Abstract
We study the osteoinductive effect of the hyaluronic acid (HA) by using an esterified low-molecular HA preparation (EHA) as a coadjuvant in the grafting processes to produce bone-like tissue in the presence of employing autologous bone obtained from intra-oral sites, to treat infra-bone defects without covering membrane.
We report on 9 patients with periodontal defects treated by EHA and autologous grafting (4 males and 5 females, all non smokers, with a mean age of 43,8 years for females, 40,0 years for males and 42 years for all the group, in good health) with a mean depth of 8.3 mm of the infra-bone defects, as revealed by intra-operative probes. Data were obtained at baseline before treatment and after 10 days, and subsequently at 6,9, and 24 months after treatment.
Clinical results showed a mean gain hi clinical attachment (gCAL) of 2.6mm of the treated sites, confirmed by radiographic evaluation. Such results suggest that autologous bone combined with EHA seems to have good capabilities in accelerating new bone formation in the infra-bone defects.
Keywords: Guided tissue regeneration, bone graft, Hyaluronic acid, biomaterials.
INTRODUCTION
Hyaluronic acid (HA) is a natural occurring linear polysaccharide of the extracellular matrix of connective tissue, synovial fluid, and other tissues. HA structure consists of polyanionic disaccharide units of glucouronic acid and N-acetyl-glucosamine connected by alternating β 1-3 and β1-4 bonds 1. There is no anti-genic specificity for species or tissues; and thus, these agents have a low potential for allergic or immunogenic reaction 2.
It is detectable in all vertebrate animals and as a “biofilm” around bacteria 3. HA have specific physical and biochemical properties in normal tissue that make them ideal structural compounds 1. In humans, thanks to its viscoelastic properties, HA is the ground substance of the synovial fluid, as well as the skin, different organs and tissues 4,5.
When HA is incorporated into aqueous solution, hydrogen bonding occurs between adjacent carboxyl and N-acetyl groups; this feature allows HA to maintain conformational stiffness and to retain water. One gram of HA can bind up to 6 L of water 6. As a physical background material, it has functions in space filling, lubrication, shock absorption, and protein exclusion. Its biochemical properties include modulation of inflammatory cells, interaction with the proteoglycans of the extracellular matrix and scavenging of free radicals 4,5.
However, recent data indicated a certain role played by undersulfated glycosaminoglycans, such as HA, on hydroxyapatite crystal formation 7. Moreover, low molecular weight HA has shown osteogenic properties when tested in vitro with bone cells, both through the intramembranous and the endochondral paths of osteogenesis, with the assumption that HA provide differentiation of stem or progenitor cells before attaching to a surface (36a and 36b) 8,9. The application of exogenous HA and HA-based biomaterials showed good results in manipulating and accelerating the wound healing process in a large number of medical disciplines, as evident in ophthalmology, dermatology, dentistry and rheumatology 10,11.
Based up on these data the aim of this study was to observe the potential of Esterified Hyaluronic Acid (EHA) as a coadjuvant of grafting processes to produce bone-like tissue in the presence of employing autologous bone obtained from intra-oral sites, in order to treat infra-bone defects without the aid of membrane, confronting data obtained with previous reported cases used as control.
PATIENTS AND METHODS
Study drug
Hyaloss® matrix, trade names of products composed entirely of an ester of hyaluronic acid with benzyl alcohol (HYAFF™) 2, a concentration ranging of from 20 to 60 mg/ml.
Surgical group
We report on 9 patients with periodontal defects treated by EHA and autologous grafting, 4 males and 5 females, all non smokers, with a mean age of 43,8 years for females, 40,0 years for males and 42 years for all the group, in good health and with a mean depth of the infra-bone defects of 8.3 mm, as revealed by intra-operative probes.
The Exclusion criteria included: smokers of more than 10 cigarettes/day, pregnant or in lactation women, severe systemic disease, plaque and bleeding indexes > 25%, infra-bone defects < 3mm, sites with stabilized teeth (no M2, M3), treatment with drugs that could interfere with the tissue regeneration processes, and patients failing to observe the recommended oral hygiene measures. All patients underwent non surgical periodontal treatment to reduce the FMPS (full mouth plaque surfaces); and FMBS (full mouth plaque surfaces) indexes.
Radiographic Examination
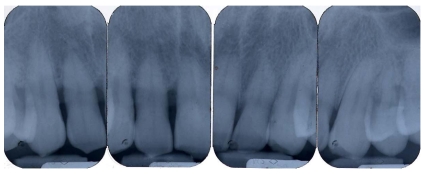
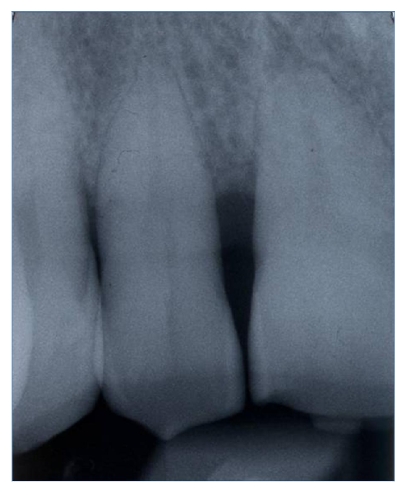
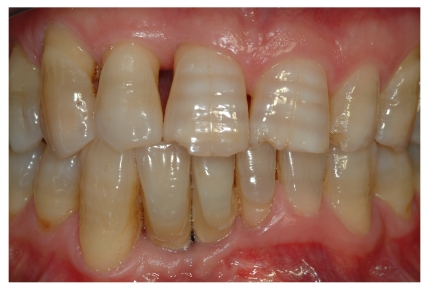
Pre-operative periapical radiography with a Rinn Centering was performed [fig. 1].
Fig 1.
Pre-operative periapical radiography
The examination technique was standardized to obtain radiographs as similar as possible. Impressions were made at the first examination and saved for follow-up control examinations. Radiographs were performed immediately before treatment and at 10 days, and 6, 9, and 24 months after treatment.
Clinical Measurements
To assess the treatment results, the following pre-operative and intra-operative clinical parameters were analyzed:
FMPS;
FMBS;
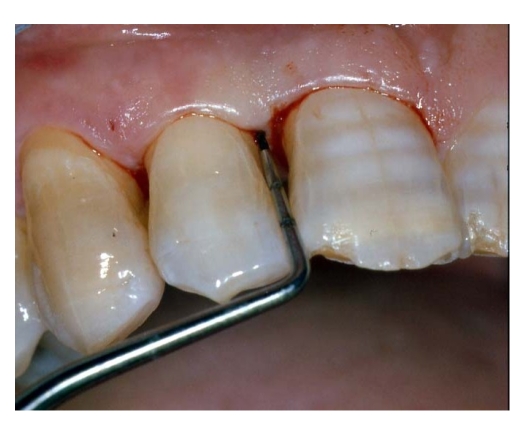
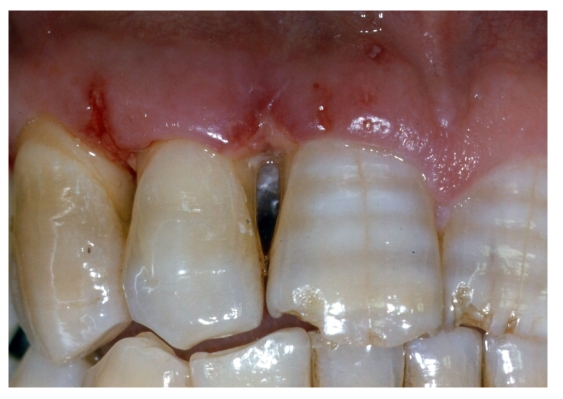
PPD (periodontal pocket depth) [fig. 2];
R: gingival recession, i.e. the position of the gingival margin with respect to the cement enamel junction (CEJ);
CAL (clinical attachment level): i.e. the position of the attachment in relationship to the CEJ;
IBPD (intrabony pocket depth): distance between the CEJ and the bone crest.
Fig 2.
Initial probing
Periodontal pockets were measured with a manual probe marked in millimiter increments. Bleeding on probing was recorded and the presence of plaque was registred mesially, buccally, distally, and lingually. Data were obtained at baseline before treatment and at 10 days, and 6,9, and 24 months after treatment.
Surgical Technique
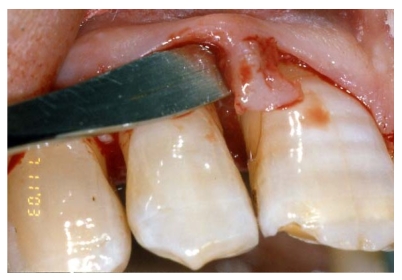
After local anaesthesia, intrasulcular incisions were made at the buccal and lingual sides with Bard-Parker surgical blade n° 15, at least one tooth away from the mesial portion, distally to the graft site, to create access for the tools and facilitate the direct clinical view of the defect [fig. 3-4].
Fig 3.
Surgical flap
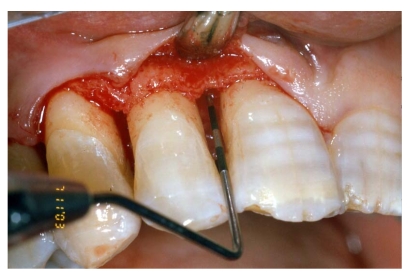
Fig 4.
Flap elevation, intra-surgical evaluation of the parameters and surgical curettage of the intra-osseous defect
A full-thickness flap was elevated and the granulation tissue was removed showing the true extension and depth of the infra-bone defect.
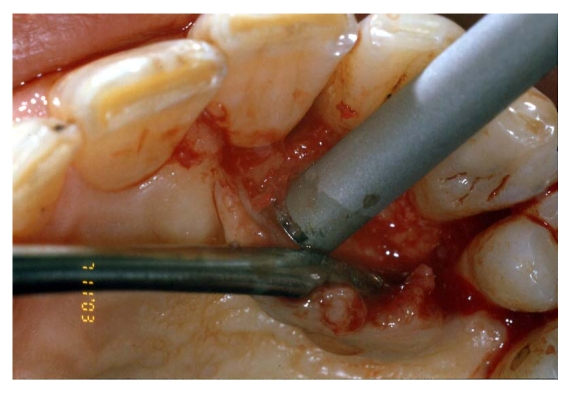
Debridement and root preparation were carried out with hand and ultrasonic instruments [fig. 5].
Fig 5.
Autogenous bone graft harvested by a mini-bone scraper (Safescraper curve or Micross)
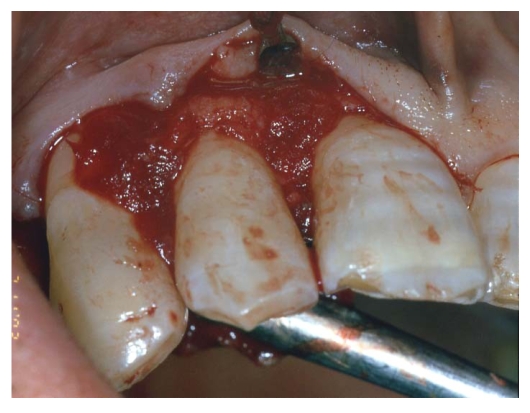
Subsequently, the graft material was prepared and positioned: 0.5 cc of autologous bone from intra-oral donor sites blended with two bundles of EHA fibres (Biopolimero Hyaloss® Matrix) and a few drops of physiological solution [fig. 6].
Fig 6.
Autogenous bone graft mixed with Hyaloss® matrix
Excess fluids were removed with a sterile gauze and the graft material was locally administered.
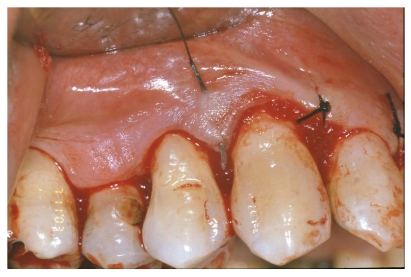
Finally, the flap was re-positioned and sutured with single stitches. Firm pressure was exerted with fingers for 2 - 3 minutes using a gauze dipped in physiological solution, to reduce the blood clot and promote healing [fig. 7-8].
Fig 7.
Flap replacement and nylon 4-0 single stitches sutures.
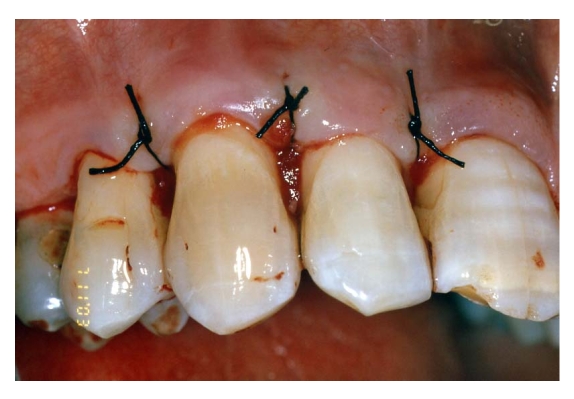
Fig 8.
Flap replacement and nylon 4-0 single stitches sutures.
After surgery, patients were instructed to rinse their mouths twice daily with 10 ml of 0.2 % chlorexidine for 6 weeks.
RESULTS
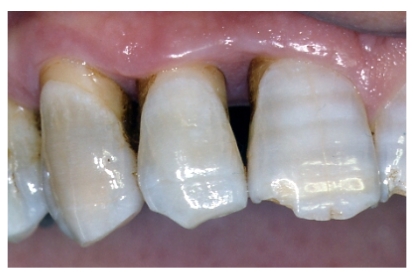
The management of the soft tissues was easy and healing almost in a high rate occurred after the first treatment. During sutures removal, no important tissues inflammations were observed. At a 10-day follow-up, post-operative clinical assessment demonstrated a gingivitis grade of 0 or 1. Thanks to the bacteriostatic properties of the tested polymer, a more effective control of the surgical wound no bacterial contamination of the surgical site was observable in all instances [fig. 9]. Post-operative radiographs showed absence of bone remodelling, and satisfactory filling of the infra-bone defects with the graft material in situ. After 6 months, radiographs showed presence of mild bone remodelling and excellent infra-bone filling [fig. 10-11].
Fig 9.
Clinical re-evaluation 10 days after surgery
Fig 10.
Clinical re-evaluation 6 months after surgery
Fig 11.
Radiographic re-evaluation 6 months from surgery
At 9 months from the procedure the dental elements were virtually stables, with a mean gCAL (gain hi clinical attachment) of 2.6mm; radiographic evaluation showed defect filling and good prognosis [fig. 12-13].
Fig 12.
Clinical re-evaluation 9 months after surgery
Fig 13.
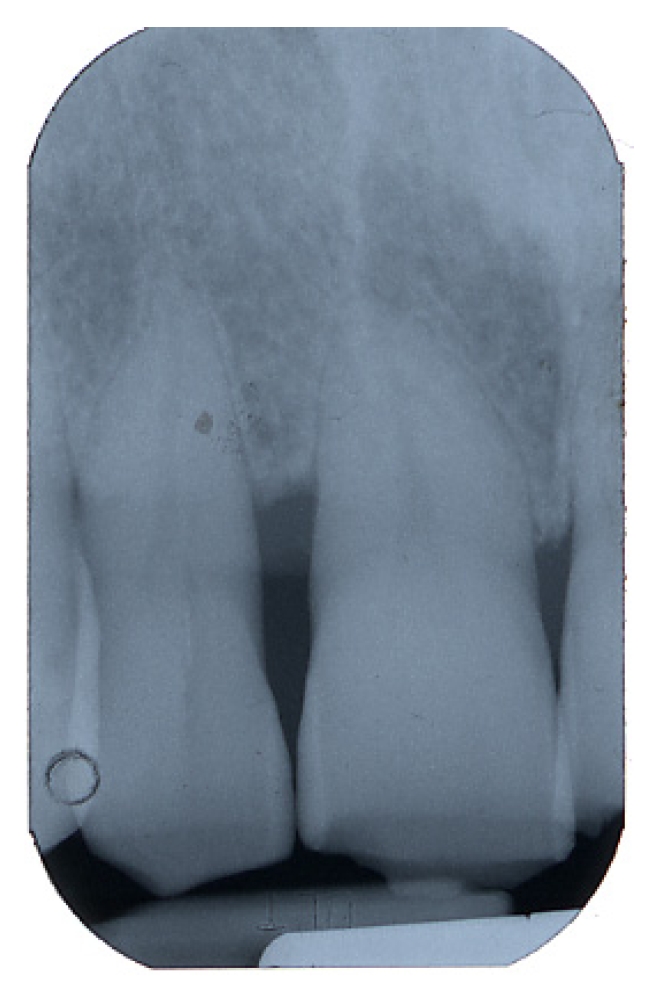
Radiographic follow up at 9 months after surgery
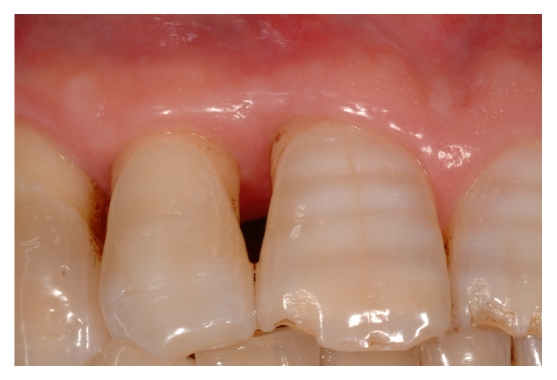
After 24 months clinical [fig. 14] and radiographic [fig. 15] re-evaluation shows a present and satisfactory filling [Tables 1 and 2].
Fig 14.
Clinical re-evaluation 24 months after surgery
Fig 15.
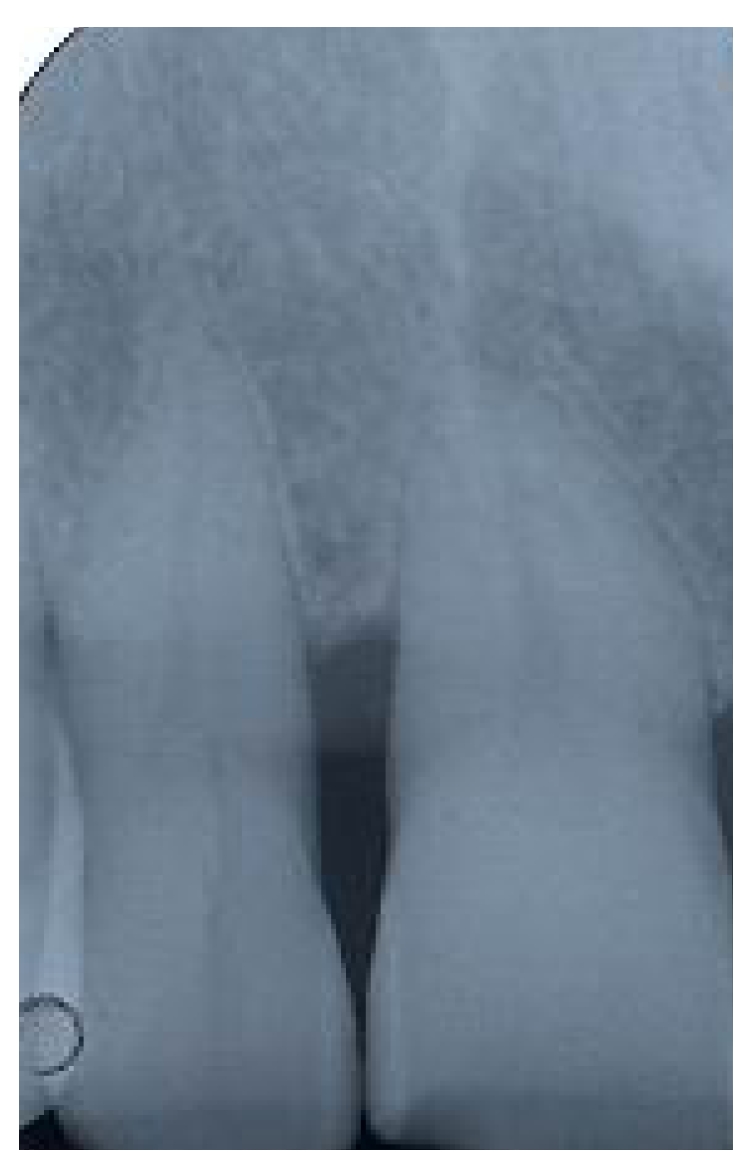
Radiographic follow up at 24 months after surgery
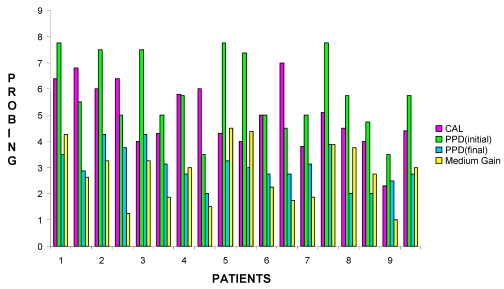
Table 1.
Medium gain obtained durig Surgical treatment with Hyaloss® matrix.
| Patient | Age | Sex | FMPS | FMBS | Osseous defect | Surgical site | PPD (initial) | PPD (final) | Cal | Medium gain | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 44 | F | 100 | 100 | Defect combined 1 and 2 walls | 32 - 33 | 7,8 | - | 5,5 | 3,5 | - | 2,9 | 6,4 | - | 6,8 | 4,3 - 2,6 | |
| 2 | 40 | M | 50 | 50 | Defect at 3 walls | 35 - 36 | 7,5 | - | 5,0 | 4,3 | - | 3,8 | 6 | - | 6,4 | 3,3 - 1,3 | |
| 3 | 40 | M | 50 | 50 | Defect at 3 walls | 44 - 45 | 7,5 | - | 5,0 | 4,3 | - | 3,1 | 4 | - | 4,3 | 3,3 - 1,9 | |
| 4 | 28 | M | 60 | 100 | Defect combined 1 and 3 walls | 41 - 42 | 5,8 | - | 3,5 | 2,8 | - | 2,0 | 5,8 | - | 6 | 3 - 1,5 | |
| 5 | 34 | F | 100 | 100 | Defect combined 1 and 2 walls | 11 - 21 | 7,8 | - | 7,4 | 3,3 | - | 3,0 | 4,3 | - | 4 | 4,5 - 4,4 | |
| 6 | 36 | F | 60 | 40 | Defect combined 1 and 3 walls | 15 - 16 | 5,0 | - | 4,5 | 2,8 | - | 2,8 | 5 | - | 7 | 2,3 - 1,8 | |
| 7 | 44 | F | 50 | 20 | Defect combined 1,2 and 3 walls | 45 - 46 | 5,0 | - | 7,8 | 3,1 | - | 3,9 | 3,8 | - | 5,1 | 1,9 - 3,9 | |
| 8 | 52 | M | 60 | 60 | Defect combined 2 and 3 walls | 11 - 12 | 5,8 | - | 4,8 | 2,0 | - | 2,0 | 4,5 | - | 4 | 3,8 - 2,8 | |
| 9 | 60 | F | 80 | 80 | Defect combined 1 and 2 walls | 42 - 43 | 3,5 | - | 5,8 | 2,5 | - | 2,8 | 2,3 | - | 4,4 | 1 - 3 | |
Table 2.
Graphical representation of Medium gain per patient for each surgical site obtained durig treatment with Hyaloss® matrix.
DISCUSSION
Through its complex interactions with matrix components and cells, HA has multifaceted roles in biology utilizing both its physicochemical and biological properties.
HA has a primary role in the principal biological processes such as cell organization and differentiation.
It is postulated that the morphogenetic effects of HA are due to its ability to act as a template for assembly of a multi-component, pericellular matrix as well as to its physical properties 1,4. This matrix would provide a hydrated environment in which cells are separated from structural barriers to morphogenetic changes and receive signals from HA itself and from associated factors 4,12.
HA is an essential component of extracellular matrix. It interacts with other macromolecules and plays a predominant role in tissue morphogenesis, cell migration, differentiation, and adhesion 4,12, 13.
Recent in vitro studies have suggested potential roles for these two molecules in various aspects of endothelial function It appears to exert its biological effects through binding interactions with at least two cell surface receptors: CD44 and receptor for HA-mediated motility (RHAMM) 14-16.
Interactions between basal epithelial cells and the extracellular matrix are mediated by special receptors on the cell surface which are known as integrins and belong to the family of cellular adhesion molecules (CAM) 17. Several studies suggest that integrin-mediated interaction plays a decisive role in the regulation of proliferation, migration, and differentiation of the epithelial cells 2,12,18,19. Following the surgical treatment of adult periodontitis, the epithelial regeneration of the periodontal attachment is non-physiological and thus unsatisfactory, if membranes or artificial bone material are not used. Re-epithelialization is based on the proliferation, migration, and differentiation of basal epithelial cells which are in contact with a wound matrix and whose molecular makeup differs from the extracellular matrix of intact regions 17.
The general physicochemical and biological properties of HA, are utilized in the various processes of wound healing: inflammation, granulation and re-epithelization 17,20,21. Inflammation occurs when the entire organism reacts to pathogenic agents penetration inside a wound, and all the possible defence systems are activated 15,16,22. When wound becomes inflamed several factors necessary to the subsequent healing phases are generated such as growth factors and cytokines, which promote migration of inflammatory cells, fibroblasts and endothelial cells into the damaged site. It has been showed that fibroblasts cultured in presence of increasing doses of HA have an increased production of pro-inflammatory cytokines such as TSG-6, TNF-α, IL-Iβ and IL-δ, triggered by CD44 receptors 12,15-17,21,22.
It has been shown that high molecular weight HA is an angiogenesis inhibitor, while low molecular weight HA oligosaccharides had a marked angiogenic effect in a series of experimental models, as well as stimulating the production of collagen in endothelial cells 23.
Thanks to its hygroscopic, rheological and viscoelastic properties, HA can influence the cell function that modify the surrounding micro and macro environment as a result of complex interactions with the cells and other extracellular matrix components 6.
This characteristic is largely responsible for the consistency of the active component that can act as a barrier to the spread of any bacteria penetrating the tissues including those of the periodontium. It is conceivable that hyaluronan administration to periodontal sites could achieve comparable benefits in periodontal healing and surgery, hence aiding treatment of periodontal disease 24,25.
HA can be an ideal vector for the bone morphogenic proteins (BMP), the only growth factors universally recognized to be able to stimulate the formation of new bone tissue 26-29. HA of appropriate molecular weight alone in optimal concentration induce osteoblast differentiation and bone formation 8. In fact HA has a molecular weight-specific and dose-specific mode of action that may enhance the osteogenic and osteoinductive properties of bone graft materials and substitutes due to its stimulatory effects on osteoblasts 30. Considering that no previous report exist reporting on the use of EHA combined with autologous bone but without the application of a membrane in the treatment of infra bone defects, our clinical results are encouraging considering that in a similar study a mean value of the increase of the bone height of 0.5 mm was obtain with membrane application 31.
CONCLUSIONS
All these properties make HA useful in periodontal regenerative therapy as a coadjuvant of autologous bone grafting. In contact with the patient's blood or saline solution, the Hyaloss® matrix forms a gel almost instantly, thus facilitating the application of the bone fragments.
The Hyaloss® matrix is highly multipurpose, because at room temperature it can form a biodegradable, biocompatible gel that can be adapted from the operator to the desired consistency, by regulating the blood and saline volume.
In fact, the Hyaloss® matrix has a dual function: on one hand its physiochemical properties facilitate the application of bone graft in the damaged site and on the other hand, it creates an environment with a rich content of HA, with all the advantages deriving from the phenomenon.
The present study's clinic and radiologic results showed positive bone formation without a significant inflammatory host response. We feel that using autologous bone and EHA is appropriate for performing clinical infra osseous defects.
Acknowledgments
Written informed consent was obtained from the patient for publication of this study and all accompanying images.
Author's Contributions: SC and AB made substantial contributions to conception and design and drafted the manuscript. SC revised it critically for important intellectual content and gave final approval of the version to be published. FRG assisted with manuscript revision. All authors read and approved the final manuscript.
References
- 1.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006 Aug;85(8):699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Benedetti L, Cortivo R, Berti A, Pea F, Mazzo M, Moras M, Abatangelo G. Biocompatibility and biodegradation of different hyaluronan derivatives (HYAFF) implanted in rats. Biomaterials. 1993;14:1154–1160. doi: 10.1016/0142-9612(93)90160-4. [DOI] [PubMed] [Google Scholar]
- 3.Pirnazar P, Wolinsky L, Nachnani S, Haake S, Pilloni A, Bernard GW. Bacteriostatic effects of hyaluronic acid. J Periodontol. 1999 Apr;70(4):370–4. doi: 10.1902/jop.1999.70.4.370. [DOI] [PubMed] [Google Scholar]
- 4.Toole BP. Proteoglycans and hyaluronan in morphogenesis and differentiation. In: Hay ED, editor. Cell Biology of Extracellular Matrix. New York: Plenum Press; 1991. pp. 305–41. [Google Scholar]
- 5.Laurent TC, Fraser JRE. Hyaluronan. FASEB J. 1992;6:2397–404. [PubMed] [Google Scholar]
- 6.Sutherland I. Novel and established applications of microbial polysaccharides. Trends Biotechnol. 1988;16:41–46. doi: 10.1016/S0167-7799(97)01139-6. [DOI] [PubMed] [Google Scholar]
- 7.Boskey AL, Dick BL. Hyaluronan interactions with hydroxyapatite do not alter in vitro hydroxyapatite crystal proliferation and growth. Matrix. 1991;11:442–6. doi: 10.1016/s0934-8832(11)80198-8. [DOI] [PubMed] [Google Scholar]
- 8.Pilloni A, Bernard GW. The effect of hyaluronan on mouse intramembranous osteogenesis in vitro. Cell Tissue Res. 1998 Nov;294(2):323–33. doi: 10.1007/s004410051182. [DOI] [PubMed] [Google Scholar]
- 9.Pilloni A, Rimondini L, De Luca M, Bernard GW. Effect of hyaluronan on calcification-nodule formation from human periodontal ligament cell culture. Journal of Applied Biomaterials & Biomechanics. 2003;1:1–7. [PubMed] [Google Scholar]
- 10.Laurent TC. The Chemistry, Biology, and Medical Applications of Hyaluronan and Its Derivatives. London: Portland Press; 1998. [Google Scholar]
- 11.Kuo JW. Practical Aspects of Hyaluronan Based Medical Products, 1 edition. CRC Press LLC. 2005.
- 12.Turley EA. The role of cell-associated hyaluronan bindig protein in fibroblast behaviour. In: Evered D, Whelan J, editors. The Biology of Hyaluronan. Chichester: J Wiley & Sons; 1989. pp. 121–37. [DOI] [PubMed] [Google Scholar]
- 13.Toole BP. Hyaluronan in morphogenesis. Semin Cell Dev Biol. 2001 Apr;12(2):79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 14.Turley EA, Austen L, Moore D, Hoare K. ras-Trasformed cells express both CD44 and RHAMM hyaluronan receptors: only RHAMM is essential for hyaluronan promoted locomotion. Exp Cell Res. 1993;207:277–82. doi: 10.1006/excr.1993.1194. [DOI] [PubMed] [Google Scholar]
- 15.Heldin P, Karousou E, Bernert B, Porsch H, Nishitsuka K, Skandalis SS. Importance of hyaluronan-CD44 interactions in inflammation and tumorigenesis. Connect Tissue Res. 2008;49(3):215–8. doi: 10.1080/03008200802143323. [DOI] [PubMed] [Google Scholar]
- 16.Lesley J, Gál I, Mahoney DJ, Cordell MR, Rugg MS, Hyman R, Day AJ, Mikecz K. TSG-6 modulates the interaction between hyaluronan and cell surface CD44. J Biol Chem. 2004 Jun 11;279(24):25745–54. doi: 10.1074/jbc.M313319200. [DOI] [PubMed] [Google Scholar]
- 17.Graber HG, Conrads G, Wilharm J, Lampert F. Role of interactions between integrins and extracellular matrix components in healthy epithelial tissue and establishment of a long junctional epithelium during periodontal wound healing: a review. J Periodontol. 1999 Dec;70(12):1511–22. doi: 10.1902/jop.1999.70.12.1511. [DOI] [PubMed] [Google Scholar]
- 18.Campoccia D, Hunt J.A, Doherty P.J, Zhong S.P, O' Regan M, Benedetti L, Williams D.F. Quantitative assessment of the tissue resposnse to films of hyaluronan. Biomaterials. 1996;17:973–975. doi: 10.1016/0142-9612(96)84670-9. [DOI] [PubMed] [Google Scholar]
- 19.Oksala O, Salo T, Tammi R, Hakkinen H, Jalkanen M, Inki P, Larjava H. Expression of proteoglycans and hyaluronan during wound healing. J Histochem Cytochem. 1995;43:125–35. doi: 10.1177/43.2.7529785. [DOI] [PubMed] [Google Scholar]
- 20.Pianigiani E, Andreassi A, Taddeucci P, Alessandrini C, Fimiani M, Andreassi L. A new model for studying differentiation and growth of epidermal cultures on hyaluronan-based carrier. Biomaterials. 1999 Sep;20(18):1689–94. doi: 10.1016/s0142-9612(99)00056-3. [DOI] [PubMed] [Google Scholar]
- 21.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–61. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 22.Wisniewski H.G, Vilcek J. TSG-6: an IL-1/TNF-inducible protein With anti-inflammatory activity. Cytokine and growth Factor Review. 1997 Jun;8(2):143–56. doi: 10.1016/s1359-6101(97)00008-7. [DOI] [PubMed] [Google Scholar]
- 23.Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem. 2001 Sep 28;276(39):36770–8. doi: 10.1074/jbc.M102273200. [DOI] [PubMed] [Google Scholar]
- 24.Jentsch H, Pomowski R, Kundt G, Göcke R. Treatment of gingivitis with hyaluronan. J Clin Periodontol. 2003 Feb;30(2):159–64. doi: 10.1034/j.1600-051x.2003.300203.x. [DOI] [PubMed] [Google Scholar]
- 25.Moseley R, Waddington RJ, Embery G. Hyaluronan and its potential role in periodontal healing. Dent Update. 2002 Apr;29(3):144–8. doi: 10.12968/denu.2002.29.3.144. [DOI] [PubMed] [Google Scholar]
- 26.Lisignoli G, Fini M, Giavaresi G, Nicolini Aldini N, Toneguzzi S, Facchini A. Osteogenesis of large segmental radium defects enhanced by basic fibroblast growth factor activated bone marrow stromal cells grown on-woven hyaluronic acid-based polymer scaffold. Biomaterials. 2002;23:1043–1051. doi: 10.1016/s0142-9612(01)00216-2. [DOI] [PubMed] [Google Scholar]
- 27.Caplan A.I. Tissue engineering design for the future: new logistic, old molecules. Tissue Engineering. 2000;6:1–8. doi: 10.1089/107632700320838. [DOI] [PubMed] [Google Scholar]
- 28.Kim H.D, Valentini R.F. Retention and activity of BMP-2 in hyaluronic acid-based scaffolds in vitro. J Biomed Mater Res. 2002;59:573–584. doi: 10.1002/jbm.10011. [DOI] [PubMed] [Google Scholar]
- 29.Hunt D.R, Jovanovic S.A, Wikesjo M.E, Wozney J.M, Bernard G.W. Hyaluronan supports recombinant human bone morphgenetic protein-2 induced bone reconstruction of advanved alveolar ridge defects in dogs. A pilot study. J Periodontol. 2001;72:651–658. doi: 10.1902/jop.2001.72.5.651. [DOI] [PubMed] [Google Scholar]
- 30.Huang L, Cheng YY, Koo PL, Lee KM, Qin L, Cheng JC, Kumta SM. The effect of hyaluronan on osteoblast proliferation and differentiation in rat calvarial-derived cell cultures. J Biomed Mater Res. 2003 Sep 15;66A(4):880–4. doi: 10.1002/jbm.a.10535. [DOI] [PubMed] [Google Scholar]
- 31.Engström PE, Shi XQ, Tronje G, Larsson A, Welander U, Frithiof L, Engstrom GN. The effect of hyaluronan on bone and soft tissue and immune response in wound healing. J Periodontol. 2001 Sep;72(9):1192–200. doi: 10.1902/jop.2000.72.9.1192. [DOI] [PubMed] [Google Scholar]