Abstract
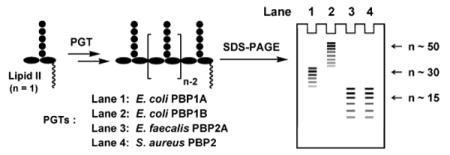
Peptidoglycan is an essential component of bacterial cell wall. The glycan strands of peptidoglycan are synthesized by enzymes called peptidoglycan glycosyltransferases (PGTs). Using a high-resolution SDS-PAGE assay, we compared the glycan strand lengths of four different PGTs from three different organisms (Escherichia coli, Enterococcus faecalis, and Staphylococcus aureus). We report that each enzyme makes a polymer having an intrinsic characteristic length that is independent of the enzyme:substrate ratio. The glycan strand lengths vary considerably depending on the enzyme. These results indicate that each enzyme must have some mechanism, as yet unknown, for controlling product length. The observation that different PGTs produce different length glycan chains may have implications for their cellular roles and for the three dimensional structure of bacterial peptidoglycan.
Bacterial cells are surrounded by a polymer matrix comprising crosslinked strands of peptidoglycan (PG). This matrix, called the sacculus, functions as an exoskeleton, maintaining cell shape and enabling the plasma membrane to withstand high internal osmotic pressures.1 The three dimensional architecture of PG is not yet clear, but is presumed to depend, among other things, on the lengths of the glycan strands, which are synthesized by processive enzymes called peptidoglycan glycosyltransferases (PGTs).2 Numerous studies have evaluated lengths of glycan strands from digested bacterial sacculi, and a range of values have been reported even for digests from the same organism.3 Because substrates and appropriate analytical methods were not available until recently, there have been no systematic studies comparing the lengths of glycan strands produced by different PGTs in vitro. Using a high-resolution gel electrophoresis assay recently developed in our laboratory,2b we report here a comparative study of the glycan strand length distributions produced by four different PGTs, Escherichia coli PBP1A (E. coli PBP1A), Escherichia coli PBP1B (E. coli PBP1B), Enterococcus faecalis PBP2A (E. faecalis PBP2A), and Staphylococcus aureus PBP2 (S. aureus PBP2) (Fig 1). We show that different PGTs produce glycan chains having a characteristic intrinsic length distribution. The intrinsic lengths are a function of the particular PGT but are independent of enzyme:substrate ratios. There is a correlation between the intrinsic in vitro product lengths and the longest strands isolated from sacculi. The implications of these observations for the architecture of the bacterial cell wall are discussed.
Figure 1.
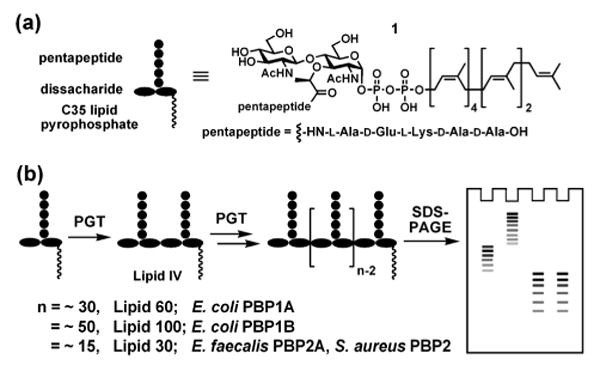
(a) The chemical structure of the heptaprenyl-[14C]-Lipid II analogue 1 used in this study. The 14C radiolabels are incorporated into the GlcNAc residue. (b) Schematic representation of the SDS-PAGE assay.
The four PGTs we studied were overexpressed, purified and subjected under similar conditions to reaction with heptaprenyl-[14C]-Lipid II (1) (Fig 1a).2,4,5,6 Unexpectedly, we found that the four PGTs produced glycan chains of different limiting lengths (i.e., the size beyond which the length does not increase even if reaction times are extended and additional substrate is added) under similar reaction conditions. For E. coli PBP1B, the limiting length was ∼50 disaccharide units (Lipid 100), whereas it was ∼30 disaccharide units for E. coli PBP1A (Lipid 60), and ∼15 disaccharide units for E. faecalis PBP2A and S. aureus PBP2 (Lipid 30) (Fig 2). These findings suggest that there are intrinsic differences among the enzymes with respect to the features that control chain length even though the reaction mechanisms are similar.
Figure 2.
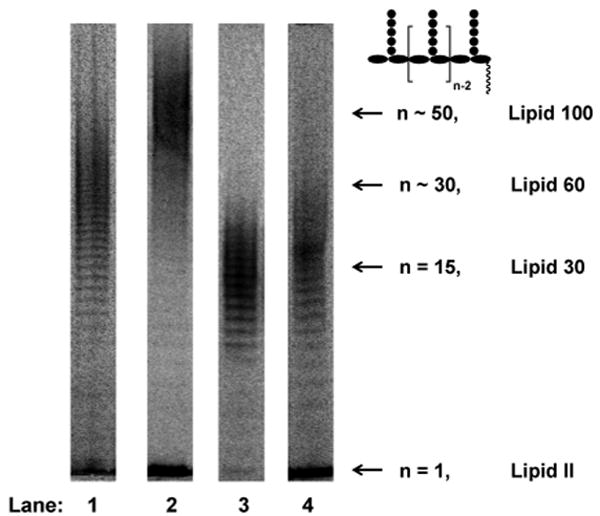
Length distributions of four full-length PGTs: E. coli PBP1A (Lane 1), E. coli PBP1B (Lane 2), E. faecalis PBP2A (Lane 3) and S. aureus PBP2 (Lane 4). The reactions in lane 1 to 3 were quenched after 3 minutes and the reaction in lane 4 was quenched after 60 minutes. The product lengths in each lane were determined by glycan chain ladders for shorter products (data not shown) or retardation factors for longer products, as described in ref. 2b.
To probe the factors that affect polymer length, we evaluated whether enzyme:substrate ratios affect the length distributions. Preliminary experiments showed that the glycan strand length distributions were identical at different enzyme:substrate ratios for each enzyme.7 The results for E. faecalis PBP2A are particularly clear because this enzyme makes relatively short glycan chains (∼15 disaccharide units) that fall within the well-resolved region of the polyacrylamide gel.4e We found that there were no significant differences in final product lengths even when the enzyme:substrate ratio varied by a factor of 100 (Fig. 3). It might be expected that an enzyme:substrate ratio of 1:1 would yield mainly single turnover products (e.g., Lipid IV, n = 2), but instead, a distribution centered around Lipid 30 (15 disaccharide units) was observed. These results imply that there is a slow step in which a small fraction of the active enzyme in the reaction couples Lipid II subunits to form Lipid IV, and this slow step is followed by a rapid elongation process during which Lipid II subunits are added until the products reach the intrinsic length threshold.8 A consequence of the slow initiation-fast polymerization process is that PGTs produce long glycan chains even when Lipid II is limiting.
Figure 3.
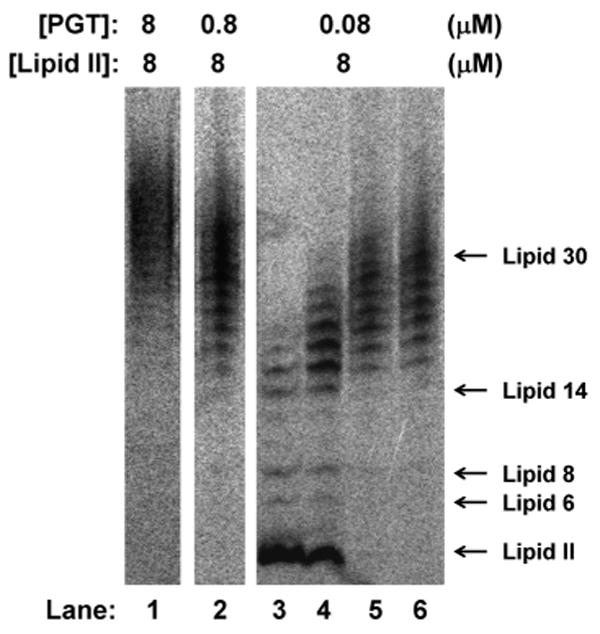
Effect of enzyme:substrate ratio on glycan chain lengths produced by E. faecalis ΔTMPBP2A. Concentrations of Lipid II and enzyme used in each reaction are shown. Reactions in lane 1 and 2 were quenched after 20 minutes. Reactions in lanes 3 to 6 were quenched after 3, 5, 20 and 65 minutes respectively. The final average lengths at all three enzyme:substrate ratios are around Lipid 30 (15 disaccharide units).
Additional evidence for an intrinsic product length distribution emerges from a comparison of experiments carried out at 1:10 or 1:100 enzyme:substrate ratios. Under these conditions, E. faecalis ΔTMPBP2A produced Lipid 30 chains even when the reaction went to complete conversion. If the oligomeric products had not released once the intrinsic length was reached, the observed product length at full conversion for the 1:100 enzyme:substrate ratio would have been ∼Lipid 200 (100 disaccharide units), whereas at the 1:10 ratio it would have been Lipid 20 (10 disaccharide units). Taken together, the enzyme:substrate ratio experiments imply that E. faecalis ΔTMPBP2A must have some mechanism to facilitate product release at a length of about Lipid 30 so that new oligomers can initiate and polymerize until all the Lipid II is used up. The results obtained for E. faecalis ΔTMPBP2A hold for all the other PGTs studied here, which also produce glycan strands of a characteristic length regardless of enzyme:substrate ratios.
The existence of an intrinsic product length for these processive glycan polymerases, which translocate rather than release products during elongation, implies that there must be a termination/release mechanism that frees the products once the length threshold is achieved. The biological significance of different PGTs producing different length distributions is unclear, but it should be pointed out that most bacteria contain multiple PGTs. Some studies have suggested that the different PGTs act at different stages of cell growth and division,9 and it is possible that the variation in glycan strand lengths reflects different cellular functions.
In closing, we note that there have been many studies aimed at determining peptidoglycan chain lengths because this information is required to assess models of cell wall architecture.10,1a Two extreme models for the structure of the bacterial cell wall have been proposed:3a,11 in the classical model, the glycan strands are parallel to the bacterial cell membrane; in the more recently proposed scaffold model, they are perpendicular. HPLC profiles of glycan strands isolated from digests of E. coli sacculi show both long (13 to >31 disaccharide units) and short (2 to 12 disaccharide units) glycan chains.3b The short strands may result from sample preparation procedures and/or may reflect processing by lytic enzymes during cell wall synthesis.3a Regardless of how these short strands arise, the longer strands isolated from E. coli sacculi are similar in length to the intrinsic glycan chain lengths produced by the E. coli PGTs in vitro. These long strands are only consistent with a model for peptidoglycan architecture in which the glycan chains are parallel to the cell surface. It is possible that the structure of peptidoglycan varies at different locations in the cell, perhaps explaining the presence of both short and long strands.11d
Supplementary Material
Supporting Information Available: Experimental procedures, including the cloning and purification of E. faecalis ΔTMPBP2A and the details of SDS-PAGE assays are described. This material is available free of charge via the Internet at http://pubs.acs.org/.
Acknowledgments
This work was supported by the NIH (GM076710).
References
- 1.(a) Vollmer W, Blanot D, de Pedro MA. FEMS Microbiol Rev. 2008;32:149–167. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]; (b) Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. FEMS Microbiol Rev. 2008;32:234–258. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 2.(a) Yuan Y, Barrett D, Zhang Y, Kahne D, Sliz P, Walker S. Proc Natl Acad Sci USA. 2007;104:5348–5353. doi: 10.1073/pnas.0701160104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Barrett D, Wang TSA, Yuan Y, Zhang Y, Kahne D, Walker S. J Biol Chem. 2007;282:31964–31971. doi: 10.1074/jbc.M705440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Vollmer W, Höltje JV. J Bacteriol. 2004;186:5978–5987. doi: 10.1128/JB.186.18.5978-5987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Harz H, Burgdorf K, Höltje JV. Anal Biochem. 1990;190:120–128. doi: 10.1016/0003-2697(90)90144-x. [DOI] [PubMed] [Google Scholar]; (c) Schindler M, Mirelman D, Schwarz U. Eur J Biochem. 1976;71:131–134. doi: 10.1111/j.1432-1033.1976.tb11098.x. [DOI] [PubMed] [Google Scholar]; (d) Glauner B, Höltje JV, Schwarz U. J Biol Chem. 1988;263:10088–10095. [PubMed] [Google Scholar]
- 4.Four full-length and one transmembrane (TM) anchor truncated PGTs (E. faecalis ΔTMPBP2A) were used in this study. See the following references for expression and purification conditions. For E. coli PBP1A:Zhang Y, Fechter EJ, Wang TSA, Barrett D, Walker S, Kahne D.J Am Chem Soc 20071293080–3081.For E. coli PBP1B:Chen L, Walker D, Sun B, Hu Y, Walker S, Kahne D.Proc Natl Acad Sci USA 20031005658–5663.For E. faecalis PBP2A:Adachi M, Zhang Y, Leimkuhler C, Sun B, LaTour JV, Kahne D.J Am Chem Soc 200612814012–14013.For S. aureus PBP2:Barrett D, Leimkuhler C, Chen L, Walker D, Kahne D, Walker S.J Bacteriol 20051872215–2217.(e) See Supporting Information for the cloning and purification of E. faecalis ΔTMPBP2A. The TM anchor has no effect on the length distributions of glycan strands produced in vitro (See Fig. S1). The truncated construct was used for experiments shown in Fig. 3 because it does not aggregate like the full-length construct
- 5.Ye XY, Lo MC, Brunner L, Walker D, Kahne D, Walker S. J Am Chem Soc. 2001;123:3155–3156. doi: 10.1021/ja010028q. [DOI] [PubMed] [Google Scholar]
- 6.The amino acid at position three of the Lipid II pentapeptide varies among organisms. In E. coli., the pentapeptide sequence is L-Ala-D-Glu-meso-A2pm-D-Ala-D-Ala, while in E. faecalis and S. aureus, it is an L-Lys modified with either L-Ala-L-Ala and (Gly)5, respectively. Previous work has suggested that PGTs are not sensitive to the identity of the amino acid in the third position of the pentapeptide. See the following references. Schwartz B, Markwalder JA, Seitz SP, Wang Y, Stein RL. Biochemistry. 2002;41:12552–12561. doi: 10.1021/bi026205x.Liu H, Wong CH. Bioorg Med Chem. 2006;14:7187–7195. doi: 10.1016/j.bmc.2006.06.058.
- 7.To verify the accuracy of the enzyme:substrate ratio, the enzyme concentration were determined by active site titration with moenomycin, as described in reference 2b. E. coli PBP1A and E. faecalis ΔTMPBP2A were >90% active.
- 8.A slow initiation step was also observed in polyhydroxybutyrate synthase and hyaluronan synthase. Wodzinska J, Snell KD, Rhomberg A, Sinskey AJ, Biemann K, Stubbe J. J Am Chem Soc. 1996;118:6319–6320.Stubbe J, Tian J, He A, Sinskey AJ, Lawrence AG, Liu P. Annu Rev Biochem. 2005;74:433–480. doi: 10.1146/annurev.biochem.74.082803.133013.Weigel PH, DeAngelis PL. J Biol Chem. 2007;282:36777–36781. doi: 10.1074/jbc.R700036200.
- 9.(a) Scheffers DJ, Pinho MG. Microbiol Mol Biol Rev. 2005;69:585–607. doi: 10.1128/MMBR.69.4.585-607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Popham DL, Young KD. Curr Opin Microbiol. 2003;6:594–599. doi: 10.1016/j.mib.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Vollmer W, Bertsche U. Biochim Biophys Acta. 2008;1778:1714–1734. doi: 10.1016/j.bbamem.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 11.(a) Dmitriev BA, Toukach FV, Schaper KJ, Holst O, Rietschel ET, Ehlers S. J Bacteriol. 2003;185:3458–3468. doi: 10.1128/JB.185.11.3458-3468.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dmitriev B, Toukach F, Ehlers S. Trends Microbiol. 2005;13:569–574. doi: 10.1016/j.tim.2005.10.001. [DOI] [PubMed] [Google Scholar]; (c) Meroueh SO, Bencze KZ, Hesek D, Lee M, Fisher JF, Stemmler TL, Mobashery S. Proc Natl Acad Sci USA. 2006;103:4404–4409. doi: 10.1073/pnas.0510182103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Young KD. Trends Microbiol. 2006;14:155–156. doi: 10.1016/j.tim.2006.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available: Experimental procedures, including the cloning and purification of E. faecalis ΔTMPBP2A and the details of SDS-PAGE assays are described. This material is available free of charge via the Internet at http://pubs.acs.org/.


