Abstract
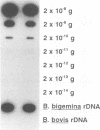
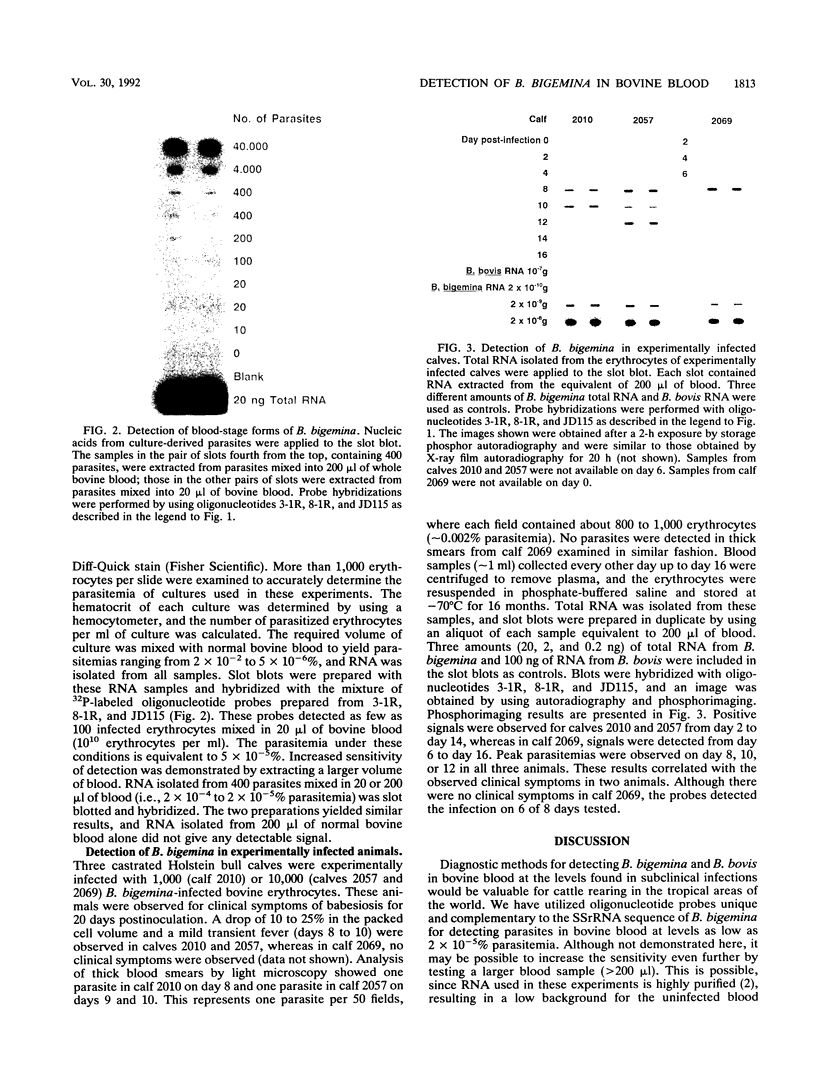
Three synthetic oligonucleotide probes complementary to unique regions of Babesia bigemina small-subunit rRNA were developed for detecting the parasite in bovine blood. These probes specifically detected a parasitemia of 2 x 10(-5)% by autoradiography in less than 24 h by using a 200-microliters sample of bovine blood. These probes did not bind to total RNA or genomic DNA isolated from another closely related species, Babesia bovis, or to bovine leukocyte RNA. This method detected B. bigemina infections in calves inoculated with as few as 1,000 infected erythrocytes from the second day onward for 16 days.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buening G. M., Barbet A., Myler P., Mahan S., Nene V., McGuire T. C. Characterization of a repetitive DNA probe for Babesia bigemina. Vet Parasitol. 1990 May;36(1-2):11–20. doi: 10.1016/0304-4017(90)90089-t. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dams E., Hendriks L., Van de Peer Y., Neefs J. M., Smits G., Vandenbempt I., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1988;16 (Suppl):r87–173. doi: 10.1093/nar/16.suppl.r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake T. A., Hindler J. A., Berlin O. G., Bruckner D. A. Rapid identification of Mycobacterium avium complex in culture using DNA probes. J Clin Microbiol. 1987 Aug;25(8):1442–1445. doi: 10.1128/jcm.25.8.1442-1445.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel U., Maas R., Haun G., Vinga-Martins C., Stanbridge E. J. Synthetic oligonucleotide probes complementary to rRNA for group- and species-specific detection of mycoplasmas. Isr J Med Sci. 1987 Jun;23(6):742–746. [PubMed] [Google Scholar]
- Jensen N. S., Casey T. A., Stanton T. B. Detection and identification of Treponema hyodysenteriae by using oligodeoxynucleotide probes complementary to 16S rRNA. J Clin Microbiol. 1990 Dec;28(12):2717–2721. doi: 10.1128/jcm.28.12.2717-2721.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A. A., Changkasiri S., Hollingdale M. R., McCutchan T. F. Ribosomal RNA-based diagnosis of Plasmodium falciparum malaria. Mol Biochem Parasitol. 1989 Aug;36(1):67–71. doi: 10.1016/0166-6851(89)90201-6. [DOI] [PubMed] [Google Scholar]
- Mahoney D. F. Bovine babesiasis: a study of factors concerned in transmission. Ann Trop Med Parasitol. 1969 Mar;63(1):1–14. doi: 10.1080/00034983.1969.11686595. [DOI] [PubMed] [Google Scholar]
- McLaughlin G. L., Edlind T. D., Ihler G. M. Detection of Babesia bovis using DNA hybridization. J Protozool. 1986 Feb;33(1):125–128. doi: 10.1111/j.1550-7408.1986.tb05571.x. [DOI] [PubMed] [Google Scholar]
- Posnett E. S., Ambrosio R. E. DNA probes for the detection of Babesia caballi. Parasitology. 1991 Jun;102(Pt 3):357–365. doi: 10.1017/s0031182000064301. [DOI] [PubMed] [Google Scholar]
- Reddy G. R., Chakrabarti D., Yowell C. A., Dame J. B. Sequence microheterogeneity of the three small subunit ribosomal RNA genes of Babesia bigemina: expression in erythrocyte culture. Nucleic Acids Res. 1991 Jul 11;19(13):3641–3645. doi: 10.1093/nar/19.13.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackebrandt E., Witt D., Kemmerling C., Kroppenstedt R., Liesack W. Designation of Streptomycete 16S and 23S rRNA-based target regions for oligonucleotide probes. Appl Environ Microbiol. 1991 May;57(5):1468–1477. doi: 10.1128/aem.57.5.1468-1477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega C. A., Buening G. M., Green T. J., Carson C. A. In vitro cultivation of Babesia bigemina. Am J Vet Res. 1985 Feb;46(2):416–420. [PubMed] [Google Scholar]
- Vega C. A., Buening G. M., Rodriguez S. D., Carson C. A. Concentration and enzyme content of in vitro-cultured Babesia bigemina-infected erythrocytes. J Protozool. 1986 Nov;33(4):514–518. doi: 10.1111/j.1550-7408.1986.tb05653.x. [DOI] [PubMed] [Google Scholar]
- Waters A. P., McCutchan T. F. Rapid, sensitive diagnosis of malaria based on ribosomal RNA. Lancet. 1989 Jun 17;1(8651):1343–1346. doi: 10.1016/s0140-6736(89)92800-6. [DOI] [PubMed] [Google Scholar]
- Waters A. P., McCuthan T. F. Ribosomal RNA: nature's own polymerase-amplified target for diagnosis. Parasitol Today. 1990 Feb;6(2):56–59. doi: 10.1016/0169-4758(90)90071-b. [DOI] [PubMed] [Google Scholar]
- Wesley I. V., Wesley R. D., Cardella M., Dewhirst F. E., Paster B. J. Oligodeoxynucleotide probes for Campylobacter fetus and Campylobacter hyointestinalis based on 16S rRNA sequences. J Clin Microbiol. 1991 Sep;29(9):1812–1817. doi: 10.1128/jcm.29.9.1812-1817.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. H., Blitchington R., Hindenach B., Greene R. C. Species-specific oligonucleotide probes for rRNA of Clostridium difficile and related species. J Clin Microbiol. 1988 Dec;26(12):2484–2488. doi: 10.1128/jcm.26.12.2484-2488.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]