Table 2). Angiotensin-converting enzyme inhibitors are recommended as first-line antihypertensive agents. Trial data support their efficacy in reducing blood pressure, and for the prevention and treatment of left ventricular dysfunction and heart failure (SOLVD Investigators, 1992; AIRE Investigators, 1993; Moyé et al, 1994; Arnold et al, 2003). There is also direct trial evidence for the efficacy of ACE inhibitors in preventing the decrease in LVEF observed in patients after high-dose chemotherapy (Cardinale et al, 2006).
Table 2. Currently available ACE inhibitors licensed for heart failure, and their recommended dosing schedules (BNF, 2007).
| ACE inhibitor | Starting dose | Dose titration |
|---|---|---|
| Captopril | 6.25–12.5 mg twice | Maintenance: 25–50 mg twice daily |
| daily | Maximum: 150 mg daily in divided doses | |
| Cilazapril | 0.5 mg once daily | Maintenance: 2.5–5 mg once daily |
| Maximum: 5 mg once daily | ||
| Enalapril maleate | 2.5 mg once daily | Maintenance: 20 mg once daily |
| Maximum: 10–20 mg twice daily | ||
| Fosinopril sodium | 10 mg once daily | Maintenance: 10–40 mg once daily |
| Maximum: 40 mg once daily | ||
| Lisinopril | 2.5 mg once daily | Maintenance: 20 mg once daily |
| Maximum: 35 mg once daily | ||
| Perindopril | 2 mg once daily | Maintenance: 4 mg once daily |
| erbumine | Maximum: 4 mg once daily | |
| Quinapril | 2.5 mg once daily | Maintenance: 10–20 mg once daily |
| Maximum: 40 mg once daily | ||
| Ramipril | 1.25 mg once daily | Maintenance: 2.5–5 mg once daily |
| Maximum: 10 mg once daily |
It is recommended that dose titration and renal function monitoring be performed in primary care in accordance with current cardiac guidance (NICE, 2003). Patients with breast cancer whose hypertension cannot be controlled with standard pharmacological treatment should be referred to a specialist.
Lifestyle recommendations
Patients should be advised by their GP and oncologist about lifestyle changes that reduce their cardiovascular risk:
Smoking cessation.
- Improving diet.
- ○ Moderate alcohol consumption (up to 14 units a week for women – heavy alcohol consumption can both increase blood pressure and reduce cardiac function).
- ○ Reducing dietary salt.
- ○ Reducing fat.
- ○ Increasing fruit and vegetable consumption (five a day).
Increasing physical activity.
Weight loss where appropriate.
Management of cardiac function during trastuzumab
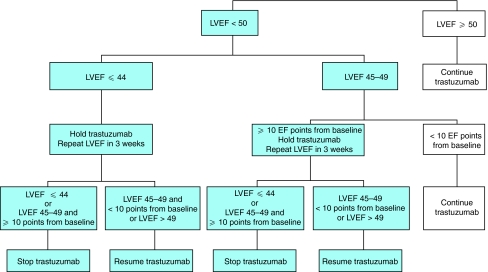
Use of the present algorithm for monitoring cardiac function in trastuzumab-treated patients (Figure 1) has resulted in a low incidence of clinical heart failure in routine practice. However, the algorithm has a number of limitations. Specifically, it:
Is susceptible to misinterpretation.
Requires the determination of LVEF with a precision and reproducibility, that cannot often be achieved in routine clinical practice.
Does not take account of the normal ranges for LVEF of different imaging modalities, in different institutions.
Requires a high frequency of monitoring compared with the risk of clinical heart failure.
Does not specify a pre-chemotherapy LVEF assessment as a baseline for the evaluation of cytotoxic drug-related cardiac damage and dysfunction.
Does not provide guidance for the optimisation of cardiac health before trastuzumab therapy.
Does not make recommendations on the treatment of patients with LVSD other than the interruption of trastuzumab therapy.
Does not facilitate successful rechallenge with trastuzumab.
Figure 1.
Current recommendations for cardiac monitoring in trastuzumab-treated patients (reproduced from Suter et al, 2007; online Appendix only). Reproduced with permission of the American Society of Clinical Oncology, from Suter et al, 2007.
Assessment of LVEF before trastuzumab treatment
Left ventricular ejection fraction should be further assessed in all patients after completion of chemotherapy and before initiating trastuzumab therapy (Figure 2). Some patients (7% in NASBP-B31) will experience a decrease in LVEF that precludes trastuzumab treatment (Romond et al, 2005). These patients are not eligible to commence trastuzumab and should be started on an ACE inhibitor and referred to a cardiologist. Repeat assessment of cardiac function should take place after 3 months (but before the time window for starting trastuzumab specified by NICE expires).
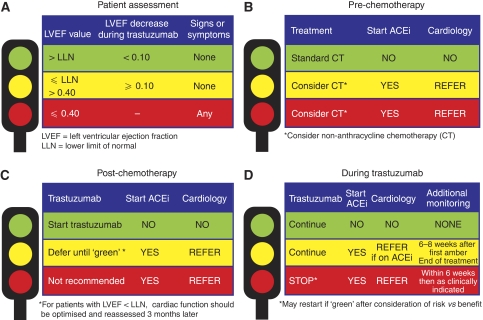
Figure 2.
Traffic light system to prevent, monitor, and manage cardiac events in patients undergoing cytotoxic chemotherapy. (A) Patient assessment during trastuzumab therapy; (B–D) indications for ACEi therapy and referral to a cardiologist before (B) and after (C) chemotherapy, and (D) during trastuzumab therapy, when additional cardiac assessments may also be required. ACEi=angiotensin-converting enzyme inhibitor.
A significant decrease in LVEF (e.g., 0.10 points) during the course of anthracycline chemotherapy is most likely to indicate a left ventricle that has been left in a damaged, haemodynamically compromised state, and is thus at increased susceptibility to trastuzumab. Prophylactic ACE inhibitor therapy may therefore be considered for such patients.
Initiation of trastuzumab therapy
Trastuzumab may be initiated in patients with LVEF above the LLN for the institution (Figure 2A and D).
Monitoring frequency
Routine LVEF monitoring is recommended at 4 and 8 months. A further assessment at the end of treatment is recommended for patients requiring cardiovascular intervention during treatment. The minimum number of LVEF assessments when following this recommendation is four, compared with five using the NCRI guidelines. Additional testing is required in patients who have LVSD, but the frequency of these additional tests is no more than in the present guidance. This slightly reduced frequency of monitoring reflects the more proactive approach to preventing cardiac events, and increased understanding of the time course and reversibility of trastuzumab-induced LVSD.
Symptomatic heart failure
Patients developing signs and symptoms of heart failure should have their trastuzumab treatment interrupted, have ACE inhibitor therapy initiated by the oncologist, and be referred to a cardiologist. Investigation and treatment is recommended in accordance with present guidelines (NICE, 2003; Bonow et al, 2005; Swedberg et al, 2005).
Asymptomatic decrease in LVEF
For the purposes of these recommendations, an LVEF of 0.40 or less has been taken to represent biologically important LVSD. This is in keeping with the many studies showing that at this level of LVEF, intervention with pharmacotherapy influences an otherwise adverse outcome.
If the LVEF decreases to ⩽0.40, trastuzumab should be interrupted, as significant LVSD has occurred. An ACE inhibitor should be started by the oncologist, and the patient should be referred to a cardiologist. Investigation and treatment is recommended in accordance with national and international guidelines on the management of CHF in adult (NICE, 2003; Bonow et al, 2005; Swedberg et al, 2005). The LVEF measurement should be repeated after 6–8 weeks. Trastuzumab may be re-initiated if the LVEF is restored to a level above the LLN.
If the LVEF decreases to below the LLN but >0.40, trastuzumab may be continued, but an ACE inhibitor should be initiated. If this decrease occurs despite pre-existing ACE inhibitor therapy, the patient should be referred to a cardiologist. Monitoring should be repeated after 6–8 weeks. This may require a cultural shift among senior oncologists, but the members of the group have embraced this change in practice, which is working well in their centres. In the past decade, ACE inhibitor therapy has become the standard of care in the treatment of hypertension, primary and secondary prevention of cardiovascular disease, heart failure, and renal impairment. There are robust trial data to support these treatments, which are now often initiated, and almost universally monitored and titrated, in primary care. Angiotensin-converting enzyme inhibitors are some of the most widely used drugs in modern cardiology. They are very effective in a wide range of cardiovascular conditions, including asymptomatic LVSD (Moyé et al, 1994), heart failure due to LVSD (CONSENSUS Trial Study Group, 1987; SOLVD Investigators, 1991), LVSD following a myocardial infarction (Pfeffer et al, 1992; AIRE Investigators, 1993; Torp-Pedersen and Køber, 1999), hypertension (Dahlöf et al, 2005), and stable coronary artery disease (Yusuf et al, 2000; Fox et al, 2003). Their use is now included in all the major national and international guidelines for the treatment of these conditions, both in primary and secondary care.
If the LVEF decreases by 0.10 points or more and remains above the LLN, trastuzumab may be continued, but an ACE inhibitor should be initiated. Monitoring should be repeated after 6–8 weeks. A decrease of 0.10 or more may suggest an increased risk of heart failure, and intervention with an ACE inhibitor is recommended to reduce this risk.
Traffic light system
Navigation through these recommendations may be facilitated by the adoption of a traffic light system (Figure 2).
A green light indicates LVEF above the LLN, no signs or symptoms of CHF and any trastuzumab-related LVEF decrease being <0.10.
An amber light indicates LVEF between the LLN and 0.40, with no signs or symptoms of CHF, or a trastuzumab-related LVEF reduction of 0.1 or more.
A red light indicates LVEF ⩽0.40 or symptoms and signs of cardiac failure.
Before chemotherapy (Figure 2B), green indicates go. Red or amber indicates careful consideration of decision to start chemotherapy, with consideration of non-anthracycline-containing regimens. Both amber and red are indications for the initiation of ACE inhibitors and referral to cardiology for the optimisation of cardiac function.
Post-chemotherapy (Figure 2C), green indicates go. Amber indicates defer until green. Red indicates that it is unlikely to be safe to start trastuzumab. Both amber and red are indications for the initiation of ACE inhibitors and referral to cardiology for the optimisation of cardiac function. It is recommended that LVEF is reassessed after 3 months, and that trastuzumab is not commenced unless LVEF is within normal limits at that point.
During trastuzumab (Figure 2D), green is an indication to continue treatment. Amber is also an indication to continue treatment, but patients should also be taking an ACE inhibitor. Patients who drop into the amber range while on an ACE inhibitor should be referred for a cardiology opinion. Red is an indication to interrupt trastuzumab, start on an ACE inhibitor if applicable, and refer for a cardiology opinion.
Patients whose trastuzumab is interrupted (i.e., red light) should not restart until LVEF is within the normal range (i.e., green light).
Monitoring procedures in early and metastatic breast cancer
These recommendations were primarily developed for use in patients with early breast cancer who are expected to complete a full 12-month course of trastuzumab therapy. In patients with metastatic breast cancer, trastuzumab is continued until disease progression, but the recommendations are still suitable for monitoring cardiac effects during the first few months of therapy. During trastuzumab therapy, patients with metastatic breast cancer should be monitored for 8 months according to our recommendations. Assuming no complications have occurred, cardiac monitoring should be performed at the discretion of the treating physician, after discussion with the patient, and in accordance with local guidelines. Patients with metastatic disease have a very different oncology risk profile to those receiving adjuvant treatment; the monitoring strategy and thresholds for treatment discontinuation should thus be individualised in consultation with the oncologist and cardiologist when appropriate.
Proposal for prospective audit
The primary aim of reviewing the cardiac monitoring guidance for trastuzumab was to re-evaluate the present guidelines, based on the latest safety data and extensive clinical experience using adjuvant trastuzumab. These recommendations consider the best available present evidence and the application of data from large cardiovascular trials. Data obtained from retrospective review of trastuzumab-treated patients will be compared with prospective audit data generated from the use of these new recommendations; a separate prospective audit of the recommendations is also planned. The oncologists who participated in the development of the recommendations have adopted the practices outlined in the treatment algorithm, and audit is already underway in their centres. Audit findings will be published as soon as sufficient follow-up data are available.
Conclusions
The present guidelines for cardiac monitoring in patients receiving trastuzumab reflect clinical trial design, rather than the whole population of patients with HER2-positive breast cancer. Experience gained through implementation of national guidance led oncologists, cardiologists, and a cardiovascular lead GP to review the evidence base for managing cardiac safety in trastuzumab-treated patients, and to draft revised recommendations. The proposed changes to the present NCRI guidelines are designed to promote a proactive approach to the management of cardiac function.
The key recommendations from this review are as follows:
Cardiac assessment, including LVEF measurement, should be performed before any chemotherapy.
Heart function measurement should be referenced to the local normal range for the modality used.
Management of cardiac risk factors including hypertension before the first cycle of chemotherapy.
Reassessment of LVEF after completing chemotherapy and before starting trastuzumab.
Repeat measurements should be performed after 4 and 8 months of trastuzumab treatment.
Simplified trastuzumab treatment interruption recommendations.
Clear advice on initiating an ACE inhibitor and when to consult a cardiologist.
Recommendations for restarting trastuzumab.
Guidance that applies to both early and metastatic breast cancer.
Protocols that promote a multidisciplinary approach to patient care.
These recommendations have the potential to improve the care of patients with HER2-positive breast cancer by increasing safe access to evidence-based treatment with proven disease-free survival and mortality benefits. Spacing of cardiac assessments and omission of the end of treatment of LVEF assessment in patients without evidence of cardiac dysfunction can reduce the overall number of LVEF assessments performed. We strongly advocate collaboration in audit across the United Kingdom to evaluate their effects.
As a first-in-class drug, trastuzumab raises the challenge of managing cardiac function in patients receiving anti-HER2 therapies. Other new targeted agents are also associated with cardiac concerns, and these recommendations may be suitable, with appropriate prospective audit, to monitor cardiac function with these other drugs.
Acknowledgments
These recommendations are endorsed by the National Cancer Research Institute, and have been reviewed by oncologists from regional cancer centres across the United Kingdom. In addition, Dr Thomas Suter, a leading European cardiologist who evaluated cardiac events in the HERA trial, and Dr Perry Elliott, Honorary Consultant Cardiologist at The Heart Hospital, University College London (UK), have advocated the practices outlined in these recommendations. Sophie Berry and David Hallett are acknowledged by the authors for their impartial writing, editorial, and administrative support during the preparation of these recommendations. The working group and the publication of its findings were funded with an educational grant from Roche Products Ltd (UK) to an independent medical communications provider. The discussions of the authors were independent of the funding organisation, which sought no control over the design or content of the subsequent recommendations.
Footnotes
Competing interests
Dr Alison Jones has received trial funding, hospitality and travel to conferences, honoraria for speaking at meetings and attending advisory boards, and payments for consultancy work from Roche. Dr Peter Canney has received consultancy and lecture fees from Pfizer, Sanofi-Aventis, and Roche. Dr Mark Verrill has received trial funding, hospitality and travel to conferences, honoraria for speaking at meetings and attending advisory boards, and payments for consultancy work from Roche. Dr Andrew Wardley has received trial funding, hospitality and travel to conferences, honoraria for speaking at meetings and attending advisory boards, and payments for consultancy work from Roche. Dr Peter Barrett-Lee has received honoraria from Sanofi-Aventis, GSK, Roche, Novartis, and Pfizer for consultancy, lectures and Advisory Board participation. Dr Marion Barlow has received travel expenses and honoraria from Roche for Advisory Boards, and a grant to audit trastuzumab-induced wall motion abnormalities from Roche. Dr Stephen Robb has received honoraria from Roche for lectures and travel expenses, and honoraria from Roche for attending the meetings required to develop these recommendations. Dr Chris Plummer has received travel expenses and honoraria from Roche for attending the meetings required to develop these recommendations. Dr Iain Gilmour has received travel expenses and honoraria from Roche for attending the meetings required to develop these recommendations.
References
- Acute Infarction Ramipril Efficacy (AIRE) Study Investigators (1993) Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. Lancet 342: 821–828 [PubMed] [Google Scholar]
- Adamo V, Franchina T, Adamo B, Ferraro G, Rossello R, Maugeri Saccà M, Scibilia C, Valerio MR, Russo A (2007) Safety and activity of trastuzumab-containing therapies for the treatment of metastatic breast cancer: our long-term clinical experience (GOIM study). Ann Oncol 18(Suppl. 6): vi11–vi15 [DOI] [PubMed] [Google Scholar]
- Arnold JM, Yusuf S, Young J, Mathew J, Johnstone D, Avezum A, Lonn E, Pogue J, Bosch J (2003) Prevention of heart failure in patients in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation 107: 1284–1290 [DOI] [PubMed] [Google Scholar]
- Bonow RO, Bennett S, Casey Jr DE, Ganiats TG, Hlatky MA, Konstam MA, Lambrew CT, Normand S-LT, Pina IL, Radford MJ, Smith AL, Warner Stevenson L (2005) ACC/AHA Clinical Performance Measures for Adults with Chronic Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures): endorsed by the Heart Failure Society of America. Circulation 112: 1853–1887 [DOI] [PubMed] [Google Scholar]
- British Cardiac Society; British Hypertension Society; Diabetes UK; HEART UK; Primary Care Cardiovascular Society; Stroke Association (2005) JBS 2: Joint British Societies’ guidelines on prevention of cardiovascular disease in clinical practice. Heart 91: v1–v52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Medical Association and the Royal Pharmaceutical Society of Great Britain. British National Formulary (BNF) 54 (2007). Pharmaceutical Press: Biggleswade, ISBN: 0853697361 [Google Scholar]
- Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, Martinelli G, Veglia F, Fiorentini C, Cipolla CM (2006) Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 114: 2474–2481 [DOI] [PubMed] [Google Scholar]
- CONSENSUS Trial Study Group (1987) Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study. N Engl J Med 316: 1429–1435 [DOI] [PubMed] [Google Scholar]
- Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O′Brien E, Östergren J (2005) Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 366: 895–906 [DOI] [PubMed] [Google Scholar]
- Department of Health (2007) Cancer Reform Strategy 2007. Available at: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_081006
- Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL (2005) Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J Clin Oncol 23: 8597–8605 [DOI] [PubMed] [Google Scholar]
- Erban JK, Lau J (2006) On the toxicity of chemotherapy for breast cancer-the need for vigilance. J Natl Cancer Inst 98: 1096–1097 [DOI] [PubMed] [Google Scholar]
- Ewer MS, Lippman SM (2005) Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol 23: 2900–2902 [DOI] [PubMed] [Google Scholar]
- Ewer MS, Vooletich MT, Durand JB, Woods ML, Davis JR, Valero V, Lenihan DJ (2005) Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol 23: 7820–7826 [DOI] [PubMed] [Google Scholar]
- Fox KM, EUROPA Investigators (2003) Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial. Lancet 362: 782–788 [DOI] [PubMed] [Google Scholar]
- Guarneri V, Lenihan DJ, Valero V, Durand JB, Broglio K, Hess KR, Michaud LB, Gonzalez-Angulo AM, Hortobagyi GN, Esteva FJ (2006) Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: the M. D. Anderson Cancer Center experience. J Clin Oncol 24: 4107–4115 [DOI] [PubMed] [Google Scholar]
- Healthcare Commission (UK) (2000) Ionising Radiation (Medical Exposure) Regulations 2000. Available at: http://www.healthcarecommission.org.uk/serviceproviderinformation/irmer2000.cfm
- Herceptin Summary of Product Characteristics (September 2008). Available at: http://emc.medicines.org.uk/document.aspx?documentId=3567
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, Utriainen T, Kokko R, Hemminki A, Tarkkanen M, Turpeenniemi-Hujanen T, Jyrkkiö S, Flander M, Helle L, Ingalsuo S, Johansson K, Jääskeläinen A-S, Pajunen M, Rauhala M, Kaleva-Kerola J, Salminen T, Leinonen M, Elomaa I, Isola J (2006) Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 354: 809–820 [DOI] [PubMed] [Google Scholar]
- Jones SE, Savin MA, Holmes FA, O′Shaughnessy JA, Blum JL, Vukelja S, McIntyre KJ, Pippen JE, Bordelon JH, Kirby R, Sandbach J, Hyman WJ, Khandelwal P, Negron AG, Richards DA, Anthony SP, Mennel RG, Boehm KA, Meyer WG, Asmar L (2006) Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol 24: 5381–5387 [DOI] [PubMed] [Google Scholar]
- Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA (1973) A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 32: 302–314 [DOI] [PubMed] [Google Scholar]
- Moyé LA, Pfeffer MA, Wun CC, Davis BR, Geltman E, Hayes D, Farnham DJ, Randall OS, Dinh H, Arnold JM (1994) Uniformity of captopril benefit in the SAVE Study: subgroup analysis. Survival and Ventricular Enlargement Study. Eur Heart J 15(Suppl B): 2–8; discussion 26–30 [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence (2003) Chronic Heart Failure. National clinical guideline for diagnosis and management in primary and secondary care (July 2003). Available at: http://www.nice.org.uk/nicemedia/pdf/Full_HF_Guideline.pdf
- National Institute for Health and Clinical Excellence (2006a) Trastuzumab for the adjuvant treatment of earlystage HER2-positive breast cancer (August 2006). Available at: http://www.nice.org.uk/nicemedia/pdf/TA107guidance.pdf
- National Institute for Health and Clinical Excellence (2006b) Implantable cardioverter defibrillators for arrhythmias. January 2006, available at: http://www.nice.org.uk/nicemedia/pdf/TA095guidance.pdf
- National Institute for Health and Clinical Excellence (2006c) Cardiac resynchronisation therapy for the treatment of heart failure (May 2006). Available at: http://www.nice.org.uk/nicemedia/pdf/TA120Guidance.pdf
- NCRI Breast Clinical Studies Group (2005) UK Clinical Guidelines for the use of adjuvant trastuzumab (Herceptin®) with or following chemotherapy in HER2-positive early breast cancer. Available at: http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4126384.pdf
- Ng R, Better N, Green MD (2006) Anticancer agents and cardiotoxicity. Semin Oncol 33: 2–14 [DOI] [PubMed] [Google Scholar]
- Partridge AH, Burstein HJ, Winer EP (2001) Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. J Natl Cancer Inst Monogr 30: 135–142 [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown Jr EJ, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC (1992) Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. N Engl J Med 327: 669–677 [DOI] [PubMed] [Google Scholar]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD, Herceptin Adjuvant (HERA) Trial Study Team (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353: 1659–1672 [DOI] [PubMed] [Google Scholar]
- Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH (2007) Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol 25: 3808–3815 [DOI] [PubMed] [Google Scholar]
- Rastogi P, Jeong J, Geyer CE, Costantino JP, Romond EH, Ewer MS, Keefe DL, Levine T, Swain SM, Wolmark N (2007) Five year update of cardiac dysfunction on NSABP B-31, a randomized trial of sequential doxorubicin/cyclophosphamide (AC) → paclitaxel (T) vs AC → T with trastuzumab (H). J Clin Oncol 25: Abstract LBA513 [Google Scholar]
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer Jr CE, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353: 1673–1684 [DOI] [PubMed] [Google Scholar]
- Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, Stewart SJ, Keefe D (2002) Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 20: 1215–1221 [DOI] [PubMed] [Google Scholar]
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Pawlicki M, Chan M, Smylie M, Liu M, Falkson C, Pinter T, Fornander T, Shiftan T, Valero V, Mackey J, Tabah-Fisch I, Buyse M, Lindsay MA, Riva A, Bee V, Pegram M, Press M, Crown J (2006) on behalf of the BCIRG006 Investigators. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC → T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC → TH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2neu positive early breast cancer patients: BCIRG006 study. Breast Cancer Res Treat 94: Abstract 1 [Google Scholar]
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783–792 [DOI] [PubMed] [Google Scholar]
- Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, Kaufmann M, Cameron D, Bell R, Bergh J, Coleman R, Wardley A, Harbeck N, Lopez RI, Mallmann P, Gelmon K, Wilcken N, Wist E, Sánchez Rovira P, Piccart-Gebhart MJ, HERA study team (2007) 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet 369: 29–36 [DOI] [PubMed] [Google Scholar]
- SOLVD Investigators (1991) Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 325: 293–302 [DOI] [PubMed] [Google Scholar]
- SOLVD Investigators (1992) Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 327: 685–691 [DOI] [PubMed] [Google Scholar]
- Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, Perren T, Passalacqua R, Bighin C, Klijn JG, Ageev FT, Hitre E, Groetz J, Iwata H, Knap M, Gnant M, Muehlbauer S, Spence A, Gelber RD, Piccart-Gebhart MJ (2007) Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol 25: 3859–3865 [DOI] [PubMed] [Google Scholar]
- Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA, Gavazzi A, Haverich A, Hoes A, Jaarsma T, Korewicki J, Lévy S, Linde C, Lopez-Sendon JL, Nieminen MS, Piérard L, Remme WJ, Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology (2005) Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): the Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J 26: 1115–1140 [DOI] [PubMed] [Google Scholar]
- Tan C, Tasaka H, Yu KP, Murphy ML, Karnofsky DA (1967) Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer 20: 333–353 [DOI] [PubMed] [Google Scholar]
- Tan-Chiu E, Yothers G, Romond E, Geyer Jr CE, Ewer M, Keefe D, Shannon RP, Swain SM, Brown A, Fehrenbacher L, Vogel VG, Seay TE, Rastogi P, Mamounas EP, Wolmark N, Bryant J (2005) Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol 23: 7811–7819 [DOI] [PubMed] [Google Scholar]
- Torp-Pedersen C, Køber L (1999) Effect of ACE inhibitor trandolapril on life expectancy of patients with reduced left-ventricular function after acute myocardial infarction. Lancet 354: 9–12 [DOI] [PubMed] [Google Scholar]
- Von Hoff DD, Layard MW, Basa P, Davis Jr HL, Von Hoff AL, Rozencweig M, Muggia FM (1979) Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 91: 710–717 [DOI] [PubMed] [Google Scholar]
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G (2000) Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 342: 145–153 [DOI] [PubMed] [Google Scholar]