Abstract
Background and objective
There are several reports of sub-standard and counterfeit antimalarial drugs circulating in the markets of developing countries; we aimed to review the literature for the African continent.
Methods
A search was conducted in PubMED in English using the medical subject headings (MeSH) terms: “Antimalarials/analysis”[MeSH] OR “Antimalarials/standards”[MeSH] AND “Africa”[MeSH]” to include articles published up to and including 26/02/07. Data were augmented with reports on the quality of antimalarial drugs in Africa obtained from colleagues in the World Health Organization. We summarised the data under the following themes: content and dissolution; relative bioavalability of antimalarial products; antimalarial stability and shelf life; general tests on pharmaceutical dosage forms; and the presence of degradation or unidentifiable impurities in formulations.
Results and discussion
The search yielded 21 relevant peer-reviewed articles and three reports on the quality of antimalarial drugs in Africa. The literature was varied in the quality and breadth of data presented, with most bioavailability studies poorly designed and executed. The review highlights the common finding in drug quality studies that 1) most antimalarial products pass the basic tests for pharmaceutical dosage forms, such as the uniformity of weight for tablets 2) most antimalarial drugs pass the content test 3) in vitro product dissolution is the main problem area where most drugs fail to meet required pharmacopoeial specifications, especially with regard to sulfadoxine-pyrimethamine products. In addition, there are worryingly high quality failure rates for artemisinin monotherapies such as dihydroartemisin (DHA); for instance all five DHA sampled products in one study in Nairobi, Kenya, were reported to have failed the requisite tests.
Conclusions
There is an urgent need to strengthen pharmaceutical management systems such as post-marketing surveillance and the broader health systems in Africa to ensure populations in the continent have access to antimalarial drugs that are safe, of the highest quality standards and that retain their integrity throughout the distribution chain through adequate enforcement of existing legislation and enactment of new ones if necessary, and provision of the necessary resources for drug quality assurance.
Keywords: Antimalarial drugs, Africa, quality, content, dissolution, bioavailability, impurity
INTRODUCTION AND BACKGROUND
Safety, quality, and efficacy of medicines are the three most important criteria used by governments to regulate pharmaceuticals (1). Quality of drugs is especially important and is one of the earliest to come under government scrutiny (2). In England for instance, although product adulteration was banned as early as 1,316 (3), drug efficacy and safety only became an issue in England and in other European countries after the thalidomide tragedy of the late 1950s (4).
Malaria is a major cause of morbidity and mortality worldwide (5). The most lethal form of the malaria parasite, Plasmodium falciparum, is prevalent in sub-Saharan Africa (SSA), infecting some 365 million people in the continent annually, mostly women and children below the age of five (6). Most people who present with malaria fever self-medicate, mostly with shop-bought antipyretic and antimalarial drugs (7), hence, the average African child will likely use antimalarial drugs at least four times in a year (8). The burden of malaria in Africa is exacerbated by the rapid emergence of drug resistance (9), with most countries changing policy often. Kenya, for instance, changed its first-line drug policy against malaria twice in the last decade (10, 11). Currently, the artemisinin-based combination therapies (ACTs) are the recommended first-line drugs for treatment of uncomplicated malaria in most of SSA, although they cost at least ten times more compared to previously available monotherapies which are no longer effective. Thus, deployment of ACTs requires external support (12).
The global problem of drug quality
About 15% of all drugs in circulation worldwide are believed to be counterfeit, with the figures rising to as high as 50% in some parts of Africa and Asia (13). Counterfeit ranitidine (anti-ulcer drug) and tadalafil (anti-impotence drug) have been reported in the United Kingdom in 1994 and 2004 respectively (14); sub-standard thyroxine has also been reported in the United States (15), but such reports are usually sporadic and not commonplace. It is largely acknowledged that sub-standard and counterfeit drugs is a problem of the developing world (16, 17).
For diseases like malaria where progression from mild to severe disease is rapid, especially in young children (18), giving drugs with little or no active ingredient has been said to be “tantamount to murder” (19, 20). Giving drugs with no active ingredient or with the wrong active ingredients means the patient will not be cured of malaria and there is a good chance such a patient will die. Giving patients antimalarial drugs with sub-therapeutic levels of the drug means drug-resistant parasites will be selected in a given population. This, in turn, means a switch to using newer and more expensive drugs. In SSA therefore, a balance has to be struck between the need to make affordable antimalarials available close to where the majority of the people live, and ensuring that in the process the quality of the drugs is not compromised.
General drug quality tests
There are many indicators of drug quality which are described in the literature. Some of these can be performed at facilities with modest infrastructure while others require more substantial investment. They can broadly be classified as a) physical tests that include visual inspection, b) chemical tests for content of active ingredients and impurities under normal and simulated storage conditions, c) in vitro disintegration and dissolution tests and d) in vivo bioavailability studies. Thus, physical tests may include tests done on liquid, semi-solid and solid pharmaceutical dosage forms. For instance, for tablets, uniformity of weight, friability (how well a tablet holds under normal conditions of transportation, measured by the proportion of the tablet that is lost as powder), tablet hardness, and so on, can all be carried out as part of the quality tests (21).
Conventionally, drug quality tests are performed using procedures outlined in official monographs such as the European, British and the United States Pharmacopoeia (USP). Such monographs state the most basic aspects of drug quality that need to be assessed for a particular drug and formulation, and the criteria to be used in the assessment (22, 23). For instance, the test for content determines the amount of active ingredient in a product, which is expressed as a percentage of the label claim, while the dissolution test determines the amount of active ingredient that is released from the dosage form and available for absorption, and is used as surrogate marker of in vivo bioavailability for oral dosage forms containing poorly aqueous-soluble drugs such as sulfadoxine-pyrimethamine (24). Other tests for quality which have been reported in the literature reviewed here are briefly described below.
Stability tests and product shelf life
Stability tests are performed as part of the quality assessment of a drug product when stored under specified conditions of temperature and moisture. They assess the ability of a drug product to remain stable with reference to identity, strength and purity throughout its stated shelf life (25). Stability studies are designed to test the integrity of the active ingredients under a range of conditions that mimic what would be expected during transportation, storage, handling, and use. Specifications for testing the stability of medicines have been standardised over the years for the purpose of regulatory approval under the auspices of the International Conference on Harmonization (ICH).There are currently three broad categories of stability studies: long term, intermediate and accelerated stability studies.
Under current ICH guidelines, long term and intermediate stability studies are carried out under the temperature of intended storage of the drug product and the active ingredients and degradation products quantified every 3 months. Studies should cover a minimum of 12 and 6 months respectively and the data is submitted to regulatory authorities, together with a schedule of when other submissions will be made post-registration for the labeled shelf life of the drug (26). However, more common is the accelerated stability studies where the drug products are subjected to elevated temperatures and humidity for 6 months. Data from the 6-month period are extrapolated using computer programmes that take into consideration the intended range of storage conditions for the given product. A minimum of three batches are analysed and the data pooled to give the estimated product shelf life. Statistical tests of heterogeneity are used to determine if the results from the test batches can be pooled or if more tests need to be done to give a better estimate of shelf life (27). The shelf life is then estimated as the time it takes for the drug product to lose 10% of the active ingredient.
Bioavailability
Bioavailability studies are important with regard to generic drugs of multiple origins. They are important, for example, for drugs with limited aqueous solubility that can lead to erratic, incomplete and unpredictable absorption profiles. But they are comparatively more expensive to plan and execute. For purposes of this review, the following definitions and brief descriptions of experimental design and data analysis methods are helpful in understanding the basis for the comments made regarding some of the studies reviewed in this paper:
Bioavailability
This is a measure of the rate and extent of availability of drug in systemic circulation. The extent of bioavailability is determined from the area under the plasma/blood drug concentration-time profile (AUC) while rate of bioavailability is determined from the peak drug concentrations achieved (Cmax) and the corresponding times (Tmax). Absolute bioavailability is a measure of bioavailability of a product administered extravascularly compared to a reference product administered intravenously. Relative bioavailability is a measure of the bioavailability from one (test) product with reference to a standard product administered via the same (extravascular) route.
Bioequivalent products
These are products of the same dosage form containing the same drug, and that show no difference (using statistical methods described below) in bioavailability. They are usually assumed to be therapeutic equivalents, i.e., show the same therapeutic effect(s).
Design of bioavailability studies
In brief, bioavailability studies involve healthy subjects (usually 24) who receive the test and reference products according to a balanced, cross over study design (i.e., each subject acts as their own control) with a suitable “wash-out” period in between the treatments. If the terminal elimination half-life of the drug is too long (a wash-out period should be equivalent or longer that 6 half-lifes) as may be the case with certain antimalarial drugs, then a parallel study design (in which each subject receives just one product) is preferred.
Statistical analysis in bioequivalence studies
For bioavailability studies, it is not enough to simply demonstrate statistically significant difference between mean bioavailability parameters for two products, but also to be able to state whether such difference is likely to be clinically significant. A two or one-sided t-Test is usually used to determine if mean AUC and Cmax for test and reference products are comparable (28). The two products are judged to be bioequivalent if the 90% confidence interval for the difference between the log-transformed AUC and Cmax for the test and reference products falls between 80-125% of the mean for the reference product for each product. For this comparison, T max is not used as it has proved too variable (28)
LITERATURE REVIEW
We searched PubMED in English for relevant articles on antimalarial drug quality in Africa to include articles published up to and including 26/02/2007. Appropriate medical subject headings (MeSH) were built from the word “antimalarials” and “Africa” by restricting sub-headings under the word “antimalarials” to the nearest MeSH connotations of antimalarial drug quality (“standards” or “analysis”):
“Antimalarials/analysis”[MeSH] OR “Antimalarials/standards”[MeSH] AND “Africa”[MeSH] ”
MeSH terms are a controlled set of vocabulary used to index articles in the United States National Library of Medicine’s MEDLINE/PubMED. They provide a consistent way of retrieving information on the same subject area regardless of the key words used in the submitted articles. This search strategy yielded 95 references; 11 of these were primary investigations of antimalarial drug quality in Africa and were therefore deemed relevant (16, 24, 29-37); the rest of the articles contained some of the MeSH terms used in the search, but were mostly on other areas of malaria therapeutics or did not involve primary work on antimalarial drug quality in the continent. Cross-referencing of the 11 key articles yielded a further 11 Africa-specific references (38-48). In addition, we contacted colleagues at the World Health Organization (WHO), Geneva and also at WHO’s Regional Office for Africa (AFRO) for any grey literature on antimalarial drug quality in the continent. This yielded 2 WHO reports that investigated antimalarial drug quality in several African countries (49, 50) and one report from Senegal by the United States Pharmacopoeia Drug Quality and Information Program (USPDQI). We have also used other search strategies using combinations of such connotations of drug quality as “content”, “dissolution”, “expiry” “counterfeit”, sub-standard”, “fake”, “stability”, “GMP”, together with “antimalarial” and “Africa”, but none of these provided better results than the MeSH strategy outlined above.
We have excluded studies that retrospectively analysed accumulated data. For instance the University of Nairobi’s Drug Analysis and Research Unit (DARU) in Kenya has been reporting on summaries of its findings including the quality of antimalarial drugs in the country (51-56). This exclusion criteria was based on the fact that samples are submitted to DARU by a wide variety of clients, ranging from those who suspect that the product of interest is of poor quality (e.g. in medico-legal cases) to those who seek first-time registration for a new product intended for the Kenyan market; therefore such samples are unrepresentative of the true picture of a particular drug’s quality in a given area and across time. In addition, one article describing work that did not use the conventional laboratory methods or monographs but which used simple colour reactions to determine drug quality, was excluded (57). Further, a Nigerian study (36) was excluded from subsequent analysis because there were no details provided as to what tests were done on sampled chloroquine (CQ) tablets, the results of tests conducted, no were any contact details provided to enable information to be obtained from the authors.
The papers reviewed differ both in their scope (number of tests done on the samples) and breadth (the details of the analytical results reported) and are therefore of varying quality. The review also identified five in vivo bioavailability studies.
RESULTS
General tests on pharmaceutical dosage forms
Only three studies reported on general tests done for a given pharmaceutical dosage form. Hebron et al (29) in Tanzania and Odeniyi et al (41) in Nigeria tested sulfadoxine-pyrimethamine (SP) tablet brands for uniformity of weight, tablet harness, friability and tablet disintegration time. In the Nigerian study, all test samples passed all tests except the friability test. Of 8 SP brands, 5 passed this test and 3 did not. In the Nigeria study, of 15 SP samples (representing different batches of 11 brands), all samples passed the uniformity of weight test, 12 passed the tablet hardness test and 14 the friability and disintegration tests. Gaudiano et al tested quinine (QN), CQ and mefloquine (MEF) tablets sampled from Angola, Burundi and the Democratic Republic of Congo(37) for uniformity of weight and disintegration time; only 1 out of 10 QN sampled failed the weight uniformity test and all samples tested, passed disintegration test.
Content and dissolution
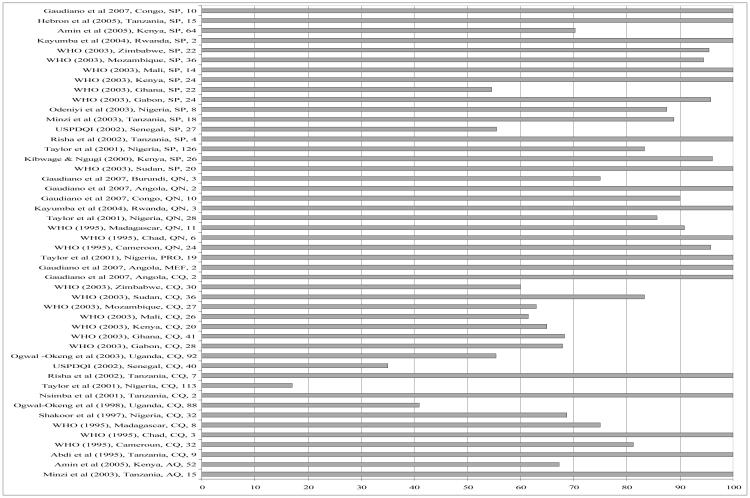
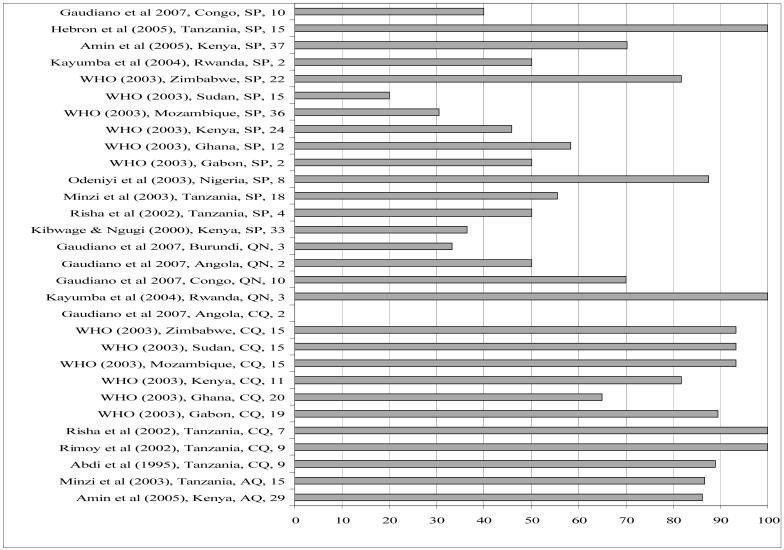
Figures 1 and 2 present content and dissolution data (the two most commonly reported tests) for the papers reviewed. The figures show the proportion of samples analysed (in any one study) that passed content or dissolution. For multi-country studies, each country and each antimalarial drug class was considered as a study of its own to enable meaningful grouping per country and per drug class. Of the studies reviewed, 48 reported on content and 30 on dissolution profiles of antimalarial drug products across different countries, years and drug classes. One of the goals of the Roll Back Malaria initiative, which has been incorporated into national malaria control programmes such as Kenya’s, is that 80% of antimalarial products should be of internationally acceptable standards of quality. We have used this framework in order to make sense of the data since these are international targets that are directly comparable across time and settings. Using this framework (or 80% cut-off), it is apparent from our results that drug content is less of a problem than the other parameters studied (Figure 1). In 31 of the studies the majority (> 80%) of samples analysed had the right amount of the active ingredient. This was true for SP, QN, proguanil (PRO) and mefloquine (MEF). The picture is very different for CQ; only in 7 of 19 studies involving CQ were the majority (> 80%) of samples analysed found to be within the limits for CQ content. There were 30 studies which reported on the dissolution of the tablet form analysed. In general, most antimalarial solid drug products, especially those containing SP have been found to have problems of dissolution but not content of the active ingredient (24, 38, 46). Consistent with this, the review showed that overall, of the 30 studies reporting on dissolution, only in 14 studies were 80% of the samples analysed within the pharmacopoeial specifications. Most of these 14 studies involved CQ, AQ and QN. Of 14 studies involving SP, only 3 were 80% of samples analysed within the pharmacopaeial specifications of ≥60% of sulfadoxine and pyrimethamine released within the dissolution medium in 30 minutes (Figure 2). Most of the SP samples which failed the dissolution test as reported in the various studies did so with respect to the pyrimethamine component.
Figure 1.
% samples passing content test (Publication, Year, Country, and Drug Class, Sample Size). SP=sulfadoxine-pyrimethamine, QN=quinine, PRO=proguanil, MEF=Mefloquine, CQ=chloroquine, AQ=amodiaquine
Figure 2.
% samples passing dissolution test (Publication, Year, Country, and Drug Class, Sample Size). SP=sulfadoxine-pyrimethamine, QN=quinine, PRO=proguanil, CQ=chloroquine, AQ=amodiaquine
With regard to artemisinin-containing products, there is only one published primary article on the quality of the artemisinins in Africa within our review window. Atemnkeng et al (48) sampled 23 artemisinin monotherapy products (tablets, dry powder suspensions, soft gelatin capsules and injections) from pharmacies in Nairobi, Kenya and Bukavu, the Democratic Republic of Congo (DR Congo). In some instances only one product of a given drug was sampled, e.g. one sample each of dihydroartemisinin from Bukavu, DR Congo and of arteether from Kenya. All five samples of dihydroartemisinin (DHA) products obtained from Kenya (one tablet formulation and four dry powder suspensions) failed the content test i.e., contained below or above the requisite 95-105% of the product label claim specified in the European Pharmacopoeia. Of 7 artemisinin monotherapies sampled from DR Congo, only one DHA tablet formulation failed the content test. The failure rates for the other antimalarial products from Kenya were as follows: artemether 20% (1/5), artesunate 25% (1/4) and arteether 100% (1/1). In addition, three products found in DR Congo and purportedly manufactured by a company in Belgium (Saphire SPRL) were counterfeits since such a company was not known to the health authorities in Belgium (48). Two of these products had the right amount of drug. Hence counterfeit in this context meant “...drugs deliberately and fraudulently mislabeled with regard to identity and/or source...” as per the WHO definition.
Bioavailability of antimalarial drugs
A number of studies have compared the bioavailability of antimalarial drugs in the market. For example, Murphy & Mberu (47) reported the comparative relative biaoavialability of Falcidin® (Cosmos Industries, Kenya) and Fansidar® (Hoffman La Roche, Switzerland), two products containing SP, in six healthy male Kenyan volunteers and concluded that the two products were bioequivalent, even though the design of this study was faulty (too few subjects and inappropriate statistical analysis of bioavalability data). In another study in Nigeria, also using six healthy male volunteers, Sowunmi et al (45) compared the absolute bioavailability of three oral salts of QN: 600mg QN sulphate capsule, 600mg QN dihydrochloride plain tablet and 600mg QN sulphate sugar-coated tablets to that of a standard 600mg of QN hydrochloride intravenous infusion. They found that the sugar-coated QN sulphate tablet had no QN, confirming the presence of counterfeit antimalarial drugs in the Nigerian market. There were no differences in bioavailability with regard to the other test drugs. But this study can also be faulted on account of using too few subjects. In the Sudan, Mahmoud et al (44) compared the bioavailability of five brands of CQ in 10 healthy male volunteers. The mean Cmax of four of these was similar (three imported products and one local product); while one locally manufactured brand was significantly less bioavailable than the rest when an ANOVA test was conducted. However, regardless of the statistical differences in Cmax, levels for all brands were well above those required to clear infection. Two studies in Tanzania have also reported the bioequivalence of antimalarials in the market. Nsimba et al (35) compared a sugar-coated CQ brand with a plain CQ tablet in 20 health male volunteers, and reported that there was no significant difference in the AUC between the two formulations. In the second Tanzanian study, Rimoy et al (34) did a similar study comparing the bioavailability of sugar-coated CQ phosphate tablets with that of plain CQ phosphate tablets in two groups of 10 healthy male volunteers; the authors concluded that the plain tablet was overall more bioavailable than the sugar-coated one, but both drugs had attained the required therapeutic plasma level. These studies can be faulted on account of using the wrong statistical methods to analyse bioavailability parameters.
Residue, degradation products and unidentifiable substances in formulations
Atemnkeng et al (48) found several unidentifiable peaks in chromatograms of some arteether injection and DHA dry powder suspensions sampled from pharmacies in Nairobi, Kenya. Hebron et al (29) analysed 11 brands (15 samples) of SP tablets in the Tanzania market, an impurity, closely related to sulfadoxine, which was barely detectable in the originator SP product (Fansidar®, Hoffmann La Roche, Switzerland) was present at about 0.5% w/w in all the generic brands, pointing perhaps to poor quality raw materials used in the manufacture of the affected products.
Antimalarial stability and shelf life
From the literature review there are only three studies in Africa which have looked at the stability of antimalarial drugs in tropical climates. Ballereau et al (39) have shown a CQ content loss >10% by day 450 (temperature 32-40°C, relative humidity 40-80%) in a rural area of Burkina Faso. They concluded that the drug was unstable under typical storage conditions in rural areas of Africa and recommended that CQ be used within one year of manufacture and not according to the labeled shelf life of three years (39). In a more recent study, Risha et al (38) looked at the stability of four samples of SP from different manufacturers and seven of CQ over time using accelerated stability testing. The samples were analysed at time 0, then stored under conditions of relative humidity of 75% ± 5% and temperature of 40°C ± 2°C. At time 0, two of the SP samples (one from Tanzania and one from India) were below the required standard for dissolution. The dissolution profiles of these two products deteriorated further with time, but the remaining two products (from Kenya and Switzerland) retained their integrity even up to the 6th month. All seven CQ products had passed the dissolution test at time 0, at 3 months one of the samples was below the required limit and this had gone further by 6 months. At 6 months also, an additional product had CQ dissolution below the required minimum limit. Using a similar approach Kayumba et al (30) tested three QN products and two SP products obtained from Rwanda. By the 6th month one SP product and one QN product failed to meet the pharmacopoeial specification (that 75% of QN is released in 30 minutes and 60% of sulfadoxine and of pyrimethamine is released within 30 minutes).
Other studies have looked at the expiry status of antimalarials at various points in the distribution chain. For example, Amin et al (24) audited antimalarial products in 880 retail outlets across four districts in Kenya. They calculated remaining shelf life for 2,167 unexpired antimalarial products audited during the survey. The authors report that there was sufficient remaining shelf life for most antimalarial drugs with a median of 46 months for AQ, 30 months for SP, 22 months for CQ, 17 months for QN and 12 months for products containing artemisinin. The authors report that overall approximately 90% of products were within their labeled shelf life. In this study, the basic storage conditions for antimalarials in the audited retail outlets was also evaluated using a simple set of indicators: stored off floor, out of direct sunlight, in a dry area and away from direct sunlight. Over 97% of the products audited satisfied these conditions. In an earlier study, Sesay (43) investigated the use of expired essential drugs (including CQ) in Sierra Leone, and the attitudes of medical practitioners to the subject matter. The author reports that there were variations in the shelf life of CQ products audited, with a range of 3 to 5 years. More worrying was the view by 67% of practitioners that expired products could be used, only that their usefulness was diminished. Only 27% of health practitioners in this study stated categorically that expired products should not be used.
DISCUSSION
We acknowledge that a broad review of antimalarial drug quality over an expansive area such as Africa is fraught with challenges. As is apparent from our review, there is few data on which to judge antimalarial drug quality as few objective studies have been conducted in the continent. Second, on first glance, it would appear that many important references have been left out. Good examples of such references are commentaries that do not contain primary data on antimalarial drug quality in Africa, but have contributed substantially to the debate (58-61), and studies which argue for the use of simple colour reactions to make a judgment on drug quality or studies that have actually used such methods in the field (57, 62, 63). The latter were excluded because they are largely qualitative or semi-quantitative and whilst such tests are very useful in the field as screening aids, especially in resource poor settings in third world countries, few if any regulatory decisions are based on them and in almost all cases where these are used, definitive, full laboratory tests are required (64). Other categories of excluded studies that merit mention are case studies such as the detection of fake halofantrine by Wolff et al in West Africa (65).
A clear distinction needs to be made between counterfeit and substandard products. There is tendency in the literature to use the two terms interchangeably as highlighted by Shakoor et al (16). The WHO defines counterfeit products as “...those deliberately and fraudulently mislabeled with regard to identity and/or source...” (17). Whilst it is the case that most counterfeit products are sub-standard, there are some that are within the specified pharmacopoeial limits as shown by Atemnkeng et al in the DR Congo (48).
These would still be counterfeit because they claim to be what they are not. From the review, it is the case, in Africa at least that most products are sub-standard and not counterfeit (16, 24, 40, 46), pointing perhaps to lack of enforcement of good manufacturing practices (GMP) rather than a deliberate attempt to defraud. However, in the advent of the artemisinins, which are used by at least 30 African countries in SSA as first-line therapeutics (66), the cases of counterfeit antimalarial drugs are likely to rise for the simple reason that these are high value products, are consumed by millions of Africans each year (67) and therefore profitable (19).
Quality of antimalarial drugs is important since it affects the ability to effectively manage one of the most important diseases in SSA. There is an intuitive link between drug quality and drug resistance. Sub-standard and counterfeit drugs could have either of two outcomes for therapeutics: a) they avail too much drug to the body thus precipitating toxic or adverse reactions, or b) avail too little resulting in sub-therapeutic levels of the drug in plasma. Drug action is such that a minimum concentration is required to elicit a physiological response (lowering of blood pressure in hypertension for instance) or parasite kill in the case of malaria and other infectious diseases. The link between sub-therapeutic levels of antimalarial drugs and antimalarial drug resistance is usually explained in terms of “selection pressure” in the literature, i.e. low levels of a drug selectively kill susceptible parasites, leaving resistant parasites to flourish in their stead (sustained and haphazard use of drugs has the same effect). This is especially true for drugs with long half-lives (such as SP) and which therefore are more likely to spend part of their time in the body below the minimum inhibitory concentration required for parasite kill. This residual and sub-therapeutic drug in the host is likely to encounter re-infecting parasites, a common feature in areas of high transmission (most of sub-Saharan Africa), resulting in selective kill of susceptible parasites (68, 69).
SP tablets have notoriously poor in vitro dissolution profiles, especially with regard to the pyrimethamine component. This is mostly a problem with the generic products rather the originator. It is thought that this is due to the poor aqueous solubility of pyrimethamine occasioned by the use of poor quality raw materials or poor choice of excipients in the formulation (38, 46). The clinical implications of a poor dissolution profile of SP products are not hard to fathom; the pharmacopoeia assume a good in vitro-in vivo correlation such that a product which failed in vitro dissolution will most likely fail in an in vivo (bioavailability) test and therefore result in a low plasma level of sulfadoxine and pyrimethamine with the attendant risks of therapeutic failure. The few studies done on SP bioavalability and reviewed here are inconclusive as they have been faulted on sample size requirements.
Antimalarial drug quality is important throughout the distribution chain, storage and dispensing outlets. A very good drug that leaves the factory gate might well be worthless a few months down the line owing to rapid deterioration as a result of exposure to excessive moisture and temperature at the point of use. There are few studies on the stability of antimalarial drugs under tropical climes such as Africa’s (only three were identified in the current review), but Ballereau et al’s study aptly demonstrates the importance of drug stability (39). The authors found rapid deterioration of CQ tablets when exposed to typical tropical conditions in rural Burkina Faso. The usual labeled shelf life of CQ tablets range from three to five years, but the authors report that the drugs had expired within a little more than a year. This has serious policy implications for the ACTs which are the favoured first-line antimalarial policies for most of SSA. The artemisinins are hygroscopic and have a short shelf life of 36 months or less. It is imperative to study how these drugs hold up under typical storage and handling conditions in the tropics as they probably constitute the last remaining armamentarium against the lethal Plasmodium falciparum malaria.
Although several studies have attempted to address the problem of quality of AMs in Africa as outlined in this review, there are fundamental problems that need to be addressed before such studies can inform antimalarial drug policy with regard to choosing between competing alternatives: a) some of these studies are poorly designed making the conclusions unreliable; there is a need, for instance, to acquaint researchers in Africa on basic requirements for a properly designed bioavailability study, b) weak legislation in most countries makes it difficult for results on quality of antimalarial drugs to inform the broad policy on antimalarial drugs; c) there are still too few drug regulatory authorities in the region that are sufficiently resourced (less than a third) to enable them undertake routine “quality audits”, including post market surveillance, on antimalarial drugs in most SSA (1, 17, 49). There is a need to establish National Drug Quality Control Laboratories in the regions where they do not exist and to strengthen existing ones. In tandem, legislation regarding procurement, manufacture, registration, donation, and distribution of antimalarial drugs need to be revised to emphasize drug quality (including, where possible, bioavailability studies) as an integral part of these processes.
ACKNOWLEDGEMENTS
We acknowledge the support provided by Lydiah Mwangi, Stephen Ochieng’ and Caroline Gitonga of the KEMRI/Wellcome Trust Research Programme, Nairobi in collecting some of the references. We also wish to thank Drs Clive Ondari and Jean-Marie Trapsida of the World Health Organization for their support in helping identify grey literature pertinent to the review. This paper is published with the permission of the Director, KEMRI.
Footnotes
CONFLICT OF INTEREST STATEMENT
None to declare
REFERENCES
- 1.WHO . Effective drug regulation: what can countries do? Geneva: 1999. pp. 1–53. [Google Scholar]
- 2.MCA . Towards safe medicines. A guide to the control of safety, quality and efficacy of human medicines in the United Kingdom. Revised edition 1997 Medicines Control Agency (MCA); London: 1997. pp. 1–93. [Google Scholar]
- 3.Penn RG. The state control of medicines: the first 3000 years. British Journal of Clinical Pharmacology. 1979;8:293–305. doi: 10.1111/j.1365-2125.1979.tb04710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah RR. Thalidomide, drug safety and early drug regulation in the UK. Adverse Drug Reaction and Toxicology Review. 2001;20:199–255. [PubMed] [Google Scholar]
- 5.Murray CJL, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 6.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCombie SC. Treatment seeking for malaria: A review of recent research. Social Science and Medicine. 1996;43:933–945. doi: 10.1016/0277-9536(95)00446-7. [DOI] [PubMed] [Google Scholar]
- 8.Spencer H, Kaseje D, Roberts J, Huong A. Consumption of chloroquine phosphate provided for treatment of malaria by volunteer village health worker in Saradidi, Kenya. Annals of Tropical Medicine and International Health. 1987;81:116–123. doi: 10.1080/00034983.1987.11812197. [DOI] [PubMed] [Google Scholar]
- 9.Winstanley PA, Ward SA, Snow RW, Breckenridge AM. Therapy of falciparum malaria in sub-Saharan Africa: from molecule to policy. Clinical Microbiology Reviews. 2004;17:612–637. doi: 10.1128/CMR.17.3.612-637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shretta R, Omumbo J, Rapuoda BA, Snow RW. Using evidence to change antimalarial drug policy in Kenya. Tropical Medicine and International Health. 2000;5:755–764. doi: 10.1046/j.1365-3156.2000.00643.x. [DOI] [PubMed] [Google Scholar]
- 11.MoH National symposium on next anti-malaria treatment policy in Kenya; Naivasha: Ministry of Health, Republic of Kenya. 5th-6th April 2004.2004. pp. 1–29. [Google Scholar]
- 12.IOM . Saving lives, buying time: economics of malaria drugs in an age of resistance. The National Academies Press; Washington: 2004. [PubMed] [Google Scholar]
- 13.Cockburn P, Newton PN, Agyarko EK, Akunyili D, White NJ. The Global Threat of Counterfeit Drugs: Why Industry and Governments Must Communicate the Dangers. PLoS Medicine. 2005;2:e100. doi: 10.1371/journal.pmed.0020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson L. Counterfeits of impotence drug appear in the United Kingdom. British Medical Journal. 2004;329:532. doi: 10.1136/bmj.329.7465.532-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong BJ, Hauck WW, Gambertoglio JG, Gee L, White JR, Bubp JL, Greenspan FS. Bioequivalence of generic and brand-name levothyroxine products in the treatment of hypothyroidism. Journal of American Medical Association. 1997;277:1205–1213. [PubMed] [Google Scholar]
- 16.Shakoor O, Taylor RB, Behrens RH. Assessment of the incidence of substandard drugs in developing countries. Tropical Medicine and International Health. 1997;2:839–845. doi: 10.1046/j.1365-3156.1997.d01-403.x. [DOI] [PubMed] [Google Scholar]
- 17.WHO . Counterfeit drugs: guidelines for the development of measures to combat counterfeit drugs. Geneva: 1999. pp. 1–60. [Google Scholar]
- 18.Greenwood BM, Bradley AK, Greenwood AM, Byass P, Jammeh K, Marsh K, Tulloch S, Oldfield FSJ, Hayes R. Mortality and morbidity from malaria among children in a rural area of The Gambia, West Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1987;81:478–486. doi: 10.1016/0035-9203(87)90170-2. [DOI] [PubMed] [Google Scholar]
- 19.Newton PN, McGready R, Fernandez F, Green MD, Sunjio M, Bruneton C, Phanouvong S, Millet P, Whitty CJ, Talisuna AO, Proux S, Christophel EM, Malenga G, Singhasivanon P, Bojang K, Kaur H, Palmer K, Day NP, Greenwood BM, Nosten F, White NJ. Manslaughter by fake artesunate in Asia--will Africa be next? PLoS Medicine. 2006;3:e197. doi: 10.1371/journal.pmed.0030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aldhous P. Murder by medicine. Nature. 2005;434:132–136. doi: 10.1038/434132a. [DOI] [PubMed] [Google Scholar]
- 21.Aulton ME, editor. Pharmaceutics. The Science of Dosage Form Design; Churchill Livingstone: 2001. [Google Scholar]
- 22.USP . United States Pharmacopoeia. USP 24 NF 19 edn. Pharmacopoeial Convention INC.; Rockville: 2000. [Google Scholar]
- 23.BP . British Pharmacopoeia. II. The Stationery Office; London: 2000. [Google Scholar]
- 24.Amin AA, Snow RW, Kokwaro GO. The quality of sulfadoxine-pyrimethamine and amodiaquine in the Kenyan retail sector. Journal of Clinical Pharmacy and Therapeutics. 2005;30:559–565. doi: 10.1111/j.1365-2710.2005.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucas TI, Bishara RH, Seevers RH. A Stability Program for the Distribution of Drug Products. Pharmaceutical Technology. 2004 Jul;:68–73. [Google Scholar]
- 26.U.S. Department of Health and Human Srevices . Guidance for industry Q1A(R2) stability testing of new drug substances and products. Nov, 2003. 2003, ICH Revision 2. In. [Google Scholar]
- 27.Vadas EB. Stability of pharmaceutical products. In: Gennaro AR, editor. Remminton’s Pharmaceutical Sciences. 18th Edition edn. Mack Publishing Company; Pennsylvania: 1990. pp. 1503–1512. [Google Scholar]
- 28.Wagner JG. Pharmacokinetics for the pharmaceutical scientist. Technomic Publishing Company; Lancaster: 1993. Bioavailability; pp. 187–197. [Google Scholar]
- 29.Hebron Y, Tettey JNA, Pournamdari M, Watson DG. The chemical and pharmaceutical equivalence of sulfadoxine/pyrimethamine tablets sold on the Tanzanian market. Journal of Clinical Pharmacy and Therapeutics. 2005;30:575–581. doi: 10.1111/j.1365-2710.2005.00687.x. [DOI] [PubMed] [Google Scholar]
- 30.Kayumba PC, Risha PG, Shewiyo D, Msami A, Masuki G, Ameye D, Vergote G, Ntawukuliryayo JD, Remon JP, Vervaet C. The quality of essential antimicrobial and antimalarial drugs marketed in Rwanda and Tanzania: influence of tropical storage conditions on in vitro dissolution. Journal of Clinical Pharmacy and Therapeutics. 2004;29:331–338. doi: 10.1111/j.1365-2710.2004.00568.x. [DOI] [PubMed] [Google Scholar]
- 31.Ogwal-Okeng JW, Okello DO, Odyek O. Quality of oral and parenteral chloroquine in Kampala. East African Medical Journal. 1998;75:692–694. [PubMed] [Google Scholar]
- 32.Ogwal-Okeng JW, Owino E, Obua C. Chloroquine in the Ugandan market fails quality test: a pharmacovigilance study. African Health Sciences. 2003;3:2–6. [PMC free article] [PubMed] [Google Scholar]
- 33.Minzi OM, Moshi MJ, Hipolite D, Massele AY, Tomson G, Ericsson O, Gustafsson LL. Evaluation of the quality of amodiaquine and sulphadoxine/pyrimethamine tablets sold by private wholesale pharmacies in Dar Es Salaam Tanzania. Journal of Clinical Pharmacology and Therapeutics. 2003;28:117–122. doi: 10.1046/j.1365-2710.2003.00470.x. [DOI] [PubMed] [Google Scholar]
- 34.Rimoy GH, Moshi MJ, Massele AY. Comparative bioavailability of oral sugar-coated and plain formulation of choloroquine phosphate marketed in Tanzania. Tropical Doctor. 2002;32:15–17. doi: 10.1177/004947550203200108. [DOI] [PubMed] [Google Scholar]
- 35.Nsimba SED, Aden-Abdi Y, Rimoy G, Massele AY, Alm C, Ericsson O, Gustafsson L. Comparative in vitro and in vivo study of a sugar-coated chloroquine preparation marketed in Tanzania versus an ordinary brand. Journal of Clinical Pharmacy and Therapeutics. 2001;26:43–48. doi: 10.1046/j.1365-2710.2001.00314.x. [DOI] [PubMed] [Google Scholar]
- 36.Idowu OA, Alapara SB, Lasisi AA. Assessment of quality of chloroquine tablets sold by drug vendors in Abeokuta, Nigeria. Tanzania Health Research Bulletin. 2006;8:45–46. doi: 10.4314/thrb.v8i1.14271. [DOI] [PubMed] [Google Scholar]
- 37.Gaudiano MC, Di Maggio A, Cocchieri E, Antoniella E, Bertocchi P, Alimonti S, Valvo L. Medicines informal market in Congo, Burundi and Angola: counterfeit and sub-standard antimalarials. Malaria Journal. 2007;6:22. doi: 10.1186/1475-2875-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Risha PG, Shewiyo D, Msami A, Masuki G, Vergote G, Vervaet C. In vitro evaluation of the quality of essential drugs on the Tanzanian market. Tropical Medicine and International Health. 2002;7:701–707. doi: 10.1046/j.1365-3156.2002.00937.x. [DOI] [PubMed] [Google Scholar]
- 39.Ballereau F, Prazuck T, Schrive I, Lafleuriel MT, Rozec D, Fisch A, Lafaix C. Stability of essential drugs in the field: results of a study conducted over a two-year period in Burkina Faso. American Journal of Tropical Medicine and Hygiene. 1997;57:31–36. doi: 10.4269/ajtmh.1997.57.31. [DOI] [PubMed] [Google Scholar]
- 40.Taylor RB, Shakoor O, Behrens RH, Everard M, Low AS, Wangboonskul J, Reid RG, Kolawole JA. Pharmacopoeial quality of drugs supplied by Nigerian pharmacies. Lancet. 2001;357:1933–1936. doi: 10.1016/s0140-6736(00)05065-0. [DOI] [PubMed] [Google Scholar]
- 41.Odeniyi MA, Adegoke OA, Adereti RB, Odeku OA, Itiola OA. Comparative analysis of eight brands of sulfadoxine-pyrimethamine tablets. Tropical Journal of Pharmaceutical Research. 2003;2:161–167. [Google Scholar]
- 42.Abdi YA, Rimoy G, Ericsson O, Alm C, Massele AY. Quality of chloroquine preparations marketed in Dar es Salaam, Tanzania. Lancet. 1995;346:1161. doi: 10.1016/s0140-6736(95)91834-5. [DOI] [PubMed] [Google Scholar]
- 43.Sesay MM. Expiry dates on pharmaceuticals-some worrying realities in Sierra Leone. International Pharmacy Journal. 1994;8:202–206. [Google Scholar]
- 44.Mahmoud BM, Ali HM, Homeida MMA, Bennett JL. Bioequivalence of five chloroquine brands marketed in Sudan. International Pharmacy Journal. 1994;8:164–167. [Google Scholar]
- 45.Sowunmi A, Salako LA, Ogunbona FA. Bioavailability of sulphate and dihydrochloride salts of quinine. African Journal of Medicine and Medical Sciences. 1994;23:275–278. [PubMed] [Google Scholar]
- 46.Kibwage IO, Ngugi JK. Sulphadoxine/pyrimethamine tablet products on the Kenyan market: quality concerns. East and Central African Journal of Pharmaceutical Sciences. 2000;3:14–19. [Google Scholar]
- 47.Murphy SA, Mberu EK. A study of the comparative bioavailability of pyrimethamine-sulfadoxine obtained from two oral preparations. East African Medical Journal. 1994;71:328–329. [PubMed] [Google Scholar]
- 48.Atemnkeng MA, De Cock K, Plaizier-Vercammen J. Quality control of active ingredients in artemisinin-derivative antimalarials within Kenya and DR Congo. Tropical Medicine and International Health. 2007;12:68–74. doi: 10.1111/j.1365-3156.2006.01769.x. [DOI] [PubMed] [Google Scholar]
- 49.WHO . The quality of antimalarials. A study in selected African countries. World Health Organization; Geneva: 2003. pp. 1–67. [Google Scholar]
- 50.WHO . La qualite des medicaments sur le marche pharmaceutique africain. Etude analytique dans trois pays: Cameroun, Madagascar, Tchad. World Health Organization; 1995. WHO/DAP/95.3. [Google Scholar]
- 51.Maitai CK, Kofi Tsekpo WM, Wakori E, Wangia C, Mkoji L, Githiga IM. Drug quality control in Kenya-preliminary observations. East African Medical Journal. 1982;59:399–403. [PubMed] [Google Scholar]
- 52.Ogeto JO, Maitai CK, Wangia C, Mkoji ML, Wakori E, Rutere GK, Mithamo RW, Ochieng’ A, Githiga IM. Practical therapeutical drug quality control in Kenya-further observations. East African Medical Journal. 1983;60:439–443. [PubMed] [Google Scholar]
- 53.Mang’era KO, Rutere GK, Thuranira JK, Mithamo R, Ochieng’ A, Vugigi S, Ogaja E, Kibwage IO. Drug quality control work at Drug Analysis Research Unit: observations during 1987-1990. Pharmaceutical Journal of Kenya. 1992;4:66–69. [Google Scholar]
- 54.Thoithi GN, Abuga KO, Nguyo JM, Mukindia G, Kin’gondu O, Ngugi JK, Kibwage IO. Drug quality control in Kenya: observations in Drug Analysis and Research Unit during the period 1996-2000. East and Central African Journal of Pharmaceutical Sciences. 2002;5:28–32. [Google Scholar]
- 55.Kibwage IO, Ogeto JO, Maitai CK, Rutere G, Thuranira J, Ochieng’ A. Drug quality control work in DARU: observations during 1983-1986. East African Medical Journal. 1992;69:577–580. [PubMed] [Google Scholar]
- 56.Kibwage IO, Thuranira JK, Gathu L, Githiga IM, Nguyo JM, Ngugi JK, King’ondu O. Drug quality control work in Drug Analysis and Research Unit: observations during 1991-1995. East and Central African Journal of Pharmaceutical Sciences. 1999;2:32–36. [Google Scholar]
- 57.Basco LK. Molecular epidemiology of malaria in Cameroon. XIX. Quality of antimalarial drugs used for self-medication. American Journal of Tropical Medicine and Hygiene. 2004;70:245–250. [PubMed] [Google Scholar]
- 58.Erhun WO, Babalola OO, Erhun MO. Drug regulation and control in Nigeria: the challenge of counterfeit drugs. Journal of Health and Population in Developing Countries. 2001;4:23–34. [Google Scholar]
- 59.Kron MA. Substandard primaquine phosphate for US Peace Corps personnel. Lancet. 1996;348:1453–1454. doi: 10.1016/S0140-6736(04)70101-4. [DOI] [PubMed] [Google Scholar]
- 60.Basco LK, Ringwald P, Manene AB, Chandenier J. False chloroquine resistance in Africa. Lancet. 1997;350:224. doi: 10.1016/S0140-6736(05)62397-5. [DOI] [PubMed] [Google Scholar]
- 61.Reithinger R. Bogus antimalarials: a forgotten tale. Trends in Parasitology. 2001;17:359. doi: 10.1016/s1471-4922(01)02078-5. [DOI] [PubMed] [Google Scholar]
- 62.Green MD, Mount DL, Wirtz RA. Authentication of artemether, artesunate and dihydroartemisinin antimalarial tablets using simple colorimetric method. Tropical Medicine and International Health. 2001;6:980–982. doi: 10.1046/j.1365-3156.2001.00793.x. [DOI] [PubMed] [Google Scholar]
- 63.Green MD, Mount DL, Wirtz RA, White NJ. A colorimetric field method to assess the authenticity of drugs sold as the antimalarial artesunate. Journal of Pharmaceutical and Biomedical Analysis. 2000;24:65–70. doi: 10.1016/s0731-7085(00)00360-5. [DOI] [PubMed] [Google Scholar]
- 64.Jahnke RWO, Kusters G. Low-cost quality assurance of medicines using the GPHF-Minilab. Drug Information Journal. 2001;35:941–945. [Google Scholar]
- 65.Wolff JC, Thomson LA, Eckers C. Identification of the ‘wrong’ active pharmceutical ingredient in a counterfeit Halfan™ drug product using accurate mass electrospray ionisation mass spectrometry, accurate mass tandem mass spectrometry and liquid chromatography/mass spectrometry. Rapid Communications in Mass Spectrometry. 2003;17:215–221. doi: 10.1002/rcm.893. [DOI] [PubMed] [Google Scholar]
- 66.Amin AA. Range, quality and costs of antimalarial drugs available in the retail sector in Kenya. Open University; 2005. [Google Scholar]
- 67.Snow RW, Eckert E, Teklehaimanot A. Estimating the needs for artesunate-based combination therapy for malaria case-management in Africa. trends in Parasitology. 2003;19:363–369. doi: 10.1016/s1471-4922(03)00168-5. [DOI] [PubMed] [Google Scholar]
- 68.Nzila AM, Nduati E, Mberu EK, Sibley CH, Monks SA, Winstanley PA, Watkins WM. Molecular evidence of greater selective pressure for drug resistance exerted by the long-acting antifolate pyrimethamine/sulfadoxine compared with the short-acting chlorproguanil/dapsone on Kenyan Plasmodium falciparum. Journal of Infectious Diseases. 2000;181:2023–2028. doi: 10.1086/315520. [DOI] [PubMed] [Google Scholar]
- 69.Watkins WM, Mosobo M. Treatment of Plasmodium falciparum malaria with pyrimethamine-sulfadoxine: selective pressure for resistance is a function of long elimination half-life. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1993;87:75–78. doi: 10.1016/0035-9203(93)90431-o. [DOI] [PubMed] [Google Scholar]