Summary
Limb development requires the elaboration of a proximodistal (PD) axis, which forms orthogonally to previously defined dorsoventral (DV) and anteroposterior (AP) axes. In arthropods, the PD axis of the adult leg is subdivided into two broad domains, a proximal coxopodite and a distal telopodite. We show that the progressive subdivision of the PD axis into these two domains occurs during embryogenesis and is reflected in the cis-regulatory architecture of the Distalless (Dll) gene. Early Dll expression, governed by the Dll304 enhancer, is in cells that can give rise to both domains of the leg as well as to the entire dorsal (wing) appendage. A few hours after Dll304 is activated, the activity of this enhancer fades, and two later-acting enhancers assume control over Dll expression. The LT enhancer is expressed in cells that will give rise to the entire telopodite, and only the telopodite. By contrast, cells that activate the DKO enhancer will give rise to a leg-associated larval sensory structure known as the Keilin's organ (KO). Cells that activate neither LT nor DKO, but had activated Dll304, will give rise to the coxopodite. In addition, we describe the trans-acting signals controlling the LT and DKO enhancers, and show, surprisingly, that the coxopodite progenitors begin to proliferate ∼24 hours earlier than the telopodite progenitors. Together, these findings provide a complete and high-resolution fate map of the Drosophila appendage primordia, linking the primary domains to specific cis-regulatory elements in Dll.
Keywords: Distalless, Drosophila melanogaster, Leg, Limb primordium
INTRODUCTION
Evolutionary and genetic studies in arthropods suggest that the proximodistal (PD) axis of the leg is initially established by defining two primary domains (Snodgrass, 1935) (reviewed by Boxshall, 2004). The coxopodite, which includes the coxa, the most proximal leg segment, is thought to have been derived as an outgrowth of the body wall and may have been the ancestral, unsegmented appendage. The telopodite, or leg proper, includes all of the more distal leg segments, and is thought to have evolved subsequently, to allow more sophisticated leg movements by bendable joints that separate each leg segment. However, despite the existence of this primary subdivision, and its importance in arthropod evolution, a molecular understanding of this process is lacking.
Most of the molecular dissection of arthropod leg development has come from studying the leg imaginal discs of the fruit fly, Drosophila melanogaster. These studies suggest that the formation of the telopodite is under the control of the Hedgehog (Hh) signaling pathway, whereas the coxopodite forms independently of this pathway (Gonzalez-Crespo et al., 1998; Gonzalez-Crespo and Morata, 1996). For the telopodite to form, Hh induces the expression of two downstream signals, Wingless (Wg), ventrally, and Decapentaplegic (Dpp), dorsally (Basler and Struhl, 1994). The combinatorial action of Wg plus Dpp creates the PD axis of the leg by activating target genes such as Distalless (Dll) and dachshund (dac) (Campbell et al., 1993; Diaz-Benjumea et al., 1994; Estella and Mann, 2008; Lecuit and Cohen, 1997). Based on these studies, the sum of the Dll and dac expression domains in a mature leg imaginal disc may correspond to the telopodite, a conclusion that is supported by studies in other arthropods (Abzhanov and Kaufman, 2000). By contrast, there is no clear molecular marker for the coxopodite. Initially, the presence of nuclear Extradenticle (nExd), a homeodomain protein that requires the co-expression of homothorax (hth) for nuclear localization, was proposed to be a marker for the coxopodite in the leg imaginal disc (Gonzalez-Crespo and Morata, 1996; Rieckhof et al., 1997). However, a true coxopodite gene should not be expressed distal to the coxa, and Hth-nExd are also expressed in the next-most distal leg segment, the trochanter (Abu-Shaar and Mann, 1998). The molecular definition of these two domains is also complicated by the observation that the relative expression patterns of Dll and Hth-nExd change over time. When Dll, the earliest marker of the leg primordium, is first activated in embryogenesis, all Dll-expressing cells co-express Hth-nExd in circular domains comprising ∼20 cells per thoracic hemisegment (Gonzalez-Crespo et al., 1998). Slightly later Hth-nExd are no longer expressed in a central subset of the Dll domain, but the three proteins remain co-expressed in the remaining cells (Bolinger and Boekhoff-Falk, 2005; Mann and Abu-Shaar, 1996). Eventually, in the third instar leg imaginal disc, the expression domains of Hth-nExd and Dll are mutually exclusive except for a thin ring of cells that co-express these genes and gives rise to the trochanter (Abu-Shaar and Mann, 1998; Gonzalez-Crespo and Morata, 1996).
We reasoned that insights into how the telopodite and coxopodite are specified might come from characterizing the cis-regulatory elements that regulate Dll in the embryonic leg primordia. Dll is initially activated at ∼6 hours of embryonic development under the control of an early-acting enhancer called Dll304 (Vachon et al., 1992). Wg provides the anteroposterior (AP) positional cue that activates Dll304 (Cohen et al., 1993). Two other signaling pathways, Dpp and Epidermal growth factor receptor (Egfr) signaling, limit the leg progenitor domain dorsally and ventrally, respectively (Goto and Hayashi, 1997; Kubota et al., 2000). Furthermore, although the Wg, Dpp and EGFR signals are deployed similarly in all embryonic trunk segments, Dll expression is limited to the thoracic segments by the abdominal Hox genes that directly repress Dll304 activity in the abdomen (Gebelein et al., 2002; Vachon et al., 1992).
Although Dll304 is activated by Wg and repressed by Dpp, Dll expression in the imaginal disc is activated by both signals (Campbell et al., 1993; Diaz-Benjumea et al., 1994), implying that additional Dll regulatory elements must exist. Recently, such a leg disc regulatory element, termed LT for `leg-trigger', has been described (Estella et al., 2008). Unlike Dll304, LT continuously requires Wg and Dpp input for its activity in the leg disc. Although LT (also called Dll215) has been reported to be active in late stage embryos (Castelli-Gair and Akam, 1995; Cohen et al., 1993), its spatial relationship compared to Dll304 and its regulation by Wg and Dpp during embryogenesis has not been described. In addition, the lineages that Dll304- and LT-expressing cells give rise to have not been examined and may help inform how the coxopodite and telopodite are specified.
Another important unresolved set of questions concerns the relationship between the development of the adult and larval legs. As a holometabolous insect, Drosophila undergoes complete metamorphosis, meaning that the tissues that give rise to the adult structures, the imaginal discs, grow within the larva but do not contribute to the larval body plan. Nevertheless, Drosophila has rudimentary larval appendages called Keilin's organs (KOs) that serve as thoracic-specific sensory organs. KOs are intimately associated with the developing leg imaginal disc (Lakes-Harlan et al., 1991; Madhavan and Schneiderman, 1977) and, like the adult telopodite, require Dll to form (Cohen and Jurgens, 1989). Although a group of cells within the Dll-expressing leg primordia express neural markers and is therefore thought to give rise to the KOs (Bolinger and Boekhoff-Falk, 2005; Cohen, 1993), its relationship to other Dll-dependent lineages has not been clearly defined.
Here we compare the spatial relationships, subsequent lineages and genetic inputs that regulate three Dll cis-regulatory elements, Dll304, LT, and a newly defined element, DKO, dedicated to the formation of the KOs. We show that when the leg primordia are first allocated, coincident with the activity of Dll304, this domain is multipotent and has the potential to give rise not only to the entire telopodite, coxopodite and KO, but also to dorsal (e.g. wing) appendage fates. A few hours later, Dll304 activity fades, and LT and DKO are activated in mutually exclusive subsets of the Dll304-expressing domain. In contrast to the multipotency of the Dll304 expression domain, LT-expressing cells give rise to the entire telopodite and only the telopodite, while DKO-expressing cells give rise to the KO. As in the leg imaginal discs, LT requires both Wg and Dpp to be activated during embryogenesis. In addition, we show that the telopodite fate is repressed by the KO fate, suggesting that these two sets of progenitor cells are mutually antagonistic. Surprisingly, we also find that the onset of coxopodite growth is advanced relative to the onset of telopodite growth, which begins only after Hth-nExd are turned off in these cells at ∼60 hours of development. These experiments thus provide a complete description of all the cell types within the Drosophila leg primordia, their temporal development, and the subsequent structures they generate.
MATERIALS AND METHODS
Transgenes
LT-lacZ and LT-Gal4 have been previously described (Estella et al., 2008). The DKO fragment was selected based on sequence conservation to other Drosophilids (Vista Genome Browser), and cloned by PCR (details are available upon request) into the hs43-nuc-lacZ vector (Estella et al., 2008). The DKO and 304 enhancers were also cloned in pChs-Gal4.
Immunostaining
Discs and embryos were stained using standard procedures. The primary antibodies used were, rabbit and mouse anti-β-Gal (Cappell and Promega), mouse anti-Wg, mouse anti-Cut, mouse anti-En, mouse anti-Dac and mouse anti-Elav (Developmental Studies Hybridoma Bank, University of Iowa). Guinea pig anti-P-Mad (a gift from E. Laufer and T. Jessell), guinea pig anti-Dll (generated against the full-length protein), rabbit anti-Hth generated against the full-length protein, mouse anti-Ubx (Crickmore and Mann, 2006), guinea pig anti-Tsh (a gift from G. Struhl).
Drosophila stocks and mutant analysis
lacZ lines, Gal4 lines and lineage analysis esg-lacZ (Hayashi et al., 1993), esg-Gal4 (Goto and Hayashi, 1999), Dll-Gal4 (line MD 23) (Calleja et al., 1996), Dll-Gal4 (line 212) (Gorfinkiel et al., 1997), tsh-Gal4 (Wu and Cohen, 2000), hth-Gal4 (GETDB-Gal4 Enhancer Trap Insertion Data Base), prd-Gal4 (Gebelein et al., 2004). Two Dll-Gal4 lines were used: MD 23 recapitulates all Dll expression whereas em 212 does not capture early (Dll304) expression.
Lineage analyses and neutral clones used act5C>stop>lacZ; UAS-flp (Struhl and Basler, 1993). In the tsh-Gal4 and hth-Gal4 lineage experiments flies of the genotype tub-Gal80ts; tsh-Gal4; tub-Gal80ts (Zirin and Mann, 2007) were crossed to act5C>stoP>lacZ; UAS-flp at 18°C. At various time points, 12-hour collections were transferred to 29°C. Female larvae were dissected at the third instar. Developmental stage at time of transfer to the restrictive temperature was determined based upon the amount of time spent at 29°C before dissection. tub-Gal80ts was also used to show that limiting LT-Gal4 activity to the embryo is sufficient to label the entire telopodite. For the Minute experiment we generated wild-type clones in a Minute heterozygous background in telopodite precursor cells using the LT-Gal4; UAS-flp.
Mutant lines
DllSA1 (Vachon et al., 1992), Df(1)sc-B57 (Dominguez and Campuzano, 1993), btdXG81 and Df(1)C52 (Cohen, 1990) were used. The wg temperature-sensitive experiments (WgIL114) were performed as previously described (Cohen et al., 1993) with modifications. Embryos were kept at the permissive temperature (18°C) until Dll expression is initially activated (∼10-14 hours), then transferred to the restrictive temperature (29°C) until stage 14.
UAS lines
UAS-hid (Zhou et al., 1997), UAS-ase (Brand and Dormand, 1995), UAS-arm (delta N) (Chan et al., 1997), UAS-tkvQD (Abu-Shaar and Mann, 1998), UAS-dad (Tsuneizumi et al., 1997), UAS-brk (Jazwinska et al., 1999), UAS-Dll (Gorfinkiel et al., 1997), UAS-btd (Schock et al., 1999) and UAS-p35 (Bloomington Center) were used.
RESULTS
The LT and DKO enhancers are active in mutually exclusive subsets of the limb primordia
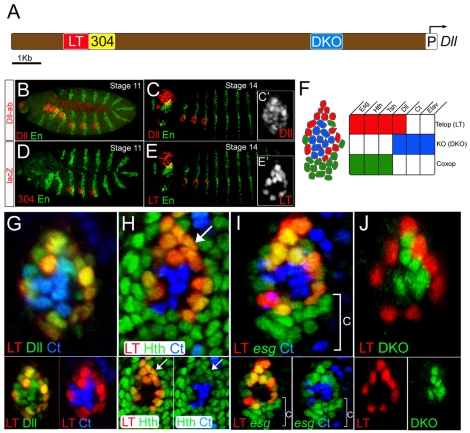
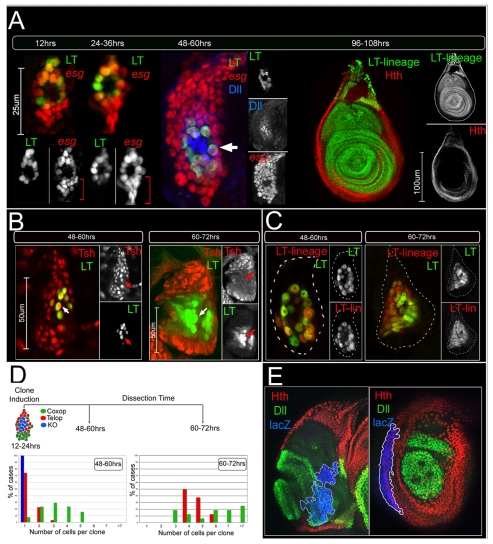
In light of the identification of the LT enhancer as a direct integrator of Wg and Dpp during leg imaginal disc development (Estella et al., 2008), we examined the activity of this enhancer relative to Dll and Dll304 during embryonic stages. Dll protein in the thorax was first detected during embryonic stage 11 (Fig. 1B), and continued to be visualized in this region until the end of embryogenesis (Fig. 1C; data not shown). Although a Dll304-lacZ transgene recapitulated the initial pattern of Dll expression (Fig. 1D), the activity of this enhancer decayed within a few hours (Cohen et al., 1993). By contrast, an LT-lacZ reporter gene became active in Dll-expressing cells of the thorax after germ-band retraction, with robust expression by stage 14 (Fig. 1E). Importantly, the LT enhancer was not active in all of the Dll-expressing cells of the thorax. LT-lacZ was expressed in the outermost ring of ∼15 cells of the Dll clusters (Fig. 1G). At this stage, LT-lacZ-expressing cells also expressed hth, esg and teashirt (tsh; Fig. 1H,I, and data not shown). Because esg is required for the maintenance of diploidy, it has been suggested that esg-expressing cells give rise to the imaginal discs (Hayashi et al., 1993). By contrast, the Dll-expressing LT-lacZ-nonexpressing cells within the LT ring did not express esg. Instead, these cells expressed cut (ct), which encodes a transcription factor required for the development of external sensory organs (Bodmer et al., 1987). Accordingly, these cells may give rise to the KO (Bolinger and Boekhoff-Falk, 2005; Cohen et al., 1993).
Fig. 1.
LT and DKO are active in mutually exclusive subsets of the limb primordia. Embryos are oriented anterior to the left and dorsal up. (A) The Dll 5′ cis-regulatory region. LT is in red, Dll304 in yellow, DKO in blue, and the Dll promoter in white. (B,C) Stage 11 (B) and stage 14 (C) embryos stained for Dll (red) and En (green). (C′) A magnification of the Dll-positive cells at stage 14. (D) A stage 11 embryo stained for Dll304 activity (red) and En (green). (E) A stage 14 embryo stained for LT activity (red) and En (green). (E′) A magnification of LT-positive cells at stage 14. LT is active in a subset of Dll-expressing cells (compare with C′). (F) Schematic representation of a leg primordium at stage 14. (G) Dll (green), Ct (blue) and LT activity (red) in a leg primordium at stage 14. LT activity is mutually exclusive with Ct expression. LT-positive and Ct-positive cells are subsets of the Dll expression domain. (H) Hth (green), Ct (blue) and LT activity (red) in a leg primordium at stage 14. LT-positive cells also express Hth (arrow). ct and hth are expressed in mutually exclusive domains. (I) esg-lacZ (green), Ct (blue) and LT activity (red) in a leg primordium at stage 14. esg and Ct are mutually exclusive and LT activity overlaps with a subset of the esg-expressing cells. The ventral esg-expressing, LT-negative cells (bracket) are the coxopodite progenitor cells. (J) LT (red) and DKO (green) activities are mutually exclusive within the Dll-positive cells of the leg primordium at stage 14.
Because the ct-expressing cells expressed Dll but not LT-lacZ, there must be additional cis-regulatory elements controlling Dll expression in these cells. To identify these elements, we cloned a conserved region of the Dll gene located approximately 3 kb 5′ to the start of transcription to create a transgenic reporter gene which we named DKO-lacZ (Fig. 1A). By stage 14 DKO was active in the Dll-expressing cells that also express ct (Fig. 1J; see Fig. S1B,C in the supplementary material). Unlike LT-lacZ, DKO-lacZ was not expressed in third instar leg discs (data not shown). However, DKO-lacZ was expressed in other cells of the embryonic peripheral nervous system that do not express Dll. The ectopic expression of DKO-lacZ indicates that this element lacks repressor input that normally limits its activity to the limb primordia. Within the leg primordia, however, the LT and DKO expression domains are mutually exclusive in stage 14 embryos, such that the sum of the LT and DKO expression domains accounts for all Dll expression (Fig. 1J). These data suggest that by stage 14 the fates of the Dll-expressing cells of the thoracic limb primordia have been determined, and they can be subdivided into two distinct populations of cells in which different Dll cis-regulatory elements are active.
Existence of different cell fates in the ventral limb primordia
To determine whether the two cell types defined above give rise to different fates, we performed lineage-tracing experiments with a panel of Gal4 drivers, including drivers made with these enhancer elements. In general, lineages were analyzed by using these Gal4 drivers to express the yeast recombinase Flp, which deleted a transcriptional stop cassette from an actin-lacZ transgene (actin>stop>lacZ; see Materials and methods for details).
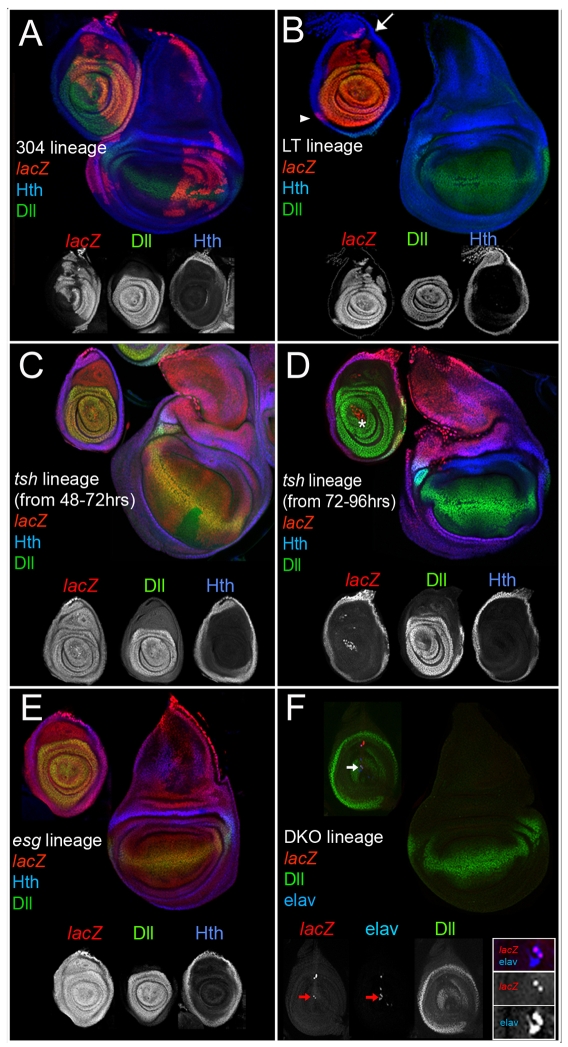
Lineage tracing using Dll304-Gal4 labeled cells in both the dorsal and ventral appendages (Fig. 2A), indicating that this enhancer was active prior to the separation of leg and wing fates, consistent with previous cell-lineage analyses (Wieschaus and Gehring, 1976). Within the leg, cells were labeled in both the coxopodite and telopodite, unlike the distal-only pattern of Dll immunostaining in mature third instar leg discs (Fig. 2A). This pattern matches that generated by Dll-Gal4, an enhancer trap into the Dll locus (line MD 23, see Material and methods; see Fig. S2A in the supplementary material) (Campbell and Tomlinson, 1998).
Fig. 2.
Lineage analyses of genes active in the ventral limb primordia. All discs except for those in F were stained for the lineage marker (red), Dll (green, subset of telopodite), and Hth (blue, coxopodite); see Materials and methods for details. (A) The progeny of cells in which Dll304 was active contribute to both dorsal (wings and halteres) and ventral (legs, both coxopodite and telopodite) thoracic limbs. Although individual wing discs show labeling in only a subset of the disc, labeled cells can contribute to any part of the disc. (B) The progeny of cells in which LT was active generate the telopodite of the leg. Expression in the dorsal coxopodite (arrow) may be due to the imperfection of the LT-Gal4 driver. The arrowhead marks a clone in the trochanter region. (C,D) The progeny of cells that expressed tsh become more restricted over time. Restricting tsh-Gal4 activity to the beginning of second instar (48-72 hours AEL) results in the labeling of both the coxopodite and telopodite (C). Allowing tsh-Gal4 to be active beginning at third instar (72-96 hours AEL) results in the labeling of only the coxopodite (D). The asterisk in D indicates lacZ-positive adepithelial cells that are not part of the disc epithelium. (E) The progeny of esg-expressing cells adopt both wing and leg (coxopodite and telopodite) fates. (F) The progeny of the cells in which DKO was active (red) occasionally contribute to larval neurons that co-express Elav (blue, arrow). All lacZ-positive cells express elav but not all elav cells are lacZ positive (see inset).
By contrast, lineage-tracing using LT-Gal4 demonstrated that LT was not active in any cells that give rise to the wing or other dorsal appendages. In both the leg disc and adult leg, the LT lineage coincided with the Dll- and dac-expressing telopodite and did not contribute to the peripodial epithelium (Fig. 2B; see Fig. S2B in the supplementary material). In the coxopodite, the LT lineage analysis consistently labeled a small group of cells in the dorsal-most stalk region (Fig. 2B). The few labeled cells in the coxopodite may be the result of the imperfection of the LT enhancer when out of its normal genomic context. Importantly, the entire telopodite was also labeled when LT-Gal4 activity was limited exclusively to embryogenesis by using a tub-Gal80ts transgene to suppress Gal4 activity during larval stages (see Material and methods for experimental details). These data suggest that the ∼15 LT-expressing cells of the embryonic limb primordia give rise to the entire telopodite. This is surprising given that, in the embryo, these cells also express hth and tsh (Fig. 1H), which are genes that are expressed only in the proximal domain of the third instar leg disc and have therefore been considered to be coxopodite markers.
To confirm that the telopodite progenitor cells also express hth and tsh, we performed lineage-tracing experiments using tsh-Gal4 and hth-Gal4. Because the entire thoracic ectoderm expresses tsh and hth prior to the initiation of Dll expression, the tub-Gal80ts transgene was used to control the activity of these Gal4 drivers. Raising the animals at the temperature where Gal80ts was active for all of development (the permissive temperature) resulted in no lacZ expression (data not shown), confirming the efficacy of the Gal80ts protein. Switching the animals to the nonpermissive temperature at the beginning of the second larval instar (∼48 hours) resulted in lacZ expression throughout the entire leg disc (Fig. 2C), indicating that tsh was active in both coxopodite and telopodite progenitors long after LT activation in the embryo. By contrast, switching the animals to the nonpermissive temperature at the beginning of the third larval instar (∼72 hours) consistently labeled the coxopodite, but rarely labeled the telopodite (Fig. 2D). Similar results were obtained using hth-Gal4 instead of tsh-Gal4 (data not shown).
Although these experiments identify the progenitors of the telopodite, they leave open the question of which embryonic cells give rise to the coxopodite. Because the product of the esg gene is required for all imaginal disc fates, we reasoned that Esg-positive, LT-nonexpressing cells would be the progenitors of the coxopodite. Such a population of cells exists just ventral to the LT-expressing ring (Fig. 1I). Consistently, all leg and wing disc cells were labeled when a lineage analysis was performed using esg-Gal4 (Fig. 2E). Thus, we conclude that the coxopodite is derived from the esg-expressing cells that are present just ventral to the LT-positive cells of the leg primordia (Fig. 1I).
Because the Dll- and DKO-lacZ-expressing cells also express ct and elav, but not esg (Fig. 1G-J; see Fig. S1A in the supplementary material), these cells were predicted to be the progenitors of the larval KO. To test this, we carried out lineage tracing using a DKO-Gal4 transgene. One-third (n=40) of these third instar leg discs had no lacZ expression, demonstrating that DKO-expressing cells did not contribute to imaginal disc fates. Approximately one third of the discs contained small numbers of lacZ-positive cells that co-expressed the neural marker Elav (Fig. 2F, see inset). These neurons may be the same as previously described, embryonically born neurons that persist until larval stages (Tix et al., 1989). The cell bodies of these neurons reside in the leg imaginal disc and project dendrites to the KO in the larval epidermis (Tix et al., 1989). Finally, approximately one-third of the discs had lacZ-expressing clones present in the disc epithelia. Because the DKO element is expressed in Dll-negative cells (see above), these clones probably result from the spurious activity of this enhancer. Altogether, these data are consistent with an earlier report (Bolinger and Boekhoff-Falk, 2005) and support the conclusion that the Dll-positive, Ct-positive cells in the center of the leg primordia, previously considered to be the progenitors of the telopodite, are the progenitors of the Keilin's organ and do not contribute to the imaginal disc. These conclusions were further confirmed by using these Gal4 drivers to express the proapoptotic gene hid (Zhou et al., 1997) to induce cell death (see Fig. S2C in the supplementary material).
In summary, when Dll304 is first activated, Dll-positive cells have the potential to give rise to all regions of the dorsal and ventral appendages. A few hours later three cell types are defined: the KO progenitors [Dll(DKO)-positive, esg-, hth- and tsh-negative], the telopodite progenitors [Dll(LT)-positive, esg-, hth- and tsh-positive], and the coxopodite progenitors (Dll-negative, esg-, hth- and tsh-positive). Together, these cells comprise the entire thoracic ventral limb primordia (Fig. 1F).
Regulation of LT and DKO activity by Wg and Dpp
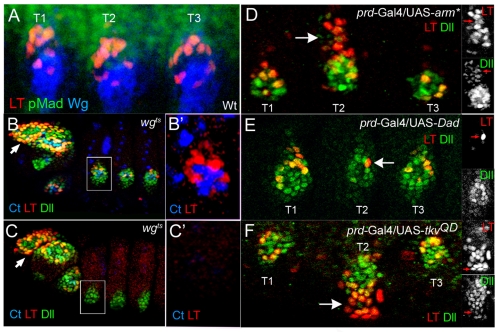
To determine how each of these cell fates in the limb primordia is specified, we carried out genetic experiments to identify the regulators of the LT and DKO enhancers. Consistent with LT's dependency on wg and dpp for leg disc expression (Estella et al., 2008), LT is activated in the embryo in cells that receive both inputs, as monitored by anti-Wg and anti-PMad staining (Fig. 3A). To determine whether wg is required for LT activity, we used a temperature-sensitive allele of wg to allow earlier Dll activation (Cohen et al., 1993). Switching the embryos to the restrictive temperature at stage 11 resulted in the absence of LT activity, despite the presence of Dll protein (probably derived from Dll304 activity; Fig. 3C). In addition, ectopic activation of the wg pathway [using an activated form of armadillo (arm*)] resulted in more LT-lacZ-expressing cells (Fig. 3D).
Fig. 3.
Regulation of LT by Wg and Dpp. (A) Cells that activate LT (red) at stage 14 in the limb primordia are close to cells expressing high levels of Wg (blue) and Dpp (visualized by pMad staining, green). (B) A wgts stage 14 embryo raised at the permissive temperature stained for Ct (blue), Dll (green) and LT activity (red). (B′) An enlargement of the leg primordium boxed in B. LT activity in the head segments (arrows) is not affected in the wgts embryos. (C) A wgts stage 14 embryo shifted to the restrictive temperature at 10-14 hours, after the initial activation of Dll and Dll304 (see methods), stained for Dll (green), Ct (blue), and LT activity (red). Dll expression is still observed, probably due to the activity of Dll304, but LT activity and Ct are not observed. (C′) An enlargement of the leg primordium boxed in C. (D-F) Dpp and Wg activate LT. prd-Gal4 is expressed in T2 but not T1 and T3. LT (red) and Dll (green) are activated dorsally by prd>arm* in T2 (arrow; D). LT activity (red) and Dll levels are reduced via prd>Dad (arrow; E). LT activity (red) and Dll (green) are expanded ventrally via prd>TkvQD (arrow; F). Insets show single channels for Dll and LT activity.
Like wg, the dpp pathway is necessary for LT-lacZ expression in leg discs. Paradoxically, dpp signaling represses Dll in the embryo because dpp mutants show an expansion in Dll304-lacZ expression (data not shown) (Goto and Hayashi, 1997). By contrast, LT-lacZ is not expressed in dpp null embryos (data not shown). LT-lacZ, but not Dll protein, was also repressed by two dpp pathway repressors, Dad and brk (Campbell and Tomlinson, 1999; Jazwinska et al., 1999; Minami et al., 1999; Tsuneizumi et al., 1997) (Fig. 3E; and data not shown). Conversely, stimulation of the dpp pathway [using an activated form of the Dpp receptor (TkvQD)] resulted in ectopic activation of LT ventrally (Fig. 3F).
Taken together, these data demonstrate that LT is activated by Wg and Dpp in the embryonic limb primordia, just as it (and Dll) is in the leg disc. Similarly, DKO activity also requires Wg and Dpp input (see Fig. S3D,E in the supplementary material).
Dll and btd confer ventral thoracic-specificity to LT expression
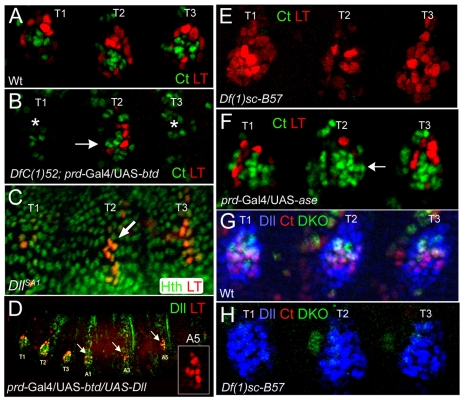
Although LT is activated by wg and dpp in the leg primordia, these signals are also present in each abdominal segment. Consequently, there must be additional factors that restrict LT activity to the thorax. One possibility is that LT is repressed by the abdominal Hox factors, such as Dll304 (Gebelein et al., 2002; Vachon et al., 1992). Alternatively, LT might be regulated by Dll, itself (Castelli-Gair and Akam, 1995). We found that in Dll null embryos LT-lacZ was initially expressed in a stripe of cells instead of a ring, but then expression decayed (Fig. 4C). Ectopic expression of Dll resulted in weak ectopic expression of LT-lacZ in the thorax and abdomen (see Fig. S3A in the supplementary material). These data suggest that LT activity is restricted to the thorax in part because of the earlier restriction of Dll304 activity to the thorax.
Fig. 4.
Regulation of DKO and LT. (A) Wild-type stage 14 limb primordia stained for LT activity (red) and Ct (green). LT activity and Ct are present in mutually exclusive domains. (B) A stage 14 prd>btd; DfC(1)52 embryo stained for LT (red) and ct (green). In the absence of btd and Sp1 both LT and ct are not activated (in T1 and T2; asterisks). Resupplying Btd in T2 rescues LT and ct activity (arrow). (C) In a stage 14 DllSA1 mutant embryo LT activity (red) initiates, but decays over time. Unlike in wild type (A), the LT expression domain is a stripe. (D) Ectopic expression of Dll (green) and Btd using prd-Gal4 activates LT (red) in the abdomen (arrows). The inset shows ectopic activation of LT in abdominal segment 5. (E) A Df(1)sc-B57 mutant embryo, deleted for the ASC, stained for LT activity (red) and Ct (green). LT activity is expanded at the expense of the ct-expressing cells, which are lost. (F) Ectopic expression of the proneural gene asense (ase) in T2 represses LT activity (red) and expands the number of cells that express ct (green; arrow). (G) Wild-type limb primordia stained for Dll (blue), DKO activity (green) and Ct (red). DKO activity and Ct expression overlap within the Dll-positive domain. (H) Df(1)sc-B57 mutant embryo stained for DKO activity (green), Ct (red) and Dll (blue). DKO activity and Ct staining are absent in the leg primordia, but the number of cells expressing Dll is not changed.
The related zinc-finger transcription factors encoded by buttonhead (btd) and Sp1 are also expressed in the limb primordia and are also required for ventral appendage specification (Estella et al., 2003). In strong btd hypomorphs, the activity of LT was still detected but the number of cells expressing LT-lacZ was decreased and its pattern was disrupted (see Fig. S3C in the supplementary material). LT-lacZ expression was completely eliminated in animals bearing a large deficiency that removes both btd and Sp1 (Fig. 4B). By contrast, Dll304 was activated normally in these animals (data not shown). Importantly, LT-lacZ expression was rescued by expressing btd in these deficiency embryos (Fig. 4B). By contrast, expressing Dll, tkvQD, or arm* did not rescue LT expression in these deficiency embryos (data not shown). Ectopic expression of btd resulted in weak ectopic activation of LT-lacZ in cells of the thorax and abdomen (see Fig. S3B in the supplementary material). Strikingly, the simultaneous expression of Dll and btd resulted in robust ectopic expression of LT-lacZ in abdominal segments in the equivalent ventrolateral position as the thoracic limb primordia (Fig. 4D). btd and Dll were not sufficient to activate LT in wg null embryos (data not shown). These data indicate that the thoracic-specific expression of the LT enhancer is controlled by the combined activities of btd and/or Sp1, Dll and the wg and dpp pathways.
Proneural genes activate DKO and repress LT
Although the above data suggest that LT is activated by a combination of Wg, Dpp, Btd and Dll, these activators are also present in the precursors of the KO, which activate DKO instead of LT. Because the KO is a sensory structure, we tested the role of members of the achaete-scute complex (ASC) that are expressed in these cells (Bolinger and Boekhoff-Falk, 2005). In embryos hemizygous for a deficiency that removes the achaete-scute complex, LT-lacZ expression was expanded at the expense of the Ct-expressing cells (Fig. 4E). Consistently, ectopic expression of the ASC gene asense (ase) repressed LT and increased the number of Ct-expressing cells (Fig. 4F). These data suggest that there is a mutual antagonism between the progenitors of the telopodite and those of the KO. We also found that DKO-lacZ expression in the leg primordia was lost in Dll or btd null embryos, consistent with the loss of KOs in these mutants (Cohen and Jurgens, 1989; Estella et al., 2003) (data not shown). DKO activity was also lost from the limb primordia in embryos deficient for the ASC (Fig. 4H). These results indicate that DKO is activated by the same genes that promote LT expression but, in addition, requires proneural input from the ASC.
Distinct cell proliferation dynamics in the coxopodite and telopodite
To follow the development of the telopodite, we performed a time-course experiment to visualize LT-expressing cells throughout larval development. esg-lacZ was used to identify all imaginal disc cells and LT-Gal4, UAS-GFP was used to mark the progenitors of the telopodite. We estimate that there are ∼15 progenitor cells each for the telopodite and coxopodite in stage 14 embryos (Fig. 5A). Previous clonal analyses suggested that the cells of the leg primordia stop dividing during embryogenesis and resume proliferation at approximately 48 hours (Bryant and Schneiderman, 1969). Consistent with these studies, the number of Esg-positive cells began increasing at the beginning of the second larval instar, about 48 hours AEL (Fig. 5A). Surprisingly, at ∼60 hours, the coxopodite progenitors (Esg-positive, LT- and Dll-negative cells) far outnumbered the telopodite progenitors (LT- and Dll-positive). In addition, the nuclei of the telopodite progenitor cells were larger than those of the coxopodite progenitor cells in these early leg discs. The telopodite progenitors also appear to be tightly associated with the larval epidermis, and they continued to express hth and tsh, in addition to Dll and esg (Fig. 5A,B). By the end of the second larval instar stage, between 60 and 72 hours AEL, the entire leg disc invaginated from the larval cuticle, and the number of LT-positive cells was dramatically increased (Fig. 5B). At this stage, these cells no longer expressed tsh or hth (Fig. 5B; and data not shown).
Fig. 5.
Distinct cell proliferation dynamics in the coxopodite and telopodite. (A) Time course of limb primordia development, staining for LT activity (green) and esg-lacZ (red). At 12 hours AEL (stage 14) the number of LT-positive telopodite progenitors is approximately equal to the number of esg-positive, LT-negative coxopodite progenitors (∼15 cells, each). At 24-36 hours AEL (early first instar) the number of LT-positive cells and esg-positive, LT-negative cells remain about the same. At 48-60 hours AEL (early second instar) the number of esg-positive, LT-negative cells is far greater than the number of LT-positive cells. LT-positive (arrow) nuclei are noticeably larger than esg-positive, LT-negative nuclei. By 96-108 hours AEL (third instar) the progeny of the LT-expressing cells now populate the entire telopodite. (B) LT activity (green) compared to tsh expression (red) during development. At early second instar (48-60 hours AEL) the number of cells that are tsh-positive but LT-negative is greater than the number of cells where LT is active. At this time the LT-positive cells also express tsh (arrow). At the end of the second instar (60-72 hours AEL) the number LT-positive cells has increased. At this time the LT-positive cells no longer express tsh (arrow). (C) At 48-60 hours AEL, the ∼15 cells in which LT is active (green) exist as a ring that is coincident with the LT lineage labeling. At 60-72 hours AEL, the LT lineage-labeled cells, although greater in number, all actively express LT. Discs have been outlined with a dotted line. (D) Randomly generated neutral clones induced at 12-24 hours of development were quantified for their position and cell number 36 and 48 hours after clone induction. Examples for each of these experiments are shown in Fig. S4 in the supplementary material. (E) Third instar discs containing neutral clones stained for Hth (red), Dll (green) and β-gal (blue, the clone marker). The subset of randomly marked neutral clones induced between 12 and 24 hours of development that reach the border do not cross between the coxopodite and the telopodite (n=25).
These data suggest that there is a difference in the time when the progenitor cells of the coxopodite and the telopodite begin to proliferate. By direct observation, we estimate that the coxopodite progenitors begin to divide between 12 and 24 hours earlier than those of the telopodite. To rule out that LT-positive cells start to proliferate at the same time, and LT is rapidly shut off in some progeny, we repeated the LT-Gal4 lineage analysis, comparing at early time points the number of cells in which LT had been active with the number that continued to express LT. Fig. 5C shows that these two cell populations are identical, arguing that the progenitors of the telopodite rarely proliferate prior to this time.
We confirmed the delay in the onset of telopodite proliferation by inducing marked clones in both domains between 12 and 24 hours AEL, and quantifying the location and number of cells 36 and 48 hours later. For these experiments, we defined the coxopodite as being Hth- or Tsh-positive and LT-GFP-negative. Conversely, the telopodite progenitors were defined as being LT-GFP-positive. At the 36-hour time point, the average number of cells in telopodite clones was 1.3 (n=31; Fig. 5D; see Fig. S4A in the supplementary material). By contrast, the average number of cells in coxopodite clones was 3.2 (n=51; Fig. 5D; see Fig. S4A in the supplementary material). All KO clones (LT-GFP-negative and Hth/Tsh-negative) remained as single cells (n=11). When measured 48 hours after clone induction, the average number of cells in telopodite clones was 4.6 (n=9) while the average number of cells located in coxopodite clones was 5.6 (n=20; Fig. 5D; see Fig. S4B in the supplementary material). These data suggest that the progenitors of the coxopodite resume proliferating approximately one to two cell divisions earlier than the progenitors of the telopodite.
Interestingly, we found that telopodite and coxopodite clones stayed within their respective domains (LT-GFP expressing or nonexpressing, respectively) (see Fig. S4A,B in the supplementary material). When clones (n=25) induced between 12 and 24 hours were allowed to grow to the third instar, their progeny continued to demonstrate a restriction in lineage (Fig. 5E). However, both sets of clones could enter the trochanter, which expresses both hth and Dll (see Fig. S5A,B in the supplementary material). By contrast, clones induced prior to stage 14 (5.5-7 hours AEL) occasionally spanned both the coxopodite and telopodite (19%, n=32; see Fig. S4C in the supplementary material). These data suggest that there is a lineage restriction along the PD axis of the developing leg that forms at stage 14, about the same time that LT is activated in the limb primordia. This lineage restriction does not constitute a compartment boundary, however, because when cells were given a growth advantage using the Minute technique (Morata and Ripoll, 1975) we observed clones that did not respect this boundary (data not shown). Moreover, this restriction is not a discreet border, but is instead defined by a region (the trochanter), that expresses both telopodite (Dll) and coxopodite (Hth) markers.
DISCUSSION
A cascade of Dll cis-regulatory elements prefigures cell fates in the appendage primordia
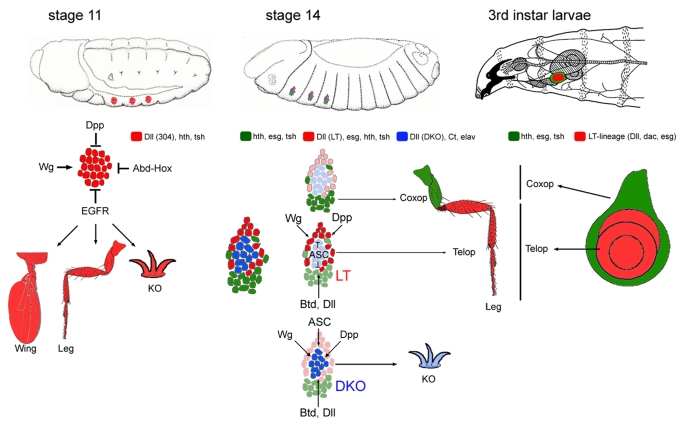
One of the most interesting findings from this work is that the temporal control of Dll expression in the limb primordia by three cis-regulatory elements is linked to cell-type specification (Fig. 6). The earliest acting element, Dll304, is active throughout the appendage primordia. At the time Dll304 is active, the cells are multipotent and can give rise to any part of the dorsal or ventral appendages, or KO. A few hours later, Dll304 activity fades, and two alternative cis-regulatory elements become active. Together, these two elements allow for the uninterrupted and uniform expression of Dll within the appendage primordia. However, their activation correlates with a higher degree of refinement in cell fate potential: LT, active in only the outer ring of the appendage primordia, is only expressed in the progenitors of the telopodite. By contrast, DKO, active in the cells within the LT ring, is only expressed in the progenitors of the KO. Thus, although the pattern of Dll protein appears unchanged, the control over Dll expression has shifted from singular control by Dll304 to dual control by LT and DKO. Moreover, not only is there a molecular handoff from Dll304 to LT and DKO, the two later enhancers both require the earlier expression of Dll. Thus, the logic of ventral primordia refinement depends on a cascade of Dll regulatory elements in which the later ones depend on the activity of an earlier one.
Fig. 6.
The fate map of the thoracic limb primordia. At stage 11 the cells of the limb primordia are multipotent and can contribute to the dorsal appendage (e.g. wing), coxopodite, telopodite, and Keilin's Organ (KO). Also shown are the genetic inputs that control the early Dll304 enhancer at this stage. Approximately 4 hours later (stage 14) the cells in the limb primordia are restricted in their potential. Cells that activate LT (red) give rise only to the telopodite. LT activation requires Wg and Dpp and is restricted to the thorax by Btd and Dll. LT is also repressed by the proneural genes of the ASC in the KO primordia. Cells that activate DKO (blue) are fated to form the KO. The DKO enhancer is controlled by Wg, Dpp, ASC, Btd and Dll. By stage 14 the coxopodite precursor cells (green) do not express Dll and are primarily located ventral to the Dll-positive cells. At the end of larval development the LT lineage gives rise to the entire telopodite (Dll and Dac, in red), while the coxopodite is marked by the restricted expression of tsh and hth (in green).
The high-resolution view of the embryonic limb primordia provided here allows us to clarify some contradictions that currently exist in the literature. Initial expression of Dll in the thorax overlaps entirely with Hth-nExd. Subsequently, hth expression is lost from most, but not all, of the Dll-expressing cells of the leg primordia. The first reports describing these changes failed to recognize the persistent overlap between Dll and Hth-nExd in some cells (Gonzalez-Crespo et al., 1998; Gonzalez-Crespo and Morata, 1996). As a result, and partly because of the analogy with the third instar leg disc, the predominant view of this fate map became that the Dll-positive, Hth-nExd-negative cells of the embryonic primordia gave rise to the telopodite, while the surrounding Hth-positive cells gave rise to the coxopodite (reviewed by Morata, 2001). The expression pattern of esg, a gene required for the maintenance of diploidy, was also misinterpreted as being a marker exclusively of proximal leg fates (Goto and Hayashi, 1997; Kubota et al., 2000; Kubota et al., 2003). Counter to these earlier studies, our experiments unambiguously show that the Dll-positive, Hth-nExd-negative cells in the center of the primordia give rise to the KO, the ring of Dll-positive, Esg-positive, Hth-nExd-positive cells gives rise to the telopodite, and the remaining Esg-positive, Dll-negative cells give rise to the coxopodite (Fig. 6).
The spurious expression of DKO-lacZ in Dll-non-expressing cells outside the leg primorida complicates the interpretation of several experiments. Attempts to refine DKO activity by changing the size of the cloned fragment proved unsuccessful. Nevertheless, our evidence supports the idea that DKO-positive, Dll-positive cells of the leg primordia give rise to the Keilin's organ, and not the adult appendage.
Regulation of proliferation along the PD axis of the developing leg
The progenitors of the coxopodite begin to proliferate at approximately 48 hours of development, consistent with previous measurements of leg imaginal disc growth, whereas the progenitors of the telopodite do not resume proliferating for an additional 12 to 24 hours. According to estimates of the cell cycle time in leg discs (Postlethwait, 1978), this difference in the onset of proliferation results in one to two additional cell divisions in the coxopodite, consistent with images of late second instar leg discs presented here. Why might the telopodite and coxopodite begin proliferation at different times? One possibility is that the cells of the coxopodite give rise to the peripodial epithelium that covers the leg imaginal disc, and therefore require additional cell divisions relative to the telopodite. It is also possible that the telopodite is delayed because the neurons of the Keilin's organ serve a pathfinding role for larval-born neurons that innervate the adult limb (Jan et al., 1985). Perhaps this pathfinding function requires that the KO and telopodite remain associated with each other through the second instar. Consistently, the leg is the only imaginal disc that has not invaginated as a sac-like structure in newly hatched first instar larvae (Madhavan and Schneiderman, 1977).
A possible explanation for the delay in the onset of telopodite proliferation is the persistent co-expression of hth and Dll in these cells; hth (and tsh) expression is turned off in these cells at about the same time they begin to proliferate. Consistent with this idea, maintaining the expression of hth throughout the primordia blocks the proliferation of the telopodite (see Fig. S5C in the supplementary material) (Azpiazu and Morata, 2002). Also noteworthy is the finding that the genes no ocelli and elbow have been shown to mediate the ability of Wg and Dpp to repress coxopodite fates (Weihe et al., 2004). Together with our findings, it is possible that the activation of these two genes in the LT-expressing progenitors is the trigger that turns off hth and tsh in these cells.
Restriction of cell lineage between coxopodite and telopodite
Our experiments suggest that once LT is activated, and under normal growth conditions, there is a lineage restriction between the telopodite and coxopodite. By contrast, previous lineage-tracing experiments using tsh-Gal4 concluded that the progeny of proximal cells could adopt more distal leg fates (Weigmann and Cohen, 1999). However, these authors were unaware that tsh is still expressed in the telopodite progenitors far into the second instar, providing an explanation for their results. In contrast to this early restriction, there is no evidence for a later lineage restriction within the telopodite. For example, the progeny of a Dll-positive cell can lose Dll expression and contribute to the dac-only domain (Gorfinkiel et al., 1997).
Interestingly, the lineage restriction between coxopodite and telopodite is not defined by the presence or absence of Hth-nExd or Tsh because both progenitor populations express hth and tsh after their fates have been specified. By contrast, when these two domains are specified, the telopodite expresses Dll, while the coxopodite does not, suggesting that Dll may be important for the lineage restriction. However, later in development, some cells in the telopodite lose Dll expression and express dac, but continue to respect the coxopodite-telopodite boundary. Thus, either Dll expression in the telopodite is somehow remembered or the telopodite-coxopodite boundary can be maintained by dac, which is expressed in place of Dll immediately adjacent to the telopodite-coxopodite boundary. Also noteworthy is our finding that clones originating in the coxopodite can contribute to the trochanter, the segment inbetween the proximal and distal components of the adult leg that expresses both Dll and hth in third instar imaginal discs (Abu-Shaar and Mann, 1998). However, the progeny of such clones do not contribute to fates more distal than the trochanter. Likewise, a clone originating in the telopodite can also contribute to the trochanter, but will not grow more proximally into the coxa (see Fig. S5A,B in the supplementary material). Thus, the lineage restriction uncovered here seems to be determined by distinct combinations of transcription factors expressed in the coxopodite and telopodite progenitors at stage 14. The progeny of cells that express Dll, tsh and hth can populate the telopodite or trochanter, whereas the progeny of cells that express tsh and hth, but not Dll, can populate the coxopodite or trochanter. In light of the Minute-positive results, however, the lineage restriction between coxopodite and telopodite does not satisfy the classical definition of a compartment boundary. A similar non-compartment lineage restriction has also been documented along the PD axis of the developing Drosophila wing (Zirin and Mann, 2007).
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/1/61/DC1
Supplementary Material
We thank S. Campuzano, G. Boekhoff-Falk, E. Laufer, T. Jessell, G. Morata, G. Struhl, M. Zecca and the Bloomington Stock Center for flies and reagents, the Developmental Studies Hybridoma Bank at The University of Iowa for antibodies, and members of the Mann and Johnston laboratories for discussions and suggestions. We also thank G. Schubiger, M. Milán, M. Giorgianni, O. Hobert and G. Struhl and for comments on the manuscript, and W. Zhang for technical assistance. This work was supported by grants from the NIH (GM058575) and March of Dimes to R.S.M. D.J.M. was partially supported by the Stem Cells and Cell Lineage Specification training grant (HD055165). Deposited in PMC for release after 12 months.
References
- Abu-Shaar, M. and Mann, R. S. (1998). Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development 125, 3821-3830. [DOI] [PubMed] [Google Scholar]
- Abzhanov, A. and Kaufman, T. C. (2000). Homologs of Drosophila appendage genes in the patterning of arthropod limbs. Dev. Biol. 227, 673-689. [DOI] [PubMed] [Google Scholar]
- Azpiazu, N. and Morata, G. (2002). Distinct functions of homothorax in leg development in Drosophila. Mech. Dev. 119, 55-67. [DOI] [PubMed] [Google Scholar]
- Basler, K. and Struhl, G. (1994). Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature 368, 208-214. [DOI] [PubMed] [Google Scholar]
- Bodmer, R., Barbel, S., Sheperd, S., Jack, J. W., Jan, L. Y. and Jan, Y. N. (1987). Transformation of sensory organs by mutations of the cut locus of D. melanogaster. Cell 51, 293-307. [DOI] [PubMed] [Google Scholar]
- Bolinger, R. A. and Boekhoff-Falk, G. (2005). Distal-less functions in subdividing the Drosophila thoracic limb primordium. Dev. Dyn. 232, 801-816. [DOI] [PubMed] [Google Scholar]
- Boxshall, G. A. (2004). The evolution of arthropod limbs. Biol. Rev. Camb. Philos. Soc. 79, 253-300. [DOI] [PubMed] [Google Scholar]
- Brand, A. H. and Dormand, E. L. (1995). The GAL4 system as a tool for unravelling the mysteries of the Drosophila nervous system. Curr. Opin. Neurobiol. 5, 572-578. [DOI] [PubMed] [Google Scholar]
- Bryant, P. J. and Schneiderman, H. A. (1969). Cell lineage, growth, and determination in the imaginal leg discs of Drosophila melanogaster. Dev. Biol. 20, 263-290. [DOI] [PubMed] [Google Scholar]
- Calleja, M., Moreno, E., Pelaz, S. and Morata, G. (1996). Visualization of gene expression in living adult Drosophila. Science 274, 252-255. [DOI] [PubMed] [Google Scholar]
- Campbell, G. and Tomlinson, A. (1998). The roles of the homeobox genes aristaless and Distal-less in patterning the legs and wings of Drosophila. Development 125, 4483-4493. [DOI] [PubMed] [Google Scholar]
- Campbell, G. and Tomlinson, A. (1999). Transducing the Dpp morphogen gradient in the wing of Drosophila: regulation of Dpp targets by brinker. Cell 96, 553-562. [DOI] [PubMed] [Google Scholar]
- Campbell, G., Weaver, T. and Tomlinson, A. (1993). Axis specification in the developing Drosophila appendage: the role of wingless, decapentaplegic, and the homeobox gene aristaless. Cell 74, 1113-1123. [DOI] [PubMed] [Google Scholar]
- Castelli-Gair, J. and Akam, M. (1995). How the Hox gene Ultrabithorax specifies two different segments: the significance of spatial and temporal regulation within metameres. Development 121, 2973-2982. [DOI] [PubMed] [Google Scholar]
- Chan, S. K., Ryoo, H. D., Gould, A., Krumlauf, R. and Mann, R. S. (1997). Switching the in vivo specificity of a minimal Hox-responsive element. Development 124, 2007-2014. [DOI] [PubMed] [Google Scholar]
- Cohen, B., Simcox, A. A. and Cohen, S. M. (1993). Allocation of the thoracic imaginal primordia in the Drosophila embryo. Development 117, 597-608. [DOI] [PubMed] [Google Scholar]
- Cohen, S. (1993). Imaginal disc development. In The Development of Drosophila Melanogaster (ed. M. Bate and A. Martinez Arias), pp. 747-842. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Cohen, S. M. (1990). Specification of limb development in the Drosophila embryo by positional cues from segmentation genes. Nature 343, 173-177. [DOI] [PubMed] [Google Scholar]
- Cohen, S. M. and Jurgens, G. (1989). Proximal-distal pattern formation in Drosophila: cell autonomous requirement for Distal-less gene activity in limb development. EMBO J. 8, 2045-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crickmore, M. A. and Mann, R. S. (2006). Hox control of organ size by regulation of morphogen production and mobility. Science 313, 63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Benjumea, F. J., Cohen, B. and Cohen, S. M. (1994). Cell interaction between compartments establishes the proximal-distal axis of Drosophila legs. Nature 372, 175-179. [DOI] [PubMed] [Google Scholar]
- Dominguez, M. and Campuzano, S. (1993). asense, a member of the Drosophila achaete-scute complex, is a proneural and neural differentiation gene. EMBO J. 12, 2049-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estella, C. and Mann, R. S. (2008). Logic of Wg and Dpp induction of distal and medial fates in the Drosophila leg. Development 135, 627-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estella, C., Rieckhof, G., Calleja, M. and Morata, G. (2003). The role of buttonhead and Sp1 in the development of the ventral imaginal discs of Drosophila. Development 130, 5929-5941. [DOI] [PubMed] [Google Scholar]
- Estella, C., McKay, D. J. and Mann, R. S. (2008). Molecular integration of wingless, decapentaplegic, and autoregulatory inputs into Distalless during Drosophila leg development. Dev. Cell 14, 86-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebelein, B., Culi, J., Ryoo, H. D., Zhang, W. and Mann, R. S. (2002). Specificity of Distalless repression and limb primordia development by abdominal Hox proteins. Dev. Cell 3, 487-498. [DOI] [PubMed] [Google Scholar]
- Gebelein, B., McKay, D. J. and Mann, R. S. (2004). Direct integration of Hox and segmentation gene inputs during Drosophila development. Nature 431, 653-659. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Crespo, S. and Morata, G. (1996). Genetic evidence for the subdivision of the arthropod limb into coxopodite and telopodite. Development 122, 3921-3928. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Crespo, S., Abu-Shaar, M., Torres, M., Martinez, A. C., Mann, R. S. and Morata, G. (1998). Antagonism between extradenticle function and Hedgehog signalling in the developing limb. Nature 394, 196-200. [DOI] [PubMed] [Google Scholar]
- Gorfinkiel, N., Morata, G. and Guerrero, I. (1997). The homeobox gene Distalless induces ventral appendage development in Drosophila. Genes Dev. 11, 2259-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, S. and Hayashi, S. (1997). Specification of the embryonic limb primordium by graded activity of Decapentaplegic. Development 124, 125-132. [DOI] [PubMed] [Google Scholar]
- Goto, S. and Hayashi, S. (1999). Proximal to distal cell communication in the Drosophila leg provides a basis for an intercalary mechanism of limb patterning. Development 126, 3407-3413. [DOI] [PubMed] [Google Scholar]
- Hayashi, S., Hirose, S., Metcalfe, T. and Shirras, A. D. (1993). Control of imaginal cell development by the escargot gene of Drosophila. Development 118, 105-115. [DOI] [PubMed] [Google Scholar]
- Jan, Y. N., Ghysen, A., Christoph, I., Barbel, S. and Jan, L. Y. (1985). Formation of neuronal pathways in the imaginal discs of Drosophila melanogaster. J. Neurosci. 5, 2453-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinska, A., Kirov, N., Wieschaus, E., Roth, S. and Rushlow, C. (1999). The Drosophila gene brinker reveals a novel mechanism of Dpp target gene regulation. Cell 96, 563-573. [DOI] [PubMed] [Google Scholar]
- Kubota, K., Goto, S., Eto, K. and Hayashi, S. (2000). EGF receptor attenuates Dpp signaling and helps to distinguish the wing and leg cell fates in Drosophila. Development 127, 3769-3776. [DOI] [PubMed] [Google Scholar]
- Kubota, K., Goto, S. and Hayashi, S. (2003). The role of Wg signaling in the patterning of embryonic leg primordium in Drosophila. Dev. Biol. 257, 117-126. [DOI] [PubMed] [Google Scholar]
- Lakes-Harlan, R., Pollack, G. S. and Merritt, D. J. (1991). From embryo to adult: anatomy and development of a leg sensory organ in Phormia regina, Meigen (Insecta: Diptera). II. Development and persistence of sensory neurons. J. Comp. Neurol. 308, 200-208. [DOI] [PubMed] [Google Scholar]
- Lecuit, T. and Cohen, S. M. (1997). Proximal-distal axis formation in the Drosophila leg. Nature 388, 139-145. [DOI] [PubMed] [Google Scholar]
- Madhavan, M. and Schneiderman, H. (1977). Histological analysis of the dynamics of growth of imaginal discs and histoblast nests during the larval development of Drosophila melanogaster. Rouxs Arch. Dev. Biol. 183, 269-305. [DOI] [PubMed] [Google Scholar]
- Mann, R. S. and Abu-Shaar, M. (1996). Nuclear import of the homeodomain protein extradenticle in response to Wg and Dpp signalling. Nature 383, 630-633. [DOI] [PubMed] [Google Scholar]
- Minami, M., Kinoshita, N., Kamoshida, Y., Tanimoto, H. and Tabata, T. (1999). brinker is a target of Dpp in Drosophila that negatively regulates Dpp-dependent genes. Nature 398, 242-246. [DOI] [PubMed] [Google Scholar]
- Morata, G. (2001). How Drosophila appendages develop. Nat. Rev. Mol. Cell. Biol. 2, 89-97. [DOI] [PubMed] [Google Scholar]
- Morata, G. and Ripoll, P. (1975). Minutes: mutants of drosophila autonomously affecting cell division rate. Dev. Biol. 42, 211-221. [DOI] [PubMed] [Google Scholar]
- Postlethwait, J. (1978). Clonal analysis of Drosophila cuticular patterns. In The Genetics and Biology of Drosophila (ed. M. Ashburner and T. Wright), pp. 359-442. London: Academic Press.
- Rieckhof, G. E., Casares, F., Ryoo, H. D., Abu-Shaar, M. and Mann, R. S. (1997). Nuclear translocation of extradenticle requires homothorax, which encodes an extradenticle-related homeodomain protein. Cell 91, 171-183. [DOI] [PubMed] [Google Scholar]
- Schock, F., Purnell, B. A., Wimmer, E. A. and Jackle, H. (1999). Common and diverged functions of the Drosophila gene pair D-Sp1 and buttonhead. Mech. Dev. 89, 125-132. [DOI] [PubMed] [Google Scholar]
- Snodgrass, R. (1935). Principles of Insect Morphology, pp. 83-99. New York: McGraw-Hill.
- Struhl, G. and Basler, K. (1993). Organizing activity of wingless protein in Drosophila. Cell 72, 527-540. [DOI] [PubMed] [Google Scholar]
- Tix, S., Bate, C. and Technau, G. (1989). Pre-existing neuronal pathways in the developing leg imaginal discs of Drosophila. Development 107, 855-862. [DOI] [PubMed] [Google Scholar]
- Tsuneizumi, K., Nakayama, T., Kamoshida, Y., Kornberg, T. B., Christian, J. L. and Tabata, T. (1997). Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nature 389, 627-631. [DOI] [PubMed] [Google Scholar]
- Vachon, G., Cohen, B., Pfeifle, C., McGuffin, M. E., Botas, J. and Cohen, S. M. (1992). Homeotic genes of the Bithorax complex repress limb development in the abdomen of the Drosophila embryo through the target gene Distal-less. Cell 71, 437-450. [DOI] [PubMed] [Google Scholar]
- Weigmann, K. and Cohen, S. M. (1999). Lineage-tracing cells born in different domains along the PD axis of the developing Drosophila leg. Development 126, 3823-3830. [DOI] [PubMed] [Google Scholar]
- Weihe, U., Dorfman, R., Wernet, M. F., Cohen, S. M. and Milan, M. (2004). Proximodistal subdivision of Drosophila legs and wings: the elbow-no ocelli gene complex. Development 131, 767-774. [DOI] [PubMed] [Google Scholar]
- Wieschaus, E. and Gehring, W. (1976). Clonal analysis of primordial disc cells in the early embryo of Drosophila melanogaster. Dev. Biol. 50, 249-263. [DOI] [PubMed] [Google Scholar]
- Wu, J. and Cohen, S. M. (2000). Proximal distal axis formation in the Drosophila leg: distinct functions of teashirt and homothorax in the proximal leg. Mech. Dev. 94, 47-56. [DOI] [PubMed] [Google Scholar]
- Zhou, L., Schnitzler, A., Agapite, J., Schwartz, L. M., Steller, H. and Nambu, J. R. (1997). Cooperative functions of the reaper and head involution defective genes in the programmed cell death of Drosophila central nervous system midline cells. Proc. Natl. Acad. Sci. USA 94, 5131-5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirin, J. D. and Mann, R. S. (2007). Nubbin and Teashirt mark barriers to clonal growth along the proximal-distal axis of the Drosophila wing. Dev. Biol. 304, 745-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.