Abstract
White matter abnormalities have been detected using diffusion tensor imaging (DTI) in a variety of locations in the brains of patients with schizophrenia. Studies that included first-episode patients report less severe or no abnormalities but more pronounced deficits in chronic patients. Here we investigated these abnormalities in a very large group of schizophrenia that had both large ranges in age and in duration of illness. A highly reproducible DTI-tractography technique was used to quantify the fractional anisotropy of the genu and splenium of the corpus callosum as well as the bilateral pyramidal tracts. We found a decline in fractional anisotropy that correlated with the duration of illness in the genu and splenium of the corpus callosum but not in the pyramidal tracts. The findings suggest that there are white matter tract-specific degenerative mechanisms that may be present at the point of illness onset and that progress throughout the illness.
Keywords: Diffusion Tensor Imaging, Schizophrenia, Fiber Tracking
Introduction
Schizophrenia is hypothesized to be the result of dysfunctional integration among discrete neuronal systems [1]. As recent evidence pointed to the abnormal expression of myelin-related genes in patients with schizophrenia[2], researchers are revisiting hypotheses of disconnectivity in schizophrenia and shifting focus from strictly neurotransmitter-based primary mechanisms to white matter abnormalities as the potential underlying neuropathology [3]. Here we investigated the temporal characteristics of white matter abnormalities in schizophrenia.
It is important to establish whether the pathogenic agent of schizophrenia is static or progressive in order to guide the design and development of novel therapeutic strategies. The temporal dynamics of white matter abnormalities in schizophrenia are not clear, but advances in medical imaging provide a window through which this phenomenon can be viewed. The advent of diffusion tensor imaging (DTI) [4] has allowed for many in vivo white matter investigations to bring attention to the anatomic interruption of connectivity that may prove to be the cardinal lesion of the schizophrenia [5,6]. If these abnormalities prove to be progressive rather than static and relate to patient outcome, then it becomes worthwhile to search for new methods to slow their progression as early as possible.
DTI studies of schizophrenia have not always been consistent and the discrepancies that exist in the literature may in part be due to the image analysis methods used [5,7]. The two most popular DTI analysis techniques, whole brain voxel-based analysis and manually placed regions of interest (ROIs), each have shortcomings that could limit the consistency of findings [7].
DTI-tractography is a relatively new method of DTI quantification by which it is possible to follow the directionality of diffusion throughout the brain and reconstruct the major white matter tracts in three-dimensions [8]. The results can then serve as tract specific 3-dimensional ROIs and have been shown to be more sensitive than both voxel-based analysis and manually placed ROIs in detecting tract specific abnormalities in patients with schizophrenia [7].
In the present study, we applied a highly reproducible quantitative DTI tractography algorithm to investigate the temporal characteristics of white matter abnormalities in a very large cohort of patients with schizophrenia. DTI parameters were quantified for the genu and splenium of the corpus callosum as well as the bilateral pyramidal tracts. We included the pyramidal tracts in this investigation as a control because this tract has not been reported to be affected in schizophrenia. With a very large dataset and an advanced DTI processing technique we discovered a significant progressive, tract-specific decline in schizophrenia that is related to illness duration.
Methods
Subjects
One hundred fifteen patients with schizophrenia were recruited from inpatient, outpatient, day treatment and vocational rehabilitation service centers following approvals by each institutional review board and receipt of informed consent. The diagnosis of schizophrenia was confirmed by a structured diagnostic interview (Comprehensive Assessment of Psychiatric Symptoms and History; CASH [9]). Drug testing and medical screening was performed to exclude patients with substance abuse and cardiovascular disease that might affect MRI results. One hundred and sixteen healthy comparison subjects, who were without any DSMIV axis I disorder, as assessed by CASH interview, were recruited from the New York area. Subjects were excluded if they had a positive urine drugs of abuse screen, a medical diagnosis which may produce white matter changes, a history of brain disorder which may produce cognitive impairment or behavioral symptoms, or had an unstable medical condition. Subjects were carefully screened with a structured assessment of symptoms, review of medical history, physical examination, laboratory studies, urine toxicology screen and electrocardiogram to ensure they fulfilled the inclusion criteria.
The necessity to match for age and gender resulted in a reduction in the total number of subjects included in the final analysis. Seventy-six patients (51 M, 24 F) that ranged in age from 18 to 78 (mean = 36 +/− 15 years) and ranged in duration of illness from less than 1 year to 52 years (mean = 15 +/− 14 years) were carefully matched for age and gender with seventy-seven healthy subjects (52 M, 25 F) that ranged in age from 18 to 82 years (mean = 37 +/− 17 years). A t test revealed no difference in the age of the groups (p = 0.7). The influence that several variables, such as medication dose and type, ethnicity and cognitive function, has on DTI parameters is not well established and were not considered in this analysis.
Image Acquisition
All imaging were performed on a 3T Allegra MRI scanner (Siemens, Ehrlangen, Germany). The following structural scans were acquired: Axial 3D-MPRage (TR = 2500 ms, TE = 4.4 ms, FOV = 21 cm, matrix size = 256×256, 208 slices with thickness = 0.82 mm); Turbo spin echo (TSE) T2-weighted Axial (TR = 5380 ms, TE = 99 ms, FOV = 18.3×21 cm, matrix = 512×448, Turbo factor = 11, 28 slices, thickness = 3 mm skip 1 mm); DTI using a pulsed-gradient spin-echo sequence with EPI-acquisition (TR = 4100 ms, TE = 80 ms, FOV = 21 cm, matrix = 128×128, 28 slices, thickness = 3 mm skip 1 mm, b-factor = 1250 s/mm2, 12 gradient directions, 5 averages).
DTI Processing and Fiber Tracking
The diffusion data set was eddy-current and motion corrected using an adaptation of the Camino/SPM package [10]. In-house software was developed in Matlab v2007a (The Mathworks Inc., Natick, MA) for further processing of the DTI and tractography.
A multiple region brute-force fiber tracking method was used [11]. First, fibers were traced using a streamline tractography algorithm from every voxel throughout the entire volume that exceeded a minimum fractional anisotropy (FA) [8]. Tracking was terminated when FA fell below a 0.1 or when the algorithm encountered a sharp angle change in the principal diffusion direction between sequential voxels (45°). Second, each tract was indexed such that a queried voxel returns all streamlines that pass through it. Multiple target regions were selected for each tract and are defined below. Only the portion of the tracts that spanned between, and not through the ROIs was used for the subsequent analysis. Tract integrity was quantified using a normalized line integral of FA along the tract.
Target Regions for Tractography
The criteria for the tractography were defined in MNI coordinates on the mean of the normalized FA images. The target regions were transformed from MNI coordinates to the original DTI space based on the normalization parameters determined by SPM2 (Wellcome Department of Cognitive Neurology, Institute of Neurology, University College London, London, UK) for each subject. The normalization parameters were computed by coregistering the no-diffusion weighted image of the DTI set to the T2-TSE image and then normalizing to the T2 MNI template provided with SPM2. Tractography criteria can be defined on the MNI image and then transformed into each subject’s DTI data for tractography. Endpoints for tractography were defined centered in MNI coordinates at (21, 52, 6) and (−21, 52, 6) for the genu, (−19, −79, 16) and (17, −79, 16) for the splenium of the corpus callosum. The pyramidal target regions were centered at (18, −31, 51) and (10, −22, −17) for the left, and (−18, −31, 51) and (−10, −22, −17) for the right. To avoid erroneous tracking additional criteria stipulated regions where streamlines must pass through for the genu and splenium of the corpus callosum that were centered at (0, 21, 5) and (0, −37, 14), respectively. The target region selection for tractography was based on the Mori MRI Atlas of Human White Matter [12], and studies of DTI tractography reproducibility [13].
Statistical Analysis
Statistical analysis was performed using Statistica 7 (Statsoft Inc., Tulsa, OK). Linear regressions were performed to determine white matter parameter and age relationships for each tract. A homogeneity of slopes test was performed to detect differences between patients and controls in the slope of DTI parameters and age. Analysis of covariance (ANCOVA) was used to calculate age and sex effects in the healthy subjects. The duration of illness analysis of the patients was performed after adjustment for age and sex by calculating the residuals from the ANCOVA model. Linear regressions were performed on the adjusted FA values and the duration of illness for patients.
Results
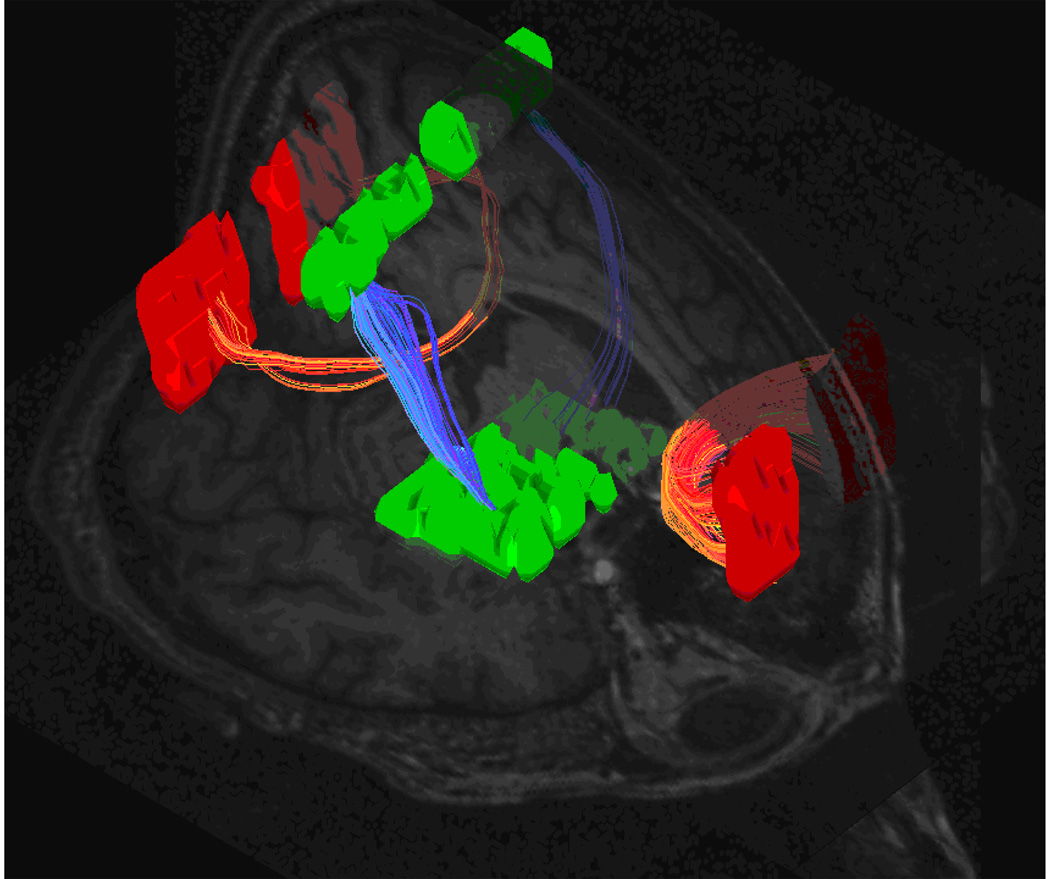
Seventy-six patients with schizophrenia and seventy-seven carefully age and gender matched healthy control subjects were included for the final analysis. The DTI data were used to quantify the white matter integrity with a brute-force tractography algorithm (Figure 1). Renderings of the images were each visually inspected and verified to be consistent with anatomical atlases [12,14].
Figure 1.
Multiple criteria brute-force fiber tracking of the genu and splenium of the corpus callosum as well as the bilateral pyramidal tracts.
Tract FA was quantified and tested for linear correlation with age for both groups (Table 1). The FA of each tract correlated with age in the healthy subjects. The homogeneity of slopes test revealed significant differences in the slopes between controls and patients for only the FA of the genu (t = −2.5, p < 0.05; Table 1).
Table 1.
Pearson correlation coefficients and slopes of white matter parameters versus age in each group. The slopes reported are FA units × 1000/yr. Differences in slopes were calculated using a homogeneity of slopes function in Statistica 7. Pearson correlation coefficients are reported for adjusted FA values (see methods) and duration of illness of the patients. No-significance (ns) is reported when p > 0.05.
| Healthy Subjects | ||||
|---|---|---|---|---|
| Left Pyramidal | Right Pyramidal | Genu | Splenium | |
| r-value | −0.26 | −0.27 | −0.72 | −0.25 |
| Slope | −0.52 | −0.52 | −1.77 | −0.59 |
| p-value | < 0.05 | < 0.05 | < 0.001 | < 0.05 |
| Patients with Schizophrenia | ||||
| Left Pyramidal | Right Pyramidal | Genu | Splenium | |
| r-value | −0.10 | −0.02 | −0.73 | −0.41 |
| Slope | −0.23 | −0.05 | −2.64 | −1.41 |
| p-value | n/s | n/s | < 0.001 | < 0.001 |
| Homogeneity of Slopes | ||||
| Left Pyramidal | Right Pyramidal | Genu | Splenium | |
| t-value | 0.87 | 1.46 | −2.51 | −1.86 |
| p-value | ns | ns | < 0.05 | ns |
| Duration of illness | ||||
| Left Pyramidal | Right Pyramidal | Genu | Splenium | |
| r-value | 0.14 | 0.19 | −0.38 | −0.24 |
| p-value | ns | ns | p < 0.001 | p < 0.05 |
An ANCOVA was used to calculate the parameters for each tract to adjust for age and sex based on the healthy control subjects. Sex was not a significant factor for any of the four tracts.
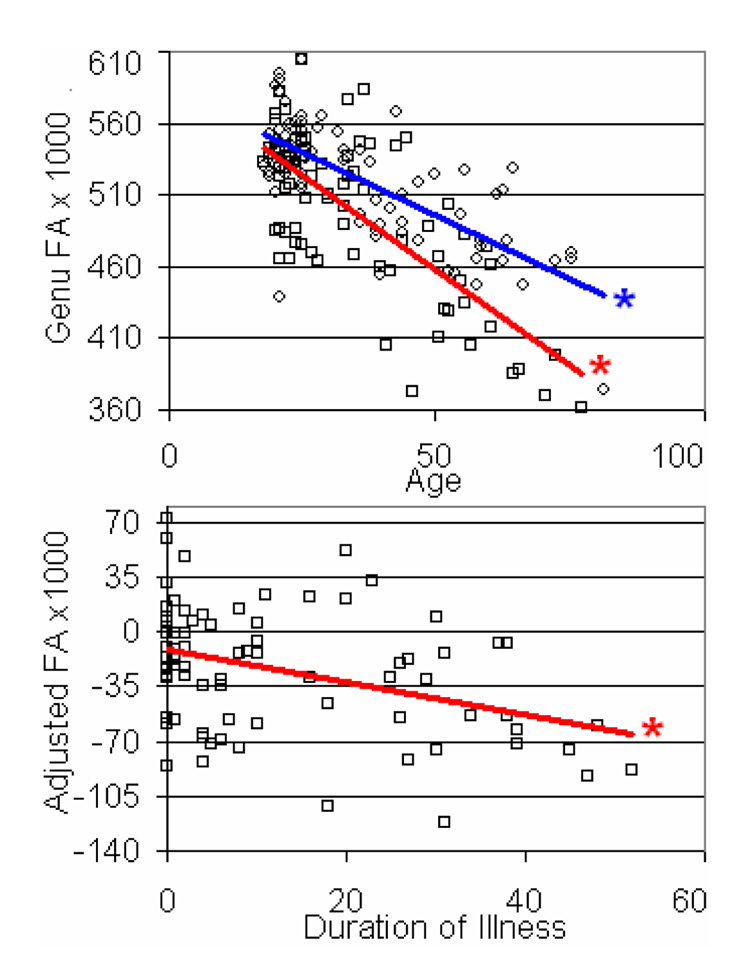
The duration of illness correlated with the age and gender adjusted FA of the genu of the corpus callosum (r = −3.8, p < 0.001; Figure 2) and revealed a trend with the splenium (r = −0.24, p < 0.05). No trend was seen in the pyramidal tracts (Table 1).
Figure 2.
FA versus age and duration of illness for the genu of the corpus callosum. Blue and red lines show the linear fit for healthy (circles) and patient (squares) data points. The FA of patients was adjusted for age and gender for the duration of illness plot (below). Asterisks indicate correlations where p < 0.001.
Discussion
Although neuroimaging has greatly advanced hypotheses of disconnectivity in schizophrenia the inconsistencies in the literature are still disconcerting. In this study, DTI-tractography was used to investigate white matter abnormalities in schizophrenia in what is the largest sample size to undergo this type of analysis to date. With this advanced technique and large sample we were able to detect a correlation between white matter integrity and the duration of illness.
Abnormality Progression Throughout Schizophrenia
It is important to understand the initial as well as the progressive abnormalities that might contribute to the psychotic symptomatology of patients with schizophrenia. Most studies to date confirm some degree of FA reduction in schizophrenia [5,6], but reports on first-episode patients are much less consistent. The discrepant findings in first-episode subjects range from region specific FA reductions [15] to no observed difference in FA when compared to healthy control subjects [16]. Several factors could contribute to these inconsistencies and include differences in imaging parameters, processing algorithms, and analysis; small sample sizes with inadequate power; and the overall heterogeneity of schizophrenia.
This is the first quantitative tractography study of this size to investigate the temporal nature of white matter abnormalities in schizophrenia. Greater FA reductions in chronic patients compared with first episode patients with schizophrenia have been demonstrated using a variety of methodologies yet many groups have failed to observe an inverse correlation between FA and illness duration [16–20]. One recent study does report such an inverse correlation with illness duration but did not include the confound of aging effects in the model [21]. In this study we sampled a very large patient cohort that spanned a great range of age and illness duration in addition to a carefully age and gender matched control group. We found that illness duration had a significant negative correlation with the FA of the genu of the corpus callosum, a finding that is in support of a progressive degenerative hypothesis [22]. The tract-specific FA decline that we showed to occur throughout the illness suggests the presence of selective degenerative mechanisms that might be present at illness onset and continue to damage the white matter throughout the illness.
Although quantitative DTI tractography seems to be more sensitive in detecting FA differences [7], the method is relatively new and there is no gold standard for comparison. In this study, the target regions for tractography were defined in standard MNI coordinates and transformed back to the original DTI space. One concern is that this method could result in improper localization of the target region in the event of poor normalization. Smaller fiber tracts, such as the cingulum bundle or the fornix, would be more susceptible to tracking errors due to misalignments from poor normalization. To avoid these problems we investigated large tracts with multiple large tractographic target regions. In addition, to prevent the inclusion of aberrant tracks and increase the validity of the tractography [11] we used the brute-force algorithm in combination with a multiple target region criteria. All normalized images as well as three-dimensional fiber renderings were visually inspected for distortions and errors.
The FA data reported for several tracts fit a linear regression with subject age, but since the ages of the subjects ranged from 18 to 80 years several points need be discussed. First, it has been shown that white matter volume [23] and anisotropy [24] increase during the first two decades of life. Second, it is possible that cerebral white matter development continues into adulthood with frontal lobe white matter volume peaking as late as in the fifth decade of life [25]. Both of these phenomena raise the possibility that FA could change between 18 and 80 years of age in a non-linear fashion. Age effects in our sample appeared to be more pronounced in the FA of the genu of the corpus callosum as it showed a greater reduction with age than the pyramidal tracts. Other aging studies have shown varying rates of decline depending on the white matter tract investigated [21]. It is important to understand the specificity of this healthy decline not only for studying aging, but also for investigating the temporal dynamics of other factors that might affect white matter, such as schizophrenia.
Conclusions
The method of FA quantification utilized in this investigation provides evidence suggestive of a progressive loss in white matter integrity in patients with schizophrenia beyond that accounted for by healthy aging. The observation of such progressive changes in patients that receive ongoing antipsychotic treatment suggests that these treatments do not arrest this deterioration.
Acknowledgments
This work was supported by the NIH grants P50 MH66392 and a GCRC grant M01 RR00071 awarded to the Mount Sinai School of Medicine.
References
- 1.Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 2.Hof PR, Haroutunian V, Friedrich VL, Jr, et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry. 2003;53:1075–1085. doi: 10.1016/s0006-3223(03)00237-3. [DOI] [PubMed] [Google Scholar]
- 3.Davis KL, Stewart DG, Friedman JI, et al. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 4.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubicki M, McCarley R, Westin CF, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang CY, Friedman J, Shungu D, et al. Correlations between Diffusion Tensor Imaging (DTI) and Magnetic Resonance Spectroscopy (1H MRS) in schizophrenic patients and normal controls. BMC Psychiatry. 2007;7:25. doi: 10.1186/1471-244X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanaan RA, Shergill SS, Barker GJ, et al. Tract-specific anisotropy measurements in diffusion tensor imaging. Psychiatry Res. 2006;146:73–82. doi: 10.1016/j.pscychresns.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- 10.Cook PA, Bai Y, Nedjati-Gilani S, et al. Camino: Open-Source Diffusion-MRI Reconstruction and Processing. 14th Scientific Meeting of the International Society for Magnetic Resonance in Medicine; Seattle, WA, USA. 2006. p. 2759. [Google Scholar]
- 11.Huang H, Zhang J, van Zijl PC, Mori S. Analysis of noise effects on DTI-based tractography using the brute-force and multi-ROI approach. Magn Reson Med. 2004;52:559–565. doi: 10.1002/mrm.20147. [DOI] [PubMed] [Google Scholar]
- 12.Mori S, Wakana S, Nagae-Poetscher L, van Zijl PC. MRI Atlas of Human White Matter. 2005 doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 13.Heiervang E, Behrens TE, Mackay CE, Robson MD, Johansen-Berg H. Between session reproducibility and between subject variability of diffusion MR and tractography measures. Neuroimage. 2006;33:867–877. doi: 10.1016/j.neuroimage.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 14.Haines DE. Neuroanatomy: An Atlas of Structures, Sections, and Systems. Philadelphia, PA: Lippincott Williams & Wilkins; 1991. [Google Scholar]
- 15.Szeszko PR, Ardekani BA, Ashtari M, et al. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry. 2005;162:602–605. doi: 10.1176/appi.ajp.162.3.602. [DOI] [PubMed] [Google Scholar]
- 16.Friedman JI, Tang CY, Carpenter DM, et al. Diffusion Tensor Imaging Findings In Schizophrenia: A Comparison of First Episode and Chronic Patients. The American Journal of Psychiatry. 2008 doi: 10.1176/appi.ajp.2008.07101640. In Press. [DOI] [PubMed] [Google Scholar]
- 17.Foong J, Maier M, Clark CA, Barker GJ, Miller DH, Ron MA. Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2000;68:242–244. doi: 10.1136/jnnp.68.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubicki M, Park H, Westin CF, et al. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26:1109–1118. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubicki M, Westin CF, Nestor PG, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003;54:1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minami T, Nobuhara K, Okugawa G, et al. Diffusion tensor magnetic resonance imaging of disruption of regional white matter in schizophrenia. Neuropsychobiology. 2003;47:141–145. doi: 10.1159/000070583. [DOI] [PubMed] [Google Scholar]
- 21.Mori T, Ohnishi T, Hashimoto R, et al. Progressive changes of white matter integrity in schizophrenia revealed by diffusion tensor imaging. Psychiatry Res. 2007;154:133–145. doi: 10.1016/j.pscychresns.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Woods BT. Is schizophrenia a progressive neurodevelopmental disorder? Toward a unitary pathogenetic mechanism. Am J Psychiatry. 1998;155:1661–1670. doi: 10.1176/ajp.155.12.1661. [DOI] [PubMed] [Google Scholar]
- 23.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee P, Miller JH, Shimony JS, et al. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol. 2002;23:1445–1456. [PMC free article] [PubMed] [Google Scholar]
- 25.Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]